Abstract
Diabetes causes many complications, including retinopathy and peripheral neuropathy, which are well understood as contributing to gait instability and falls. A less understood complication of diabetes is the effect on the vestibular system. The vestibular system contributes significantly to balance in static and dynamic conditions by providing spatially orienting information. It is noteworthy that diabetes has been reported to affect vestibular function in both animal and clinical studies. Pathophysiological changes in peripheral and central vestibular structures due to diabetes have been noted. Vestibular dysfunction is associated with impaired balance and a higher risk of falls. As the prevalence of diabetes increases, so does the potential for falls due to diabetic complications. The purpose of this perspective article is to present evidence on the pathophysiology of diabetes-related complications and their influence on balance and falls, with specific attention to emerging evidence of vestibular dysfunction due to diabetes. Understanding this relationship may be useful for screening (by physical therapists) for possible vestibular dysfunction in people with diabetes and for further developing and testing the efficacy of interventions to reduce falls in this population.
Diabetes mellitus affects 29.1 million people in the United States—about 9.3% of the US population.1 Over the next 40 years, the prevalence of diabetes in the United States will increase from its current level of 1 in 10 to 1 in 3 because of the aging of the US population, longer life spans of adults with diabetes, and the increasing prevalence of obesity and physical inactivity.2 The medical expenditures of people with diabetes are about 2.3 times higher than those of people without diabetes, with more than half of those expenditures being directly related to diabetes.3 The high prevalence and chronicity of complications make diabetes a health care concern around the world.2
People with diabetes often develop multiorgan anatomic, structural, and functional changes due to microvascular and macrovascular complications.4 Two common microvascular complications, peripheral neuropathy and retinopathy, are well established as contributors to increased postural sway and falls.5,6 Although vestibular system dysfunction is not commonly recognized as a microvascular complication of diabetