Abstract
Diabetic foot infections (DFIs) constitute a major complication of diabetes mellitus. DFIs contribute to the development of gangrene and non-traumatic lower extremity amputations with a lifetime risk of up to 25%. The aim of the present study was to identify the presence of neuropathy and determine the ulcer grade, microbial profile and phenotypic and genotypic prevalence of the methicillin-resistance gene mecA and extended spectrum β-lactamase (ESBL)-encoding genes in bacterial isolates of DFI in patients registered at the Pakistan Institute of Medical Sciences (Islamabad, Pakistan). The results indicated that 46/50 patients (92%), exhibited sensory neuropathy. The most common isolate was Staphylococcus aureus (25%), followed by Pseudomonas aeruginosa (P. aeruginosa; 18.18%), Escherichia coli (16.16%), Streptococcus species (spp.) (15.15%), Proteus spp. (15.15%), Enterococcus spp. (9%) and Klebsiella pneumoniae (K. pneumoniae; 3%). The prevalence of the mecA gene was found to be 88% phenotypically and 84% genotypically. K. pneumoniae was shown to have the highest percentage of ESBL producers with a prevalence of 66.7% by double disk synergy test, and 100% by the cefotaxime + clavulanic acid/ceftazidime + clavulanic acid combination disk test. P. aeruginosa and K. pneumoniae had the highest (100%) proportion of metallo β-lactamase producers as identified by the EDTA combination disk test. The overall prevalence of β-lactamase (bla)-CTX-M, bla-CTX-M-15, bla-TEM, bla-OXA and bla-SHV genes was found to be 76.9, 76.9, 75.0, 57.7 and 84.6%, respectively, in gram-negative DFI isolates. The prevalence of mecA and ESBL-related genes was found to be alarmingly high in DFIs, since these genes are a major cause of antibiotic treatment failure.
Keywords: diabetic foot ulcer, prevalence, antibiotic resistance, extended spectrum β-lactamase
Introduction
Diabetes, including Type I and Type II, is the most common non-communicable disease (NCD), with an overall prevalence of 8.3% worldwide, and is the fourth or fifth leading cause of mortality in developed countries (1,2). Type II diabetes accounts for the majority (>85%) of the total diabetes prevalence (3). It is estimated that 15–25% of patients with diabetes will develop a diabetic foot ulcer (DFU) at some point during their lifetime (1), and this includes patients with Type I or Type II diabetes (4). According to the International Diabetic Federation Report of 2005 (5), 85% of diabetes-related lower extremity amputations were preceded by DFUs.
Hyperglycemia causes microvascular complications, including neuropathy, retinopathy and nephropathy (6). The primary function of normal, intact skin is to control microbial populations that live on the skin surface and to prevent underlying tissues from becoming colonized and invaded by potential pathogens (7). In diabetes, a loss of sensation in the lower extremities may occur, which is known as neuropathy (8). Neuropathic individuals are highly prone to physical injuries in their lower extremities (9). Any such injury is a potential cause of a DFU, since hyperglycemia reduces blood flow and the phagocytic activity of neutrophils and macrophages (10). The most grave consequence of DFUs is limb amputation, which occurs 10–30 times more frequently in patients with diabetes than in the general population (46.1–9,600 individuals/105 vs. 5.8–31 individuals/105, respectively) (11–13). The mortality rates following amputation are 13–40% in the first year, 35–65% in the first 3 years, and 39–80% in the first 5 years (14).
In addition to the maintenance of glycemic control, surgical debridement, wound care, pressure offloading and adequate blood flow maintenance, it is also important to evaluate the type of microorganisms in infected wounds (15). Infection can convert simple injuries to gangrene and cause osteomyelitis, leading to lower extremity amputation (16). The majority of mild infections are monomicrobial and caused by aerobic gram-positive cocci, such as Staphylococcus aureus (S. aureus) and Streptococcus species (spp.). By contrast, the most severe infections are polymicrobial and caused by aerobic gram-positive cocci, gram-negative bacilli and anaerobes (17,18).
The emergence of antibiotic resistance in infecting bacteria can complicate and prolong the treatment regime, and may even cause chronic wounds to become gangrenous (19). It is noteworthy that methicillin-resistant S. aureus (MRSA) infections are associated with a higher mortality rate compared with methicillin-susceptible S. aureus infections (20). The mecA gene, which is responsible for methicillin resistance, encodes an altered penicillin-binding protein (PBP2A) with a low affinity for β-lactam antibiotics (21,22). Extended spectrum β-lactamases (ESBLs) are a group of enzymes encoded by genes that are common among Enterobacteriaceae (23). Most ESBLs are mutants of Temoneira (TEM)-, sulfhydryl variable (SHV)- and cefotaximase (CTX-M)-type lactamases, which hydrolyze cefotaxime and ceftriaxone, and are weakly active against ceftazidime (24,25). Metallo β-lactamases (MBLs), which require divalent cations, usually zinc, as metal cofactors for enzyme activity, are very broad spectrum β-lactamases with the ability to hydrolyze virtually all classes of β-lactams, including extended spectrum cephalosporins and carbapenems (26).
Genetic variations and the high incidence of resistance genes in microbes hinder the control of infections in DFUs and have an important role in the manifestation of the disease (27). It is therefore important to investigate the prevalence of these genes in patients with DFUs in order to facilitate the prompt control of infection. The aim of the present study was to investigate the phenotypic and genotypic prevalence of mecA and ESBLs in bacteria isolated from DFU samples.
