Abstract
The differentiation of cardiac fibroblasts (CFs) into myofibroblasts and the subsequent deposition of the extracellular matrix is associated with myocardial fibrosis following various types of myocardial injury. In the present study, the effect of curcumin, which is a pharmacologically-safe natural compound from the Curcuma longa herb, on transforming growth factor (TGF)-β1-induced CFs was investigated, and the underlying molecular mechanisms were examined. The expression levels of α-smooth muscle actin (SMA) stress fibers were investigated using western blotting and immunofluorescence in cultured neonatal rat CFs. Protein and mRNA expression levels of α-SMA and collagen type I (ColI) were determined by western blotting and reverse transcription-quantitative polymerase chain reaction. In addition, the activation of Smad2 and p38 was examined using western blotting. Curcumin, SB431542 (a TGF-βR-Smad2 inhibitor) and SB203580 (a p38 inhibitor) were used to inhibit the stimulation by TGF-β1. The results demonstrated that the TGF-β1-induced expression of α-SMA and ColI was suppressed by curcumin at the mRNA and protein levels, while SB431542 and SB203580 induced similar effects. Furthermore, phosphorylated Smad-2 and p38 were upregulated in TGF-β1-induced CFs, and these effects were substantially inhibited by curcumin administration. In conclusion, the results of the present study demonstrated that treatment with curcumin effectively suppresses TGF-β1-induced CF differentiation via Smad-2 and p38 signaling pathways. Thus, curcumin may be a potential therapeutic agent for the treatment of cardiac fibrosis.
Keywords: curcumin, cardiac fibroblast, transforming growth factor-β1, Smad2, p38 mitogen-activated protein kinase
Introduction
Cardiac fibroblast differentiation, excessive biosynthesis and destruction of the interstitial extracellular matrix (ECM) in the ventricles of the heart are key features of cardiac fibrosis, which is a consequence of cardiac remodeling initiated by pathological events associated with various cardiovascular disorders (1). Induced by transforming growth factor (TGF)-β1 and other factors, cardiac fibroblasts (CFs) differentiate into α-smooth muscle actin (SMA) fiber-rich cardiac myofibroblasts that facilitate contractility and increase ECM modulation ability (1). Although these changes are important for wound repair and are beneficial for the maintenance of cardiac function, continuous myocardial fibrosis may result in abnormal myocardial stiffness and, ultimately, ventricular dysfunction (1).
Curcumin, 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, is a natural polyphenolic compound from the Curcuma longa herb, possessing multiple biological and medicinal activities (2). The pharmacological safety of curcumin has previously been demonstrated in various animal models, and curcumin is nontoxic even at high doses (3). Previous studies have demonstrated that curcumin has antioxida, anti-inflammatory, anti-proliferative, pro-apoptotic and anti-carcinogenic properties (4–8). Furthermore, previous investigations into the effects of curcumin in heart disease have indicated that curcumin has a regulatory role in the cardiac remodeling process; curcumin has been demonstrated to ameliorate and reverse cardiac fibrosis, cardiac hypertrophy and heart failure in animal models (9–13). Therefore, curcumin may represent a novel therapeutic strategy for the treatment of cardiac remodeling.
TGF-β1 is a key mediator of the differentiation of fibroblasts to myofibroblasts (14), and this TGF-β1-induced effect has been demonstrated to be associated with the Smad2 and p38 mitogen-activated protein kinase (MAPK) signaling pathways (15–17). Although it has previously been demonstrated that curcumin has an inhibitory effect on ECM secretion in cultured CFs (10), the effect of curcumin on the differentiation of CFs and the underlying mechanisms are yet to be fully elucidated. In the present study, using cultured CFs from neonatal rats, it was demonstrated that curcumin has an inhibitory effect on TGF-β1-induced cardiac fibroblast differentiation. Furthermore, the role of Smad2 and p38 MAPK signaling in the activation of CFs and the anti-fibrotic mechanism of curcumin in the modulation of the TGF-β1 induced effects was addressed.
