Abstract
The aim of the present study was to investigate the plasma levels of C-reactive protein (CRP) and copeptin, in addition to the acute physiology and chronic health evaluation II (APACHE II) scores, in patients with acute organophosphorus pesticide poisoning (AOPP). A total of 100 patients with AOPP were included and divided into mild, moderate and severe groups according to AOPP diagnosis and classification standards. Blood samples were collected from all patients on days 1, 3 and 7 following AOPP. The concentrations of CRP and copeptin in the plasma were determined using enzyme-linked immunosorbent assay. All AOPP patients underwent APACHE II scoring and the diagnostic value of these scores was analyzed using receiver operating characteristic curves (ROCs). On days 1, 3 and 7 after AOPP, the levels of CRP and copeptin were increased in correlation with the increase in AOPP severity, and were significantly higher compared with the control groups. Furthermore, elevated CRP and copeptin plasma levels were detected in patients with severe AOPP on day 7, whereas these levels were reduced in patients with mild or moderate AOPP. APACHE II scores, blood lactate level, acetylcholine esterase level, twitch disappearance time, reactivating agent dose and inability to raise the head were the high-risk factors that affected the prognosis of AOPP. Patients with plasma CRP and copeptin levels higher than median values had worse prognoses. The areas under curve for ROCs were 0.89, 0.75 and 0.72 for CRP levels, copeptin levels and APACHE II scores, respectively. In addition, the plasma contents of CRP and copeptin are increased according to the severity of AOPP. Therefore, the results of the present study suggest that CRP and copeptin levels and APACHE II scores may be used for the determination of AOPP severity and the prediction of AOPP prognosis.
Keywords: acute organophosphorus pesticide poisoning, C-reactive protein, copeptin, acute physiology and chronic health evaluation II scoring, receiver operating characteristic curve
Introduction
Acute organophosphorus pesticide poisoning (AOPP) is among the most common medical acute conditions with complex symptoms and a high mortality rate (1–3). Patients with AOPP typically exhibit mortality-associated complications such as secondary infections, myocardial injury, liver-kidney dysfunction and multiple organ failure (4). Currently, AOPP severity is usually evaluated on the basis of patient symptoms, including dizziness, headaches, nausea, vomiting, salivation, sweating, blurred vision and signs of fatigue (5), and routine blood and urine laboratory tests. Urine tests typically assess the organophosphorus metabolic product content. In addition, the determination of cholinesterase levels in the plasma of patients with AOPP is widely used in the clinical diagnosis, treatment and prognosis prediction of AOPP (6,7).
C-reactive protein (CRP) is a reactive substance in acute lesions, and elevated plasma levels of CRP are a result of inflammation and trauma. In AOPP, toxins may cause lesions in tissues and organs in the body, leading to increased plasma CRP levels (8). In addition, copeptin has good stability in the plasma, usually indicating the levels of antidiuretic hormone (9). Copeptin is considered to be a novel marker that indicates individual stress and is thus used in the prognostic evaluation of diseases caused by system disorders (10). Therefore, plasma CRP and copeptin may reflect the degree of lesions caused by AOPP. Furthermore, acute physiology and chronic health evaluation II (APACHE II) scoring, as a commonly used clinical scoring method, may be applied to evaluate the severity and prognosis of acute critical diseases (9,11,12). However, to the best of our knowledge, there has been no reports investigating the changes in plasma CRP and copeptin levels in patients with AOPP, or evaluating the AOPP prognosis using APACHE II scoring. Thus, the aims of the present study were to evaluate the changes of CRP and copeptin levels in the plasma of AOPP patients, and to determine the prognosis of AOPP using APACHE II scoring.
Materials and methods
Patients
Between April 2012 and May 2014, 100 patients with AOPP admitted to the Affiliated Hospital of Taishan Medical University (Tai'an, China) were selected for the study. The diagnosis of AOPP was based on the following criteria: i) History of exposure to toxins; ii) typical clinical manifestations and symptoms (5); iii) symptom improvement following treatment with reactivating agent; and iv) reduction in activity of plasma cholinesterase. All patients had accidentally ingested organophosphorus pesticides (50–450 ml). According to the AOPP diagnosis and classification standards (13), 54 patients presented mild AOPP, 32 presented moderate AOPP and 14 presented severe AOPP. The basic clinical characteristics of all patients are listed in Table I. In addition, 100 healthy subjects, including 45 men and 55 women with an average age of 39.6±8.17 years (age range, 30–50 years), were included as the control group. The gender ratio and average age of the control group was not significantly different, as compared with the AOPP group. The present study was approved by the Ethics Committee of Taishan Medical University. Written informed consent was obtained from all patients or their families.
Table I.
Clinical characteristics of patients.
| Characteristic | Severe (n=14) | Moderate (n=32) | Mild (n=54) |
|---|---|---|---|
| Female (n) | 9 | 18 | 34 |
| Male (n) | 5 | 14 | 20 |
| Unable to raise head (n) | 13 | 28 | 43 |
| Age (years) | 17.1±5.12 | 29.4±7.41 | 30.1±10.27 |
| APACHE II score | 20.91±2.65 | 12.54±3.56 | 9.75±5.63 |
| Blood lactate level (mmol/l) | 5.67±0.52 | 3.53±0.49 | 2.26±0.21 |
| Acetylcholine esterase level (IU/l) | 324.4±107.8 | 862.7±98.6 | 2,011.1±185.6 |
| Twitch disappearance time (h) | 29.51±5.68 | 12.52±7.52 | 9.24±8.23 |
| Atropine dose (mg) | 301.4±120.4 | 256.8±96.4 | 150.4±63.8 |
| Reactivating agent dose (g) | 15.8±3.4 | 12.5±5.2 | 9.5±2.5 |
Data are presented as n or the mean ± standard deviation. Normal values are as follows: Blood lactate level, <2.4 mmol/l; acetylcholine esterase level, 130–310 U/l; normal twitch disappearance time, <4 times/min. APACHE II, acute physiology and chronic health evaluation II.
All AOPP patients received routine gastric lavage with water, oxygen intake, electrocardiogram monitoring and intravenous injection with 1–1.5 g pralidoxime chloride (Sihuan Pharmaceutical Holdings Group, Co., Ltd., Beijing, China) and 1–2 mg atropine (China Resources Double-Crane Pharmaceutical, Co., Ltd., Beijing, China). In cases of respiratory failure, the patients were subjected to intubation and ventilation, assisted by the S9 VPAP™ Auto double level automatic ventilator (ResMed, San Diego, CA, USA). In cases of asystole, the patients were treated with chest compression and cardiopulmonary resuscitation, in addition to water and electrolyte imbalance rectification, nutritional improvement and appropriate dehydration. Patients with severe symptoms underwent plasma purification therapy.
APACHE II scoring
APACHE II scoring was conducted in order to measure the severity of AOPP in patients (14). APACHE II scores are determined from a patient's age and physiological indicators, including the alveolar-arterial oxygen gradient or partial pressure of oxygen in arterial blood, body temperature (rectal), mean arterial pressure, arterial pH, heart rate, respiratory rate, serum levels of sodium and potassium, levels of creatinine, the hematocrit, white blood cell count, and the Glasgow Coma Scale (14). The worst value for each indicator within 1 day after hospitalization was recorded into a electronic table produced by Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA). Patients with one missing/unrecorded indicator received an APACHE II score of zero, while those with more than two missing/unrecorded indicators were not scored. Factors associated with a high-risk of disease progression and mortality, including blood lactate level, acetylcholine esterase level, twitch disappearance time and an inability to raise the head, as determined by our hospital, were measured via routine blood and urine tests upon admission of the patients to the hospital. If these factors were exacerbated following active treatment, the patient was considered to have toxification progression. A higher APACHE II score corresponded to a more severe disease and indicated an elevated risk of mortality (14)
Enzyme-linked immunosorbent assay (ELISA)
On days 1, 3 and 7, 5 ml plasma was obtained from fasting peripheral blood. The concentrations of plasma CRP and copeptin were measured using the CRP ELISA kit (DRG Instruments GmbH, Marburg, Germany) and the Copeptin ELISA kit (48T/96T; MLBIO, Shanghai, China), respectively, according to the manufacturer's protocol. The protein expression levels were quantified using the SpectraMax® M5 Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA, USA) and analyzed using the SoftMax® Pro Data Acquisition and Analysis software, version 5.4.1 (Molecular Devices, LLC).
Statistical analysis
All the results were analyzed using SPSS software, version 19.0 (IBM SPSS, Armonk, NY, USA). The data are expressed as the mean ± standard deviation. Differences between groups were compared using the Student's t-test, and multivariate analysis was performed using logistic regression analysis. The Kaplan-Meier method was used to analyze the prognostic asymptomatic survival curves in relation to the CRP and copeptin levels in the patient plasma. Analysis of prognostic determination was performed using the area under the receiver operating characteristic (ROC) curve. P<0.05 was considered to indicate a statistically significant difference.
Results
Levels of CRP and copeptin in patients with severe AOPP are increased with time, but not in patients with moderate or mild AOPP
To measure the changes of CRP and copeptin contents in the plasma after treatment, ELISA was conducted. The plasma CRP and copeptin levels were increased with the increase of AOPP severity on days 1, 3 and 7 after AOPP, and were significantly higher compared with those in the control group (P<0.05). Patients with severe AOPP exhibited increasing plasma CRP and copeptin contents over time (P<0.01), while patients with mild and moderate AOPP exhibited reduced plasma CRP and copeptin contents over time. On day 7 after AOPP, plasma CRP and copeptin contents in all severity groups of AOPP patients remained significantly increased compared with the levels in the control group (P<0.05; Table II). These results suggest that the plasma levels of CRP and copeptin in patients with severe AOPP increased over time; however, this is did not occur in patients with moderate or mild AOPP.
Table II.
Changes in plasma CRP and copeptin contents in AOPP patients following treatment.
| CRP level in different groups (mg/l) | Copeptin level in different groups (ng/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| Time | Severe | Moderate | Mild | Control | Severe | Moderate | Mild | Control |
| Day 1 | 23.5±11.2 | 15.2±9.4 | 7.3±4.2 | 3.2±2.3 | 11.4±5.9 | 7.2±3.6 | 2.3±1.1 | 0.8±0.3 |
| Day 3 | 27.6±15.2 | 12.3±5.4 | 5.3±3.5 | 3.2±2.3 | 13.2±6.3 | 5.9±2.4 | 1.5±0.8 | 0.8±0.3 |
| Day 7 | 63.5±29.5a | 8.3±3.8a | 4.3±2.1a | 3.2±2.3 | 33.5±12.7b | 4.1±1.6b | 1.1±0.7b | 0.8±0.3 |
P<0.01 vs. control for CRP group on the same day
P<0.05 vs. control for copeptin group on the same day. CRP, C-reactive protein; AOPP, acute organophosphorus pesticide poisoning.
Amelioration of relevant factors improves the prognosis of AOPP
To evaluate the effect of relative factors on the prognosis of AOPP, logistic regression analysis was used. The data showed that higher APACHE II scores, low blood lactate and acetylcholine esterase levels, an increased twitch disappearance time, a higher reactivating agent dose and an inability to raise the head were high risk factors (P<0.05; Table III). Therefore, the amelioration of these parameters may improve the prognosis of AOPP.
Table III.
Logistic regression analysis of the effect of multiple factors on prognosis.
| 95.0% CI for Exp (B) | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | SE | Wald | Sig | Exp (B) | Upper limit | Lower limit |
| Blood lactate level | 2.298 | 0.618 | 30.417 | 0.036 | 4.463 | 1.491 | 9.293 |
| APACHE II scores | 4.153 | 0.385 | 20.154 | 0.001 | 3.163 | 1.141 | 10.698 |
| Acetylcholine esterase level | 3.396 | 0.601 | 7.986 | 0.002 | 3.038 | 1.642 | 8.125 |
| Twitch disappearance time | 1.150 | 0.516 | 4.977 | 0.026 | 3.160 | 1.150 | 8.681 |
| Inability to raise head | 3.012 | 0.412 | 27.138 | 0.001 | 5.934 | 1.118 | 8.637 |
| Reactivating agent dose | 2.146 | 0.634 | 25.141 | 0.005 | 4.362 | 1.637 | 9.153 |
CI, confidence interval; Exp (B), odds ratio; B, coefficient of regression; SE, standard error; Wald, index of regression effect; Sig, P-value; APACHE II, acute physiology and chronic health evaluation II.
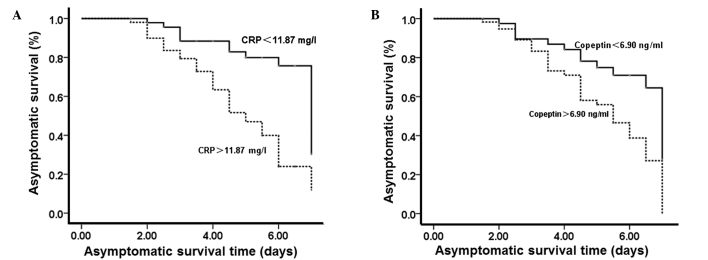
Increased plasma levels of CRP and copeptin indicate a poorer prognosis
To test the effect of plasma CRP and copeptin on prognosis, Kaplan-Meier analysis was performed on the prognostic asymptomatic survival curves. According to the plasma CRP and copeptin levels at 1 week after AOPP, the number of patients with CRP levels that were higher and lower than the median value (11.87 mg/l) was 15 and 85, respectively. In addition, the number of patients with copeptin levels that were higher and lower than the median value (6.90 ng/ml) was 19 and 81, respectively. Kaplan-Meier analysis of prognostic asymptomatic curves showed that patients with plasma CRP and copeptin levels higher than the median values had a worse prognosis (P<0.05; Fig. 1). The results suggest that higher CRP and copeptin levels in the plasma indicate a poorer prognosis.