Materials and methods
Sample size and inclusion and exclusion criteria
In total, 50 diabetic patients with DFU were included in the study between January 21, 2013 and July 20, 2013. The study was approved by the Ethics Committee and Institutional Review Board of the Atta-ur-Rahman School of Applied Biosciences (Islamabad, Pakistan). Patients of all ages and genders with type 2 diabetes with a foot infection or DFU who visited the Diabetic Foot Clinic and Out-Patient Department of Pakistan Institute of Medical Sciences (Islamabad, Pakistan) were included. The exclusion criteria were as follows: Non-diabetic patients with foot infection, diabetic patients that had previously received antibiotic treatment, patients with type 1 diabetes with foot infection, and DFUs with a duration of >3 weeks. Information on the duration of the diabetes and DFUs, glycemic control, and history of previous hospitalization due to the DFU was obtained from all patients. The patients were examined for the presence of sensory neuropathy and peripheral vascular disease by measuring the ankle brachial index. The foot ulcers were classified according to the New University of Texas classification system (28).
Isolation, identification and antimicrobial susceptibility testing of the microbes
Cultures of the specimens were obtained at the time of admission, after the surface of the wound had been washed vigorously with saline, followed by debridement of the superficial tissue from the exudates to avoid the isolation of colonizing flora. Bacteria were isolated through the inoculation of specimens on a set of selective and non-selective media, such as blood agar and MacConkey and chocolate agars (Difco; BD Biosciences, Franklin Lakes, NJ, USA). All inoculated plates were incubated at 37°C for 24–48 h. The bacterial isolates were identified using conventional biochemical tests.
The Kirby-Bauer disk diffusion method (29) was used for antibiotic susceptibility testing. The Clinical and Laboratory Standard Institute (CLSI) guidelines were followed for the selection of media, inoculum turbidity and preparation of media plates along with the application of disks and the interpretation of the zone of inhibition. Suspension was inoculated on the media plate with the assistance of a sterile glass spreader. Oxoid™ antibiotic disks (Thermo Fisher Scientific, Inc., Waltham, MA, USA) were applied using sterile forceps. The zone of inhibition around the tested antibiotics was measured, and interpretations were made using the breakpoints elaborated in the CLSI guidelines (30).
Phenotypic detection of ESBL
Isolates that showed intermediate resistance to third-generation cephalosporin were screened to detect ESBL production. A double disk synergy test (DDST) was performed for the phenotypic detection of ESBL production (31). A third-generation cephalosporin disk was placed on the plate with another disk containing amoxicillin + clavulanic acid; other combination disks, such as ampicillin + salbactam and piperacillin + tazobactam, were also tested. Plates were incubated at 37°C for 18–24 h and the shape of the zone of inhibition was noted. Isolates that exhibited a distinct potentiation towards the amoxicillin + clavulanic acid disk were considered potential ESBL producers. Escherichia coli (E. coli) ATCC 25922 and Klebsiella pneumoniae (K. pneumoniae) ATCC 700603 were used as negative and positive controls for ESBL, respectively. Phenotypic detection of MBLs was performed using an ethylenediaminetetraacetic acid (EDTA) disk synergy test (32). For this, an imipenem disk was placed on a plate inoculated with bacterial suspension, and 5 µl 0.5 M EDTA was poured on another imipenem disk and placed on the same plate; differences in the size of inhibition zone were then observed.
Molecular detection of antibiotic resistance genes
For the molecular detection of antibiotic resistance genes, the phenol-chloroform method was used to extract DNA from bacterial samples (33). Bacterial cells were grown in Luria Bertani broth overnight at 37°C and suspended in lysis buffer (0.2 mg/ml proteinase K in 1% sodium dodecyl sulfate). The suspension was then incubated for 1 h at 55°C and DNA was extracted twice using phenol-chloroform. Subsequently, sodium acetate (0.3 M) and cold ethanol were added to precipitate DNA. The precipitate was centrifuged (Sigma 1–14; Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany) at 14,462 × g for 2 min at room temperature, after which the DNA pellet was suspended in 100 µl Tris-EDTA buffer. mecA, β-lactamase (bla)-SHV, bla-CTX-M, bla-CTX-M-15, bla-TEM and bla-oxacillinase (OXA) genes were detected using polymerase chain reaction (PCR). For PCR, a total reaction mixture volume of 25 µl was prepared containing 2 mM deoxyribonucleotide triphosphates, 10X PCR buffer, 50 mM MgCl2, 50 pM of each primer, 5 µl DNA sample, 1 unit of thermo stable Taq DNA polymerase (Fermentas; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and nuclease-free water to adjust the final volume. The reaction mixture was centrifuged (Sigma 1–14; Sigma Laborzentrifugen GmbH) at 14,462 × g for 30 secs at room temperature for thorough mixing. Subsequently, PCR was conducted using the Swift™ MaxPro thermal cycler (Applied Biosystems; Thermo Fisher Scientific, Inc., Foster City, USA). The primer sequences are presented in Table I (34–37). The PCR products were separated by 1% agarose gel electrophoresis, in which a 100 bp DNA ladder was used as a reference (Invitrogen; Thermo Fisher Scientific, Inc.).
Table I.