Materials and methods
Reagents
TGF-β1 was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Curcumin and rabbit monoclonal anti-α-SMA antibody (SP171) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal anti-collagen type I (ColI; ab34710) and -von Willebrand factor (ab6994) primary antibodies were purchased from Abcam (Cambridge, MA, USA), whereas mouse monoclonal anti-vimentin antibody (zm-0260), -glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (TA-08), and horseradish peroxidase (HRP)-conjugated goat anti-rabbit and goat anti-mouse immunoglobulin (Ig)G secondary antibodies (ZB-2301 and ZB-2305) were purchased from Zhongsan Jinqiao Biotechnology Co., Ltd. (Beijing, China). Phosphorylated and non-phosphorylated monoclonal rabbit anti-rat Smad2 (#3108 and #5339) and p38 (#9215 and #2371) primary antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). SB431542, a TGF-βR-Smad2/3 inhibitor was purchased from Cayman Chemical Company (Ann Arbor, MI, USA) and SB20358, a p38 inhibitor, was purchased from Merck Millipore (Darmstadt, Germany). Cell culture materials and TRIzol® reagent were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The DNase and primers used for the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) were purchased from Sunbiotech (Beijing, China).
Cell culture and treatment
The present study was conducted with the approval of the Ethics Committee of Experimental Animal Center of Shanxi Cardiovascular Hospital (Taiyuan, China), according to the regulations outlined by the National Institutes of Health Guidelines on the Use of Laboratory Animals. CFs were isolated from neonatal (1–3-days-old) Sprague-Dawley rats via trypsin digestion methods, as previously described (18). CFs were cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum, and were passaged using trypsin (1:3). Second passage CFs were used in the present study and were serum-starved for 24 h at 80% confluence in order to induce quiescence. Immunocytochemical analysis demonstrated that the purity of the CFs used was >95%, according to positive staining for vimentin and negative staining for von Willebrand factor.
In order to elucidate the potential effects of curcumin on the various signaling pathways in TGF-β1-induced CFs, CFs were pretreated with 20 µmol/l curcumin, 10 µM TGF-βR-Smad2 inhibitor (SB431542) or 10 µM p38 MAPK inhibitor (SB203580) for 30 min, prior to treatment with recombinant 10 ng/ml TGF-β1 for 24 h. In order to investigate the activation of Smad2 and p38, CFs were treated with 10 ng/ml TGF-β1 for 1 h. Cells were subsequently harvested and stored at −80°C prior to the determination of protein and mRNA expression levels.
Immunofluorescent staining
CFs were cultured on coverslips in 6-well plates (2.5×105 cells/well). Growth was arrested and CFs were treated as described earlier. Following a 24-h treatment, CFs were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 (Applygen Technologies, Inc., Beijing, China). Non-specific binding was blocked via incubation with 10% normal goat serum (Applygen Technologies, Inc.). Subsequently, α-SMA was detected using a Cy5-conjugated goat anti-rabbit α-SMA polyclonal antibody (Abcam; ab6564) and stained cells were visualized using the BX51 Fluorescence Microscope (Olympus Corporation, Tokyo, Japan).
Western blotting
CFs were harvested in lysis buffer (Applygen Technologies, Inc.) containing 20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM Na3VO4 (pH 7.5), 1 mM phenylmethanesulfonyl fluoride, 1 mM benzamidine, 10 µg/ml leupeptin and 10 µg/ml aprotinin. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA), according to the manufacturer's protocol. Following boiling for 1 min to denature, 20 µg protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes were blocked with 5% fat-free milk in Tris-buffered saline with Tween-20 (TBST; 10 mM Tris HCl, 150 mM NaCl and 0.1% Tween-20) for 1 h at room temperature, and incubated overnight with primary antibodies (1:2,500) at 4°C. Following each incubation, membranes were washed three times for 10 min with TBST and subsequently incubated with HRP-conjugated secondary antibody (1:5,000) for 1 h at room temperature. Using chemiluminescence (Applygen Technologies, Inc.), the membranes were scanned and quantified using Quantity-One software, version 4.2 (Bio-Rad Laboratories, Inc.), and the results were presented as the optical density of phosphorylated-protein/total protein or of the target protein/GAPDH.