Figure 1.
Effect of plasma levels of (A) CRP and (B) copeptin on the asymptomatic survival time. One week after acute organophosphorus pesticide poisoning, the median CRP level was 11.87 mg/l and the median copeptin level was 6.90 ng/ml. Kaplan-Meier analysis of prognostic asymptomatic curves was performed. CRP, C-reactive protein.
CRP and copeptin levels and APACHE II scores can be used as predictors of AOPP patient survival
To evaluate the predictive role of CRP levels, copeptin levels and APACHE II scores on the survival of AOPP patients, ROC curves were plotted using SPSS software. The areas under the ROC curves were 0.89, 0.75 and 0.72 for CRP, copeptin and APACHE II scores, respectively (P<0.01). In addition, the optimum diagnostic cut-off points for CRP, copeptin and APACHE II scores were 10.2 mg/l, 6.3 ng/ml and 10.5, respectively. Notably, all the CRP levels, copeptin levels and APACHE II scores had a sensitivity and specificity of >90% (Fig. 2). The results indicate that CRP levels, copeptin levels and APACHE II scores may be used for the prediction of survival in AOPP patients.
Figure 2.

ROC curve analysis of plasma levels of CRP and copeptin, and APACHE II scores. ROC curves were plotted using SPSS software. Statistical significance was indicated when the area under the ROC curve was >0.5. ROC, receiver operating characteristic; APACHE II, acute physiology and chronic health evaluation II; CRP, C-reactive protein.
Discussion
The present study showed that the levels of CRP and copeptin on days 1, 3 and 7 after AOPP were increased according to the severity of AOPP, and were significantly increased compared with the control group. In addition, the levels of CRP and copeptin in patients with severe AOPP increased over time, while those in patients with mild or moderate AOPP decreased over time. This may be due to the degree of toxification. Patients with mild or moderate AOPP exhibit reduced acetylcholine stimulation of the cholinergic nerves, stress responses, lesions on organs and inflammation, leading to relatively low plasma CRP levels. By contrast, patients with severe AOPP have severe poisoning of tissues and organs, multiple organ failure and severe inflammation, leading to relatively high plasma CRP levels. A previous study demonstrated that AOPP patients have elevated plasma copeptin levels, as compared with normal subjects, and that its levels increase with increasing poisoning severity (15). In addition, this difference is negatively correlated with the levels of acetylcholine esterase, an indicator that reflects AOPP severity (16). Consistent with this observation, the results of the present study indicated that the plasma copeptin content was closely associated with poisoning lesions in AOPP patients, and was variable at different time points.
A previous study indicated that APACHE II scoring reflects the degree of lesions in organophosphate poisoning, with higher scores indicating a higher possibility of respiratory failure (17). Another study suggested that acetylcholine accumulation in AOPP patients affects the peripheral cholinergic nerves and inhibits the central nervous system (8). Logistic regression analysis in the present study demonstrated that APACHE II scores, blood lactate level, acetylcholine esterase level, twitch disappearance time, inability to raise head and reactivating agent dose are high-risk factors for the prognosis of AOPP patients. Kaplan-Meier analysis of asymptomatic survival curve suggested that patients with plasma CRP or copeptin levels higher than the median values were more likely to present other clinical symptoms and reduced treatment efficacy, leading to poorer prognosis. In addition, ROC analysis in the present study demonstrated that the plasma CRP and copeptin levels and APACHE II scoring have good sensitivity and specificity for the evaluation of AOPP prognosis.
The treatment of AOPP is a clinical challenge for emergency physicians. Active CRP and copeptin plasma levels, APACHE II scoring, evaluation of disease and prognosis, and effective treatment plans are directly associated with the survival of patients. In conclusion, the present study revealed that changes in the CRP and copeptin plasma levels, and the APACHE II scoring may be associated with the prediction of AOPP prognosis. However, the present study consisted of a relatively small number of patients, and did not investigate the effect of combined cardiovascular, diabetic, liver or kidney dysfunctions on the plasma levels of CRP and copeptin; thus, further studies are required to confirm the findings.