Primer sequences of mecA and extended spectrum β-lactamase genes.
| Target gene | Primer | Sequence (5′-3′) | Tm, °C | GC, % | Ref. |
|---|---|---|---|---|---|
| bla-SHV | F | CTTTATCGGCCCTCACTCAA | 60.4 | 50 | (25) |
| R | AGGTGCTCATCATGGGAAAG | 60.4 | 50 | ||
| bla-OXA | F | GGCACCAGATTCAACTTTCAAG | 60.8 | 45 | (26) |
| R | GACCCCAAGTTTCCTGTAAGTG | 62.7 | 50 | ||
| bla-CTX-M | F | ATGTGCAGTACCAGTAAAGTGATGGC | 64.6 | 46 | (27) |
| R | TGGGTAAAATAAGTCACCAGAATCAGCGG | 66 | 45 | ||
| bla-TEM | F | CGCCGCATACACTATTCTCAGAATGA | 64.6 | 46 | (28) |
| R | ACGCTCACCGGCTCCAGATTTAT | 64.6 | 52 | ||
| bla-CTX-M-15 | F | AGGCAGACTGGGTGTGGCAT | 64.5 | 60 | – |
| R | TTACCCAGCGTCAGATTCCG | 62.4 | 55 | ||
| mecA | F | GTAGAAATGACTGAACGTCCGATAA | 54.4 | 40 | – |
| R | ATTGGCCAATTCCACATTGTTTCG | 54 | 42 |
bla, β-lactamase; SHV, sulfhydryl variable; OXA, oxacillinase; CTX-M, cefotaximase; TEM, Temoneira; F, forward; R, reverse; Ref., reference; Tm, melting temperature; GC, GC nucleotide content.
Results
Neuropathy and ulcer grade
A total of 50 patients fulfilled the inclusion criteria and were included in the study. Out of those 50 subjects, 29 (58%) were men and 21 (42%) women (age range, 36–80 years; mean age, 58 years). According to the University of Texas Classification, 11 patients had stage-B grade-I ulcer, 16 had stage-B grade-II ulcer, 7 had stage-B grade-III ulcer, 2 had stage-C grade-I ulcer, 1 had stage-C grade-II, 1 had stage-D grade-I ulcer, 5 had stage-D grade-II ulcer and 7 had stage-D grade-III ulcer (Table II). The specimens containing clinically significant pathogens included wound swabs (37/50; 74%), tissue (7/50; 14%), pus (4/50; 8%) and bone (2/50; 4%). Of the 50 patients, 46 patients (92%) had sensory neuropathy while 4 patients (8%) did not (Fig. 1).
Table II.
Stages and grades of DFU in the patients according to the University of Texas Classification System (19).
| DFU grade | ||||
|---|---|---|---|---|
| Stage of ulcer | I | II | III | Total |
| A | 0 | 0 | 0 | 0 |
| B | 11 | 16 | 7 | 34 |
| C | 2 | 1 | 0 | 3 |
| D | 1 | 5 | 7 | 13 |
| Total | 14 | 22 | 14 | 50 |
DFU, diabetic foot ulcer.
Figure 1.
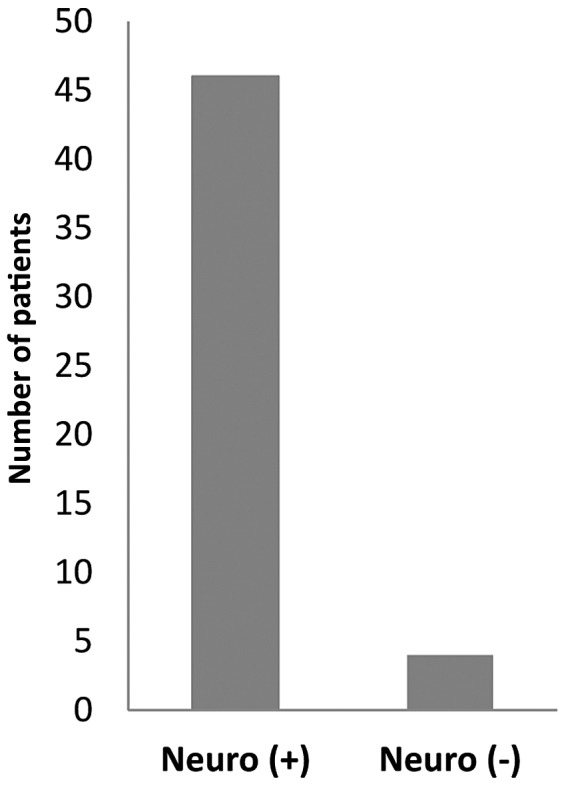
Prevalence of sensory neuropathy in diabetic foot ulcer patients. There were 46 patients (92%) with sensory neuropathy [Neuro (+)] and 4 patients (8%) without [Neuro (−)].
Microbiology and antimicrobial susceptibility testing of diabetic foot wounds sample
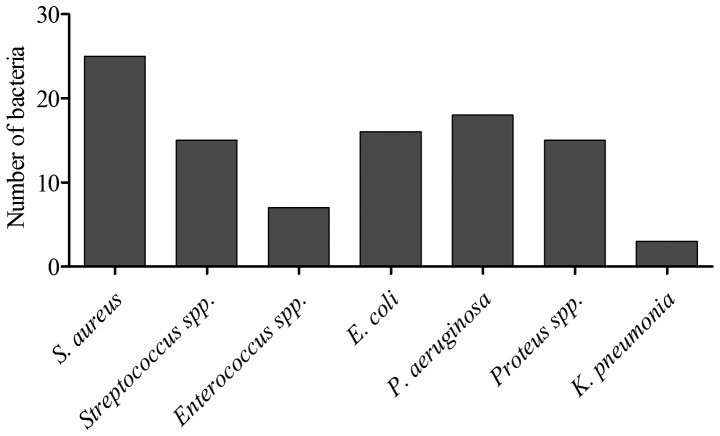
A total of 99 bacterial isolates were obtained from the 50 patients with DFUs. In these patients, gram-negative bacilli (59.59%) were isolated more frequently than gram-positive cocci (40.4%). The most common isolate was S. aureus (25%), followed by Pseudomonas aeruginosa (P. aeruginosa; 18.18%) and E. coli (16.16%). The other isolated organisms were Streptococcus spp. (15.15%), Enterococcus spp. (9%), Proteus spp. (15.15%) and K. pneumoniae (3%) (Fig. 2). Single organisms were isolated from 14 samples (28%) and mixed bacterial growths were identified in 36 samples (72%). The details of the organisms isolated from the infected foot lesions are presented in Fig. 2. The mean number of isolates per culture-positive sample was 1.9.
Figure 2.
Microbiology of DFUs. Distribution of microbes isolated from DFU samples. DFU, diabetic foot ulcer; SA, Staphylococcus aureus; E. coli, Escherichia coli; PA, Pseudomonas aeruginosa; KP, Klebsiella pneumoniae; spp, species.
All S. aureus isolates were resistant to penicillin, out of which 90% exhibited resistance against oxacillin, cefoxitin and ceftazidime. Vancomycin showed inhibitory effects for only 20% of the S. aureus isolates. By contrast, 100% of the Streptococcus spp. isolates exhibited resistance to oxicillin, cefotaxime, ceftriaxone, cefepime, cefoxitin, ceftazidime and penicillin, while 40% showed susceptibility to vancomycin. According to the sensitivity data, 70% of the S. aureus and 80% of Streptococcus spp. isolates were multidrug-resistant (Table III). All P. aeruginosa and K. pneumoniae isolates, as well as 83.33% of the Proteus spp. and 75% of the E. coli isolates were found to be resistant to ceftazidime. All gram-negative isolates exhibited high susceptibility to chloramphenicol and meropenem. K. pneumoniae and P. aeruginosa were found to be the most resistant, with >60% of strains exhibiting antibiotic resistance, whereas for Proteus spp. and E. coli, <55% of strains were resistant (Table IV).
Table III.
Antibiotic resistance (%) pattern of gram-positive bacteria.
| Antibiotics | S. aureus | Streptococcus spp. |
|---|---|---|
| Oxicillin | 90 | 100 |
| Cefotaxime | 80 | 100 |
| Ceftriaxone | 80 | 100 |
| Cefepime | 70 | 100 |
| Cefoxitin | 90 | 100 |
| Ceftazidime | 90 | 100 |
| Aztreonam | 30 | 80 |
| Imepenem | 50 | 80 |
| Gentamicin | 30 | 0 |
| Cefoperazone | 50 | 60 |
| Amoxicillin + clavulanic acid | 40 | 60 |
| Ampicillin + sulbactam | 30 | 60 |
| Tazobactam + piperacillin | 0 | 40 |
| Chloramphenicol | 0 | 20 |
| Penicillin | 100 | 100 |
| Vancomycin | 80 | 60 |
S. aureus, Staphylococcus aureus; spp., species.
Table IV.
Antibiotic resistance (%) pattern of gram-negative bacteria.
| Antibiotics | K. pneumoniae | P. aeruginosa | Proteus spp. | E. coli |
|---|---|---|---|---|
| Oxicillin | 100 | 66.7 | 83.3 | 50 |
| Cefotaxime | 80 | 100 | 83.3 | 75 |
| Ceftriaxone | 100 | 100 | 100 | 50 |
| Cefepime | 100 | 100 | 83.3 | 50 |
| Cefoxitin | 80 | 100 | 100 | 75 |
| Ceftazidime | 100 | 100 | 83.3 | 75 |
| Aztreonam | 60 | 33.3 | 50 | 50 |
| Imepenem | 60 | 66.7 | 33.3 | 50 |
| Cefoperazone | 60 | 100 | 66.7 | 50 |
| Amoxicillin + clavulanic acid | 80 | 100 | 16.7 | 50 |
| Ampicillin + sulbactam | 60 | 66.7 | 33.3 | 50 |
| Tazobactam + piperacillin | 80 | 66.7 | 33.3 | 75 |
| Amikacin | 60 | 33.3 | 33.3 | 25 |
| Meropenim | 40 | 0 | 16.7 | 25 |
| Chloramphenicol | 20 | 0 | 0 | 25 |
K. pneumoniae, Klebsiella pneumoniae; P. aeruginosa, Pseudomonas aeruginosa; E. coli, Escherichia coli; spp., species.
Phenotypic and genotypic detection of MRSA and ESBL
The phenotypic prevalence of the mecA gene was found to be 88%. No inhibition zone was observed around the 30-µg cefoxitin disk. Out of 25 S. aureus strains, 21 were found to be positive for the mecA gene PCR, with an overall prevalence of 84%. DDST results showed that 66.66, 33.33, 66.7 and 50% of the Proteus spp., P. aeruginosa, K. pneumoniae and E. coli populations, respectively, were ESBL producers. In the case of the cefotaxime + clavulanic acid/ceftazidime + clavulanic acid combination disk test for ESBL detection, a >5-mm increase was observed in the size of the zone of inhibition around the cefotaxime or ceftazidime disk containing the ESBL inhibitor clavulanic acid, as compared with the simple cefotaxime or ceftazidime disk. According to the cefotaxime + clavulanic acid/ceftazidime + clavulanic acid combination disk test, K. pneumoniae and E. coli had the highest prevalence (100%) of ESBL producers, while the EDTA disk synergy test confirmed P. aeruginosa and K. pneumoniae as having the highest prevalence of MBL producers; E. coli was found to have the lowest prevalence, with only 37% of strains producing MBL (Table V).
Table V.
Phenotypic detection of methicillin resistance, extended spectrum β-lactamase and metallo β-lactamase production (%).
| Bacterial isolates | Double disk synergy test | CTX + CL/CAZ + CL combination disk test | EDTA combinationt disk test |
|---|---|---|---|
| Proteus spp. | 66.7 | 73 | 80 |
| P. aeruginosa | 33.3 | 33 | 100 |
| K. pneumoniae | 66.7 | 100 | 100 |
| E. coli | 50 | 100 | 37 |
CTX, cefotaxime; CL, clavulanic acid; CAZ, ceftazidime; EDTA, ethylenediaminetetraacetic acid; P. aeruginosa, Pseudomonas aeruginosa; K. pneumoniae, Klebsiella pneumoniae; E. coli, Escherichia coli; spp., species.
PCR showed that all K. pneumoniae and E. coli isolates were positive for bla-CTX-M, bla-CTX-M-15, bla-TEM, bla-OXA and bla-SHV resistance genes (Fig. 3). In addition, 72.2% of the P. aeruginosa strains were positive for bla-SHV and 66.7% for bla-CTX-M, bla-CTX-M-15 and bla-TEM. However, only 33.3% of the P. aeruginosa strains were positive for the bla-OXA gene. In Proteus spp., the resistance gene with the highest prevalence (80%) was bla-SHV (Table VI).
Figure 3.
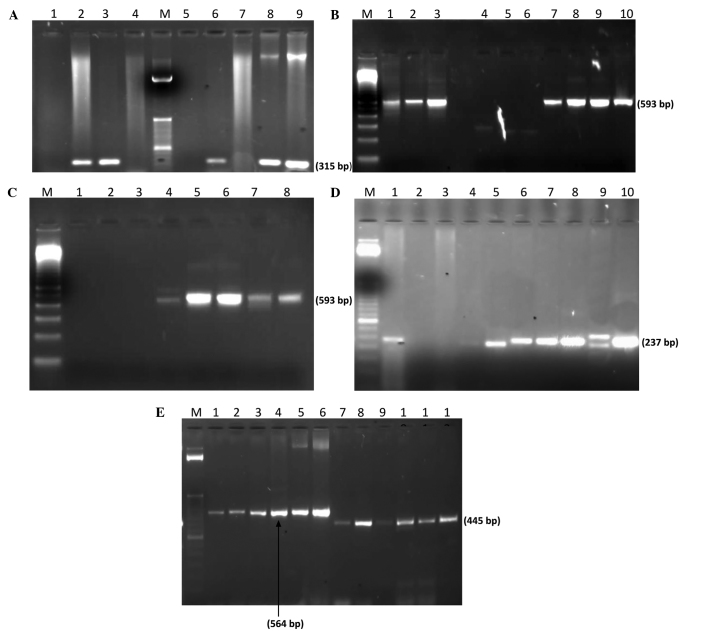
Molecular detection of antibiotic resistance genes. (A) PCR amplification of mecA gene: Lane M; 100 bp ladder (Invitrogen); lane 1, negative control (K. pneumoniae ATCC BAA-1144); lane 2, positive control (S. aureus ATCC BAA-1026); lanes 3, 6, 8 and 9, mecA gene expression in S. aureus. (B) PCR amplification of bla-CTXM gene: Lane M, 100 bp Ladder; lane 1, Proteus spp.; lanes 2 and 3, E. coli; lane 7, P. aeruginosa; lanes 8–11, K. pneumoniae. (C) PCR amplification of bla-CTXM-15 gene: Lane M, 100 bp ladder; lane 1, negative control (K. pneumoniae ATCC BAA-1144); lane 4, P. aeruginosa; lanes 5 and 6, E. coli; lane 7, K. pneumoniae; lane 8, Proteus spp. (D) PCR amplification of bla-SHV gene: Lane M, 50 bp ladder (Invitrogen); lane 10, positive control (K. pneumoniae ATCC 700603); lane 2, negative control (E. coli ATCC 25922); lane 5, P. aeruginosa; lanes 6–8, E. coli; lanes 9 and 10, K. pneumoniae. (E) PCR amplification of bla-TEM and bla-OXA genes: Lane M; 50 bp ladder; lane 1, Proteus spp.; lane 2, P. aeruginosa; lanes 3 and 4, K. pneumoniae; lanes 5 and 6, E. coli; lane 7, Proteus spp.; lane 8, P. aeruginosa; lanes 9 and 10, K. pneumoniae; lane 11 and 12, E. coli. PCR, polymerase chain reaction; bla, β-lactamase; CTX-M, cefotaximase; SHV, sulfhydryl variable; TEM, Temoneira; OXA, oxacillinase; ATCC, American Type Culture Collection; K. pneumoniae, Klebsiella pneumoniae, S. aureus, Staphylococcus aureus; P. aeruginosa, Pseudomonas aeruginosa; E. coli, Escherichia coli, spp., species.
Table VI.
Molecular detection of extended spectrum β-lactamase production (%).
| Bacterial isolates | bla-CTX-M | bla-CTX-M15 | bla-TEM | bla-OXA | bla-SHV |
|---|---|---|---|---|---|
| Proteus spp. | 60 | 60 | 53.3 | 33.3 | 80 |
| P. aeruginosa | 66.7 | 66.7 | 66.7 | 33.3 | 72.2 |
| K. pneumoniae | 100 | 100 | 100 | 100 | 100 |
| E. coli | 100 | 100 | 100 | 100 | 100 |
| Total | 76.9 | 76.9 | 75 | 57.7 | 84.6 |
bla, β-lactamase; CTX-M, cefotaximase; SHV, sulfhydryl variable; OXA, oxacillinase; TEM, Temoneira; P. aeruginosa, Pseudomonas aeruginosa; K. pneumoniae, Klebsiella pneumoniae; E. coli, Escherichia coli; spp., species.