RNA isolation and RT-qPCR
Total RNA was extracted from CFs using TRIzol® reagent and treated with DNase, according to the manufacturer's protocols. RT-qPCR analyses were performed using an ABI 7300 system (Applied Biosystems; Thermo Fisher Scientific, Inc.). For each sample, 1 µg RNA was utilized to synthesize cDNA using the Reverse Transcriptase kit (Promega Corporation, Madison, WI, USA), according to the manufacturer's protocol. The specific primer sequences used were as follows: α-SMA forward, 5′-CATCAGGAACCTCGAGAAGC-3′, and reverse, 5′-TCGGATACTTCAGGGTCAGG-3′; ColI forward, 5′-CATAAAGGGTCATCGTGGCTTC-3′, and reverse, 5′-GTGATAGGTGATGTTCTGGGAG-3′; and GAPDH forward, 5′-AACCTGCCAAGTATGATGACATCA, and reverse, 5′-TTCCACTGATATCCCAGCTGCT-3′. Target genes were amplified using SYBR Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). The PCR cycling conditions were as follows: 95°C for 5 min, followed by 40 cycles of 95°C for 35 sec and 60°C for 1 min, with a final extension step of 72°C for 5 min. Relative mRNA levels were calculated using the 2−ΔΔCq method (19), where ΔCq was the difference between GAPDH and target gene critical threshold cycle (Cq) values.
Statistical analyses
Statistical analyses in this study were conducted using the SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). The results are presented as the mean ± standard error of the mean. Between-groups differences were assessed using one-way factorial analysis of variance. P<0.05 was considered to indicate a statistically significant difference. Each assay in the present study was performed in triplicate.
Results
Treatment with curcumin impairs TGF-β1-induced cardiac fibroblast differentiation
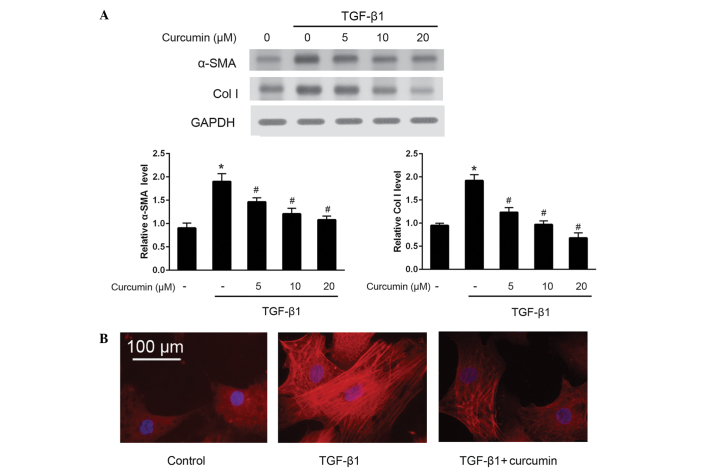
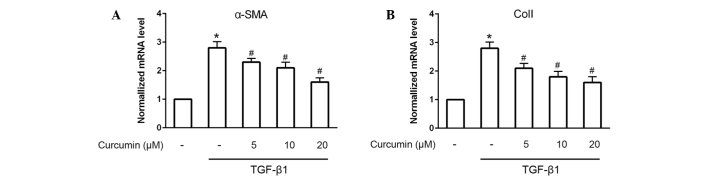
To investigate the effects of curcumin on the differentiation of CFs into myofibroblasts, western blotting was used to detect the protein expression levels of α-SMA and ColI (Fig. 1A). The results demonstrated that curcumin significantly suppressed the TGF-β1-induced protein expression of α-SMA and ColI in CFs (P<0.05), in a dose-dependent manner. These anti-differentiation effects were further validated by immunofluorescence staining of α-SMA (Fig. 1B). As compared with untreated CFs, cells induced by 10 ng/ml TGF-β1 exhibited bright fluorescence staining signals for α-SMA and prominent stress fibers; by contrast, treatment with 20 µM curcumin significantly attenuated α-SMA fluorescence signals and morphological characteristics of CFs induced by 10 ng/ml TGF-β1 (P<0.05). Furthermore, RT-qPCR was performed to analyze the mRNA expression levels of α-SMA and ColI. The results demonstrated that α-SMA and ColI mRNA expression levels in TGF-β1-induced CFs were also significantly and dose-dependently decreased following treatment with curcumin (P<0.05; Fig. 2).
Figure 1.
Curcumin suppressed α-smooth muscle actin (SMA) and collagen I (ColI) protein expression levels. (A) Western blotting and corresponding densitometric quantification of α-SMA, ColI and internal reference GAPDH expression in cardiac fibroblasts (CFs) treated with 10 ng/ml transforming growth factor (TGF)-β1 in the presence of curcumin at various concentrations (0, 5, 10 and 20 µmol/l) for 24 h. (B) Immunofluorescence staining of α-SMA in CFs following treatment with 20 µmol/l curcumin. α-SMA and nuclei were stained with Cy5 (red) and DAPI (blue), respectively (bar, 100 µm). *P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group.
Figure 2.
Reverse transcription-quantitative polymerase chain reaction analysis of (A) α-smooth muscle actin (SMA) and (B) collagen I (ColI) mRNA expression levlels in cardiac fibroblasts (CFs) treated with or without 5, 10 or 20 µmol/l curcumin for 30 min and incubated with 10 ng/ml transforming growth factor (TGF)-β1 for 24 h was performed. Curcumin was found to suppress the α-SMA and ColI mRNA expression levels. mRNA expression control CFs was normalized to 1-fold, *P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group.
TGF-β1-activated Smad2 and p38 signaling pathways in CFs
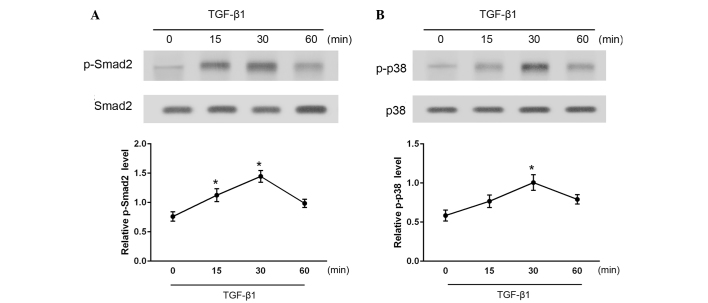
To investigate the downstream signaling pathways in TGF-β1-stimulated CFs, the levels of activated (phosphorylated) Smad2 and p38 were measured at various time points between 15 and 60 min after TGF-β1 administration (Fig. 3). As determined by western blotting, phosphorylated Smad2 and p38 (p-Smad2 and p-p38) levels were significantly increased following stimulation with TGF-β1 (P<0.05). Total Smad2 and p38 protein expression levels were also examined and found to be unaffected by TGF-β1 administration. These results indicate that treatment with TGF-β1 activates the Smad2 and p38 signaling pathways in CFs.
Figure 3.
Effects of transforming growth factor (TGF)-β1 on the phosphorylation of (A) Smad2 and (B) p38 over 60 min, as determined by western blotting. Total Smad2 and p38 was used as the control. Densitometric analysis was performed to quantify the phosphorylation levels, which are presented as the ratio between the optical densities of the p-Smad2 and total Smad2 and p-p38 and p38 bands, respectively. *P<0.05 vs. control. p-, phosphorylated.
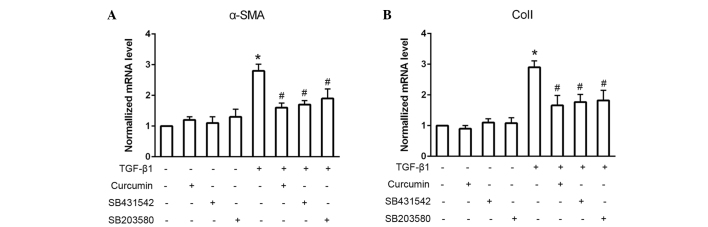
Smad2 inhibitor, SB431542, and p38 inhibitor, SB203580, suppress TGF-β1-induced α-SMA and ColI expression levels
To assess whether Smad2 and p38 were associated with the TGF-β1-induced differentiation of CFs, the Smad2 and p38 inhibitors, SB431542 and SB203580, respectively, were used to block these signaling pathways (Fig. 4). When administered alone, SB431542 and SB203580 exhibited no significant effect on cardiac fibroblast differentiation, as detected by consistent mRNA α-SMA and ColI expression levels (P>0.05). However, pretreatment with SB431542 or SB203580 prior to TGF-β1 administration significantly suppressed the TGF-β1-induced mRNA expression levels of α-SMA and ColI (P<0.05).
Figure 4.