Acknowledgements
The authors would like to thank Dr Changqin Li and Dr Jixue Shi at the Affiliated Hospital of Taishan Medical University for their assistance with the design of the experiments and write-up.
References
- 1.Wang Y, Lin K, Wu D, Wang Z, Wang Q, Mi X, Liu C. Clinical investigations of changes in serum enzymes and C-reactive protein in patients with acute toxification. Zhong Guo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16:55–56. (In Chinese) [Google Scholar]
- 2.Wang Z, Lv Y, Cui T. Analysis of cause of death by acute organophosphorus pesticide poisoning. Zhong Guo She Qu Yi Shi. 2012;14:106–107. (In Chinese) [Google Scholar]
- 3.Han L, Cui X. Application of intestinal protection in adjuvant therapy of severe organophosphorus poisoning. Shi Yong Yao Wu Yu Lin Chuang. 2010;13:154–156. (In Chinese) [Google Scholar]
- 4.Katan M, Morgenthaler N, Widmer I, Puder JJ, König C, Müller B, Christ-Crain M. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29:341–346. [PubMed] [Google Scholar]
- 5.Li H, Zhao C, Qiu Q. Clinical characteristics and prognosis of ICU patients with severe acute organic phosphorus poisoning. Xian Dai Zhong Xi Yi Jie He Za Zhi. 2008;17:1812–1813. (In Chinese) [Google Scholar]
- 6.Zhang X, Li Q, Liu Y, Zhao J. Determination of blood amylase in patients with acute organophosphorus pesticide poisoning. Zhong Guo Wei Zhong Bing Ji Jiu Yi Xue. 2000;12:433. (In Chinese) [Google Scholar]
- 7.Wang J, Liu H, Yang Y, Zhang R, Zhang Z. Clinical meaning of morphologic alteration of neutrophils in the patients with acute organic phosphorus poisoning. Zhong Guo Ji Jiu Yi Xue. 2002;22:561–562. (In Chinese) [Google Scholar]
- 8.Pan D, Zhang Z. Clinical value of high sensitivity C-reactive protein on AOPP and prognosis of patients with the disease to determine. Hua Bei Mei Tan Yi Xue Tuan Xue Bao. 2011;13:599–600. (In Chinese) [Google Scholar]
- 9.Zhang H, Ren G, Yin J, Sun W. Copeptin expression in patients with acute organophosphorus poisoning and its clinical significance. Shi Yong Yi Xue Xa Zhi She. 2013;29:576–578. (In Chinese) [Google Scholar]
- 10.Zheng X. Current situation and advance of APACHE-II score in the clinical application. Yi Xue Zong Shu. 2011;17:3297–3298. (In Chinese) [Google Scholar]
- 11.Sam KG, Kondabolu K, Pati D, Kamath A, Kumar Pradeep G, Rao PG. Poisoning severity score, APACHE II and GCS Effective clinical indices for estimating severity and predicting outcome of acute organophosphorus and carbamate poisoning. J Forensic Leg Med. 2009;16:239–247. doi: 10.1016/j.jflm.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Akdur O, Durukan P, Ozkan S, Avsarogullari L, Vardar A, Kavalci C, Ikizceli I. Poisoning severity score, Glasgow coma scale, corrected QT interval in acute organophosphate poisoning. Hum Exp Toxicol. 2010;29:419–425. doi: 10.1177/0960327110364640. [DOI] [PubMed] [Google Scholar]
- 13.Dai Z, Shi Y, Nei KX. Practice of Internal Medicine. 13th. Beijing, China: People's Health Publishing House; 2009. pp. 1723–1724. [Google Scholar]
- 14.Knaus WA, Draper EA, Wanger DP, Zimmerman JE. APACHE II. A severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Wang R, He J. Copeptin and ischemia modified albumin expression and significance of early in acute paraquat poisoning. Shi Yong Lin Chuang Yi Xue Za Zhi. 2014;18:40–43. (In Chinese) [Google Scholar]
- 16.Hu N, Huang M, Li W. The value of APACHE II scoring system in assessment of severity and prognosis of patients with organophosphate insecticide poisoning. Hua Zhong Ke Ji Da Xue Tong Ji Yi Xue Yuan. 2011;12:25–29. (In Chinese) [Google Scholar]
- 17.Deng Y, Wang F, Sun H, Wang X, Wang X. Risk factor in the patients with intermediate syndrome after acute organophosphorus pesticides poisoning. Zhong Guo Ji Jiu Yi Xue. 2008;28:704–706. (In Chinese) [Google Scholar]