Discussion
The high occurrence of DFUs and amputation within the diabetic population has become an increasingly alarming public health concern in the developed and developing worlds (38). These complications begin with neuropathy, which occurs as a result of hyperglycemia and involves a loss of sensation in the lower extremities (8). Neuropathic individuals are highly prone to physical injuries in their lower extremities (9), which lead to diabetic foot infections (DFIs), which in turn may result in the amputation of the lower extremities and subsequent mortality (11–13). DFUs impose a tremendous medical and financial burden on the health care system in the USA, with a cost as high as $45,000 per patient; in addition, the impaired mobility and substantial loss of productivity associated with DFUs affects the quality of life of the patient (39).
Diabetic neuropathy, along with poor blood circulation in the lower extremities, nerve damage and foot wounds, constitutes one of the leading causes of DFUs (40), which is consistent with previous studies in which up to 92% of patients with DFUs also had neuropathy (41,42). The treatment prognosis is exacerbated when an ulcer is infected with multiple microbes (43), since little is known about multi-species interactions or the ideal antibiotic regimen for the treatment of multi-species infections. In the present study gram-positive bacteria, including S. aureus, were observed to be dominant in these infections; which is consistent with previous reports (44,45). The assays performed in the present study also identified high percentages of multidrug-resistant P. aeruginosa, which is troubling as P. aeruginosa is an aggressive gram-negative bacillus (46).
High rates of antibiotic resistance have previously been reported in patients with diabetes (47). Richard et al (48) found that the most common causative agent of DFIs, S. aureus, represented 36.5% of isolates from DFUs and, notably, 37.4% of these were MRSA. In the present study, however, it was found that 84% of S. aureus isolates were MRSA, while 20% were vancomycin resistant. S. aureus isolates have been associated with prolonged bacteremia, greater rates of infection-associated complications and vancomycin treatment failure (49). Among the K. pneumoniae isolates of the present study, a very high incidence of ESBL in the bacteria was detected by phenotypic testing (100% by the cefotaxime + clavulanic acid/ceftazidime + clavulanic acid combination disk test), which is comparable with previous studies, where up to 97% prevalence has been reported (50,51). The highest prevalence of MBL producers was found in P. aeruginosa and K. pneumoniae (100% in the EDTA combination disk test). In a study from India, 74.5% of the ceftazidime-resistant P. aeruginosa isolates were found to be MBL producers (52). In another study from Iran, 53% of the 94 ceftazidime resistant P. aeruginosa isolates were found to be MBL producers (53).
The PCR analysis conducted in the present study revealed that 21 out of 25 S. aureus isolates (84%) harbored the mecA gene, a considerably high proportion compared with that observed in the study by Bukhari et al (54), which found an MRSA prevalence of 41.9% in clinical isolates in Lahore, Pakistan. In addition, in the present study all K. pneumoniae and E. coli isolates were 100% positive for all ESBL genes. The high prevalence of the CTX-M gene in the present study was in concordance with the results of the study of Šeputienė et al (55), who reported CTX-M encoding genes in the majority of E. coli (96%) and K. pneumoniae (71%) isolates showing the ESBL phenotype. ESBL-positive E. coli isolates investigated in Sweden encoded mainly CTX-Ms (92%), followed by TEM-type (63%), OXA-type (59%) and SHV-type (6%) β-lactamases (34). According to a study by Bali et al (56), TEM-type ESBLs were found in 72.72% of E. coli and 75% of K. pneumoniae. A study conducted by Umadevi et al (57) observed that ESBL production in P. aeruginosa is less prevalent than that in Enterobacteriaceae, which is consistent with the results of the present study. This finding limits treatment options considerably, causing great concern regarding the lack of adequate treatment and the spread of mecA- and ESBL-carrying isolates in DFIs.
In conclusion, infections caused by multidrug-resistant bacteria that produce mecA and ESBL enzymes have been reported with an increasing frequency in DFIs and are associated with amputation. Epidemiological information helps in the design of better programs for infection control. Due to the resistance of DFIs to numerous antimicrobial agents, treatment can be challenging. Hence, it is recommended that active surveillance for ESBL-producing pathogens in populations at high-risk for DFUs is performed using appropriate antimicrobial techniques.
Acknowledgements
This study was supported by the Higher Education Commission and Ministry of Science and Technology of Pakistan. The authors would like to thank Mr. Muhammad Shafique from the Microbiology Laboratory of the Pakistan Institute of Medical Sciences, for generously helping with the isolation and characterization of bacterial strains from DFU samples.