Smad2 and p38 inhibitors, SB431542 and SB20380 respectively, suppressed (A) α-smooth muscle actin (SMA) and (B) collagen I (ColI) expression levels. Cardiac fibroblasts (CFs) were treated with 10 ng/ml transforming growth factor (TGF)-β1 for 24 h in the presence or absence of 10 µM SB431542 or SB20380 inhibitors. mRNA expression of the control CFs was normalized to 1-fold. *P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group.
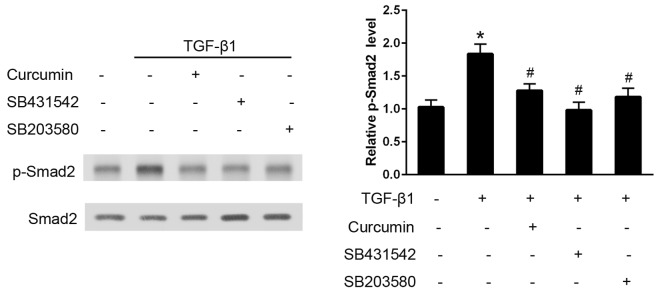
Curcumin inhibits TGF-β1-induced activation of the Smad2 signaling pathway
In CFs, TGF-β1 administration induced an early and significant increase in p-Smad2 expression levels over the baseline value in 30 min, as analyzed using western blotting (P<0.05; Fig. 5). Pre-treatment with curcumin induced a significant decrease in the degree of Smad2 phosphorylation and, as hypothesized, SB431542 (P<0.05), which is a well established TGF-βR-Smad2 inhibitor, effectively eliminated the activation of Smad2. Furthermore, pre-treatment with the p38 inhibitor, SB203580, also significantly attenuated the TGF-β1-induced Smad2 phosphorylation (P<0.05).
Figure 5.
Curcumin administration inhibited the transforming growth factor (TGF)-β1-induced activation of Smad2 in cardiac fibroblasts (CFs). CFs were pretreated with 20 µmol/l curcumin, 10 µM SB431542 (Smad2 inhibitor) or 10 µM SB203580 (p38 inhibitor) for 30 min, followed by treatment with 10 ng/ml TGF-β1 for 30 min. Densitometric analysis was performed to quantify the phosphorylation levels, which are presented as the ratio between the optical densities of the p-Smad2 and total Smad2 bands. *P<0.05 vs. the control group; #P<0.05 vs. the TGF-β1 group.
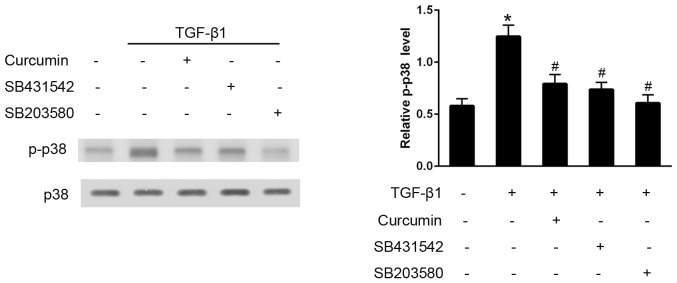
Curcumin inhibits TGF-β1-induced activation of the p38 pathway
The effects of curcumin on the TGF-β1-induced activation of the p38 signaling pathway were also examined. Western blot analysis demonstrated that, in TGF-β1-stimulated CFs, curcumin pre-treatment significantly decreased p-p38 expression levels, and SB203580 effectively eliminated p38 activation (P<0.05). Furthermore, SB431542 administration also significantly inhibited the phosphorylation of p38, thus preventing its activation (P<0.05; Fig. 6).
Figure 6.
Curcumin administration inhibited the transforming growth factor (TGF)-β1-induced activation of p38 in cardiac fibroblasts (CFs). CFs were pretreated with 20 µmol/l curcumin, 10 µM SB431542 (Smad2 inhibitor) or 10 µM SB203580 (p38 inhibitor) for 30 min, followed by treatment with 10 ng/ml TGF-β1 for 30 min. Densitometric analysis was performed to quantify the phosphorylation levels, which are presented as the ratio between the optical densities of the p-p38 and total p38 bands. *P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group.
Discussion
In the present study, curcumin was demonstrated to dose-dependently inhibit the TGF-β1-induced cardiac fibroblast differentiation. Furthermore, the results indicated that these effects may be mediated by the Smad2 and p38 signaling pathways. Therefore, the present results suggested that curcumin may be a potential therapeutic agent for the treatment of myocardial fibrosis, which has been associated with the pathogenesis of heart failure (1).
The transformation of CFs to cardiac myofibroblasts is a key event in cardiac fibrosis. Cardiac myofibroblasts are absent from normal myocardium, however, they are the predominant source of excessive extracellular collagen in cardiac fibrosis (20,21). In addition to aggravating cardiac dysfunction, previous studies have demonstrated that the persistence of cardiac myofibroblasts also contributes to malignant arrhythmia (22–24). Therefore, factors associated with the formation of cardiac myofibroblasts are of considerable clinical interest.
High expression levels of α-SMA are a hallmark of the formation of cardiac myofibroblasts (24,25). Collagen, particularly fibrillar ColI, is the predominant component of the ECM and is excessively synthesized by cardiac myofibroblasts (26,27). The results of the present study demonstrated that curcumin administration effectively suppressed TGF-β1-induced cardiac fibroblast differentiation, as determined by the decreased expression of α-SMA and ColI, at the protein and mRNA levels. These findings are consistent with previous studies which have demonstrated that curcumin is capable of inducing anti-fibrotic effects in cultured CFs (10,28).
TGF-β1, which is the most well-characterized cytokine, induces the differentiation of CFs to cardiac myofibroblasts (29,30). Activation of the Smad cascade has an essential role in the differentiation of myofibroblasts (15,16,31) and is regarded as the classical mediator of TGF-β1. Following TGF-β1 stimulation, the TGF-β1RI serine-threonine kinase phosphorylates the receptor-Smads (R-Smads), and Smad2 subsequently forms a complex with Smad3 that, in turn, associates with a Co-Smad (Smad4) and translocates into the nucleus, where it acts as a transcription factor (32). In addition to Smad-dependent pathways, previous studies have suggested that Smad independent pathways, such as p38 MAPK, may also play a pivotal role in TGF-β1-mediated differentiation (17,33). In the present study, inhibition of Smad2 and p38 consistently attenuated the TGF-β1-induced cardiac fibroblast differentiation, whereas curcumin was able to reduce the activation of Smad2 and p38 molecular signaling. Therefore, curcumin may have inhibited TGF-β1-induced activation via the Smad2 and p38 signaling pathways. Furthermore, the Smad2 inhibitor SB431542 effectively prohibited the phosphorylation of p38, and the p38 inhibitor SB203580 reduced the phosphorylation of Smad2. These results demonstrated that there is cross-talk or interaction between these two signaling pathways; however, the detailed mechanism and biological consequences of Smad2 and p38 activation remain unclear. This potential interaction among Smad2, p38 and other associated pathways requires further investigation.
In conclusion, the results of the present study suggested that curcumin has fibrosis suppressor properties and an inhibitory effect on TGF-β1-induced cardiac fibroblast differentiation, which may be mediated via the suppression of the Smad2 and p38 signaling pathways. Therefore, curcumin may be a potential novel therapeutic agent for the treatment of cardiac fibrosis.