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Mutlu F, Bener A, Eliyan A, Delghan H, Nofal E, Shalabi L, Wadi N. Projection of Diabetes Burden through 2025 and Contributing Risk Factors of Changing Disease Prevalence: An Emerging Public Health Problem. J Diabetes Metab. 2014;5:341. doi: 10.1016/j.dsx.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine. 2010;38:602–606. doi: 10.1016/j.mpmed.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cusick M, Meleth AD, Agrón E, Fisher MR, Reed GF, Knatterud GL, Barton FB, Davis MD, Ferris FL, Chew EY. Early Treatment Diabetic Retinopathy Study Research Group: Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: Early treatment diabetic retinopathy study report no. 27. Diabetes Care. 2005;28:617–625. doi: 10.2337/diacare.28.3.617. [DOI] [PubMed] [Google Scholar]
- 5.Clayton W, Elasy TA. A Review of the Pathophysiology, Classification, and Treatment of Foot Ulcers in Diabetic Patients. Clin Diabetes. 2009;27:52–58. doi: 10.2337/diaclin.27.2.52. [DOI] [Google Scholar]
- 6.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clinical diabetes. 2008;26:77–82. doi: 10.2337/diaclin.26.2.77. [DOI] [Google Scholar]
- 7.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: Clinical and quality-of-life issues. Mayo Clin Proc. 2006;81(Suppl 4):S3–S11. doi: 10.1016/S0025-6196(11)61474-2. [DOI] [PubMed] [Google Scholar]
- 9.Podwall D, Gooch C. Diabetic neuropathy: Clinical features, etiology, and therapy. Curr Neurol Neurosci Rep. 2004;4:55–61. doi: 10.1007/s11910-004-0013-9. [DOI] [PubMed] [Google Scholar]
- 10.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Siitonen OI, Niskanen LK, Laakso M, Siitonen JT, Pyörälä K. Lower-extremity amputations in diabetic and nondiabetic patients: A population-based study in eastern Finland. Diabetes care. 1993;16:16–20. doi: 10.2337/diacare.16.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Trautner C, Haastert B, Giani G, Berger M. Incidence of lower limb amputations and diabetes. Diabetes care. 1996;19:1006–1009. doi: 10.2337/diacare.19.9.1006. [DOI] [PubMed] [Google Scholar]
- 13.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy. Eur Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiber GE. Epidemiology of foot ulcers and amputations in the diabetic foot. The diabetic foot. 2001;6:13–32. [Google Scholar]
- 15.Tiwari S, Pratyush DD, Dwivedi A, Gupta SK, Rai M, Singh SK. Microbiological and clinical characteristics of diabetic foot infections in northern India. J Infec Dev Ctries. 2012;6:329–332. doi: 10.3855/jidc.1827. [DOI] [PubMed] [Google Scholar]
- 16.Lipsky B, Pecoraro R, Wheat L. The diabetic foot. Soft tissue and bone infection. Infect Dis Clin North Am. 1990;4:409–432. [PubMed] [Google Scholar]
- 17.Frykberg RG. An evidence-based approach to diabetic foot infections. Am J Surg. 2003;186(Suppl):S44–S54. doi: 10.1016/j.amjsurg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Benwan K, Al Mulla A, Rotimi VO. A study of the microbiology of diabetic foot infections in a teaching hospital in Kuwait. J Infect Public Health. 2012;5:1–8. doi: 10.1016/j.jiph.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Roberts RR, Hota B, Ahmad I, Scott RD, 2nd, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: Implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 20.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 21.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 22.Askari E, Soleymani F, Arianpoor A, Tabatabai SM, Amini A, Nasab Naderi M. Epidemiology of mecA-methicillin resistant Staphylococcus aureus (MRSA) in Iran: A systematic review and meta-analysis. Iran J Basic Med Sci. 2012;15:1010–1019. [PMC free article] [PubMed] [Google Scholar]
- 23.Poole K. Resistance to beta-lactam antibiotics. Cell Mol Life Sci. 2004;61:2200–2223. doi: 10.1007/s00018-004-4060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnet R. Growing group of extended-spectrum beta-lactamases: The CTX-M enzymes. Antimicrob Agents Chemother. 2004;48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez F, Endimiani A, Hujer KM, Bonomo RA. The continuing challenge of ESBLs. Curr Opin Pharmacol. 2007;7:459–469. doi: 10.1016/j.coph.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: The quiet before the storm? Clin Microbiol Rev. 2005;18:306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahi SK, Singh VK, Kumar A. Detection of Escherichia coli and associated β-lactamases genes from diabetic foot ulcers by multiplex PCR and molecular modeling and docking of SHV-1, TEM-1, and OXA-1 β-lactamases with clindamycin and piperacillin-tazobactam. PLoS One. 2013;8:e68234. doi: 10.1371/journal.pone.0068234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: The Wagner and the University of Texas wound classification systems. Diabetes care. 2001;24:84–88. doi: 10.2337/diacare.24.1.84. [DOI] [PubMed] [Google Scholar]
- 29.Bauer AW, Perry DM, Kirby WM. Single-disk antibiotic-sensitivity testing of Staphylococci: An analysis of technique and results. AMA Arch Intern Med. 1959;104:208–216. doi: 10.1001/archinte.1959.00270080034004. [DOI] [PubMed] [Google Scholar]
- 30.Performance Standards for Antimicrobial Susceptibility Testing: Twenty-first Informational Supplement (M100-S21) Wayne, PA: CLSI; 2011. Clinical and Laboratory Standards Institute (CLSI) [Google Scholar]
- 31.Singhal S, Mathur T, Khan S, Upadhyay DJ, Chugh S, Gaind R, Rattan A. Evaluation of methods for AmpC beta-lactamase in gram negative clinical isolates from tertiary care hospitals. Indian J Med Microbiol. 2005;23:120–124. doi: 10.4103/0255-0857.16053. [DOI] [PubMed] [Google Scholar]
- 32.Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. Imipenem-EDTA disk method for differentiation of metallo-beta-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40:3798–3801. doi: 10.1128/JCM.40.10.3798-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahcheraghi F, Nikbin VS, Feizabadi MM. Prevalence of ESBLs genes among multidrug-resistant isolates of Pseudomonas aeruginosa isolated from patients in Tehran. Microb Drug Resist. 2009;15:37–39. doi: 10.1089/mdr.2009.0880. [DOI] [PubMed] [Google Scholar]
- 34.Fang H, Ataker F, Hedin G, Dornbusch K. Molecular epidemiology of extended-spectrum beta-lactamases among Escherichia coli isolates collected in a Swedish hospital and its associated health care facilities from 2001 to 2006. J Clin Microbiol. 2008;46:707–712. doi: 10.1128/JCM.01943-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 36.Boyd DA, Tyler S, Christianson S, McGeer A, Muller MP, Willey BM, Bryce E, Gardam M, Nordmann P, Mulvey MR. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob Agents Chemother. 2004;48:3758–3764. doi: 10.1128/AAC.48.10.3758-3764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monstein HJ, Ostholm-Balkhed A, Nilsson M, Nilsson M, Dornbusch K, Nilsson L. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS. 2007;115:1400–1408. doi: 10.1111/j.1600-0463.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues BT, Gilhotra RA, Vangaveti VN, Malabu UH. Prevalence and risk factors of lower limb amputation amongst diabetic food ulcer patients at the Townsville Hospital. Annals of the ACTM. 2014;15:57. [Google Scholar]
- 39.Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care. 2004;27:2129–2134. doi: 10.2337/diacare.27.9.2129. [DOI] [PubMed] [Google Scholar]
- 40.Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62:923–930. doi: 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, LeFrock JL, Lew DP, Mader JT, Norden C, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885–910. doi: 10.1086/424846. [DOI] [PubMed] [Google Scholar]
- 42.Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M. Risk stratification systems for diabetic foot ulcers: A systematic review. Diabetologia. 2011;54:1190–1199. doi: 10.1007/s00125-010-2030-3. [DOI] [PubMed] [Google Scholar]
- 43.James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 44.Mantey I, Hill R, Foster A, Wilson S, Wade JJ, Edmonds ME. Infection of foot ulcers with Staphylococcus aureus associated with increased mortality in diabetic patients. Commun Dis Public Health. 2000;3:288–290. [PubMed] [Google Scholar]
- 45.Dang CN, Prasad YD, Boulton AJ, Jude EB. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: A worsening problem. Diabet Med. 2003;20:159–161. doi: 10.1046/j.1464-5491.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- 46.Gadepalli R, Dhawan B, Sreenivas V, Kapil A, Ammini AC, Chaudhry R. A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes care. 2006;29:1727–1732. doi: 10.2337/dc06-0116. [DOI] [PubMed] [Google Scholar]
- 47.Trivedi U, Parameswaran S, Armstrong A, Burgueno-Vega D, Griswold J, Dissanaike S, Rumbaugh KP. Prevalence of Multiple Antibiotic Resistant Infections in Diabetic versus Nondiabetic Wounds. J Pathog. 2014;2014:173053. doi: 10.1155/2014/173053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richard JL, Sotto A, Lavigne JP. New insights in diabetic foot infection. World J Diabetes. 2011;2:24–32. doi: 10.4239/wjd.v2.i2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satola SW, Lessa FC, Ray SM, Bulens SN, Lynfield R, Schaffner W, Dumyati G, Nadle J, Patel JB. Active Bacterial Core surveillance (ABCs) MRSA Investigators: Clinical and laboratory characteristics of invasive infections due to methicillin-resistant Staphylococcus aureus isolates demonstrating a vancomycin MIC of 2 micrograms per milliliter: Lack of effect of heteroresistant vancomycin-intermediate S. aureus phenotype. J Clin Microbiol. 2011;49:1583–1587. doi: 10.1128/JCM.01719-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harish BN, Menezes GA, Shekatkar S, Parija SC. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae from blood culture. J Med Microbiol. 2007;56:999–1000. doi: 10.1099/jmm.0.47072-0. [DOI] [PubMed] [Google Scholar]
- 51.Parveen RM, Khan MA, Menezes GA, Harish BN, Parija SC, Hays JP. Extended-spectrum β-lactamase producing Klebsiella pneumoniae from blood cultures in Puducherry, India. Indian J Med Res. 2011;134:392–395. [PMC free article] [PubMed] [Google Scholar]
- 52.Umadevi S, Joseph NM, Kumari K, Easow JM, Kumar S, Stephen S, Srirangaraj S, Raj S. Detection of extended spectrum beta lactamases, AmpC beta lactamases and metallobetalactamases in clinical isolates of ceftazidime resistant Pseudomonas aeruginosa. Braz J Microbiol. 2011;42:1284–1288. doi: 10.1590/S1517-83822011000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saderi H, Karimi Z, Owlia P, Bahar MA, Rad SMBA. phenotypic detection of metallo-beta-lactamase producing Pseudomonas aeruginosa strains isolated from burned patients. Iranian Journal of Pathology. 2008;3:20–24. [Google Scholar]
- 54.Bukhari SZ, Ahmed S, Zia N. Antimicrobial susceptibility pattern of Staphylococcus aureus on clinical isolates and efficacy of laboratory tests to diagnose MRSA: MRSA: A multi-centre study. J Ayub Med Coll Abbottabad. 2011;23:139–142. [PubMed] [Google Scholar]
- 55.Šeputienė V, Linkevičius M, Bogdaitė A, Povilonis J, Plančiūnienė R, Giedraitiene A, Pavilonis A, Sužiedėlienė E. Molecular characterization of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates from hospitals in Lithuania. J Med Microbiol. 2010;59:1263–1265. doi: 10.1099/jmm.0.021972-0. [DOI] [PubMed] [Google Scholar]
- 56.Bali EB, Accedil L, Sultan N. Phenotypic and molecular characterization of SHV, TEM, CTX-M and extended-spectrum-lactamase produced by Escherichia coli, Acinobacter baumannii and Klebsiella isolates in a Turkish hospital. African Journal of Microbiology Research. 2010;4:650–654. [Google Scholar]
- 57.Umadevi S, Kandhakumari G, Joseph NM, Kumar S, Easow JM, Stephen S, Singh UK. Prevalence and antimicrobial susceptibility pattern of ESBL producing gram negative bacilli. J Clin Diagn Res. 2011;5:236–239. [Google Scholar]