Glossary
Abbreviations
- CFs
cardiac fibroblasts
- α-SMA
α-smooth muscle actin
- ColI
collagen type I
References
- 1.Daskalopoulos EP, Janssen BJ, Blankesteijn WM. Myofibroblasts in the infarct area: Concepts and challenges. Microsc Microanal. 2012;18:35–49. doi: 10.1017/S143192761101227X. [DOI] [PubMed] [Google Scholar]
- 2.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-κB. Nutrition. 2009;25:964–972. doi: 10.1016/j.nut.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Kolodziejczyk J, Olas B, Saluk-Juszczak J, Wachowicz B. Antioxidative properties of curcumin in the protection of blood platelets against oxidative stress in vitro. Platelets. 2011;22:270–276. doi: 10.3109/09537104.2010.547637. [DOI] [PubMed] [Google Scholar]
- 5.Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- 6.Teiten MH, Gaascht F, Cronauer M, Henry E, Dicato M, Diederich M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int J Oncol. 2011;38:603–611. doi: 10.3892/ijo.2011.905. [DOI] [PubMed] [Google Scholar]
- 7.Hartojo W, Silvers AL, Thomas DG, Seder CW, Lin L, Rao H, Wang Z, Greenson JK, Giordano TJ, Orringer MB, et al. Curcumin promotes apoptosis, increases chemosensitivity, and inhibits nuclear factor kappaB in esophageal adenocarcinoma. Transl Oncol. 2010;3:99–108. doi: 10.1593/tlo.09235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanvorachote P, Pongrakhananon V, Wannachaiyasit S, Luanpitpong S, Rojanasakul Y, Nimmannit U. Curcumin sensitizes lung cancer cells to cisplatin-induced apoptosis through superoxide anion-mediated Bcl-2 degradation. Cancer Invest. 2009;27:624–635. doi: 10.1080/07357900802653472. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh SS, Salloum FN, Abbate A, Krieg R, Sica DA, Gehr TW, Kukreja RC. Curcumin prevents cardiac remodeling secondary to chronic renal failure through deactivation of hypertrophic signaling in rats. Am J Physiol Heart Circ Physiol. 2010;299:H975–H984. doi: 10.1152/ajpheart.00154.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li HL, Liu C, de Couto G, Ouzounian M, Sun M, Wang AB, Huang Y, He CW, Shi Y, Chen X, et al. Curcumin prevents and reverses murine cardiac hypertrophy. J Clin Invest. 2008;118:879–893. doi: 10.1172/JCI32865. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Wang NP, Wang ZF, Tootle S, Philip T, Zhao ZQ. Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br J Pharmacol. 2012;167:1550–1562. doi: 10.1111/j.1476-5381.2012.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, Hasegawa K. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. 2008;118:868–878. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soetikno V, Sari FR, Sukumaran V, Lakshmanan AP, Mito S, Harima M, Thandavarayan RA, Suzuki K, Nagata M, Takagi R, Watanabe K. Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: Possible involvement of PKC-MAPK signaling pathway. Eur J Pharm Sci. 2012;47:604–614. doi: 10.1016/j.ejps.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD (P) H oxidase 4 mediates transforming growth factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 16.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deaton RA, Su C, Valencia TG, Grant SR. Transforming growth factor-β1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J Biol Chem. 2005;280:31172–31181. doi: 10.1074/jbc.M504774200. [DOI] [PubMed] [Google Scholar]
- 18.Yan-Hong F, Hui D, Qing P, Lei S, Hai-Chang W, Wei Z, Yan-Jie C. Effects of arginine vasopressin on differentiation of cardiac fibroblasts into myofibroblasts. J Cardiovasc Pharmacol. 2010;55:489–495. doi: 10.1097/FJC.0b013e3181d706ae. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 21.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 22.Sabbah HN, Sharov VG, Lesch M, Goldstein S. Progression of heart failure: A role for interstitial fibrosis. Mol Cell Biochem. 1995;147:29–34. doi: 10.1007/BF00944780. [DOI] [PubMed] [Google Scholar]
- 23.McDowell KS, Arevalo HJ, Maleckar MM, Trayanova NA. Susceptibility to arrhythmia in the infarcted heart depends on myofibroblast density. Biophys J. 2011;101:1307–1315. doi: 10.1016/j.bpj.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohr S. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm. 2009;6:848–856. doi: 10.1016/j.hrthm.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 25.Leask A. Potential therapeutic targets for cardiac fibrosis TGFβ, angiotensin, endothelin, CCN2 and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 26.Chapman D, Weber KT, Eghbali M. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res. 1990;67:787–794. doi: 10.1161/01.RES.67.4.787. [DOI] [PubMed] [Google Scholar]
- 27.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 28.Meng Z, Yu Xh, Chen J, Li L, Li S. Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-γ activation. Acta Pharmacol Sin. 2014;35:1247–1256. doi: 10.1038/aps.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leask A. TGFbeta, cardiac fibroblasts and the fibrotic response. Cardiovasc Res. 2007;74:207–212. doi: 10.1016/j.cardiores.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a induces myofibroblast differentiation by upregulating TGF-β signaling through SMAD2 in a β-catenin-dependent manner. PloS one. 2011;6:e19809. doi: 10.1371/journal.pone.0019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 33.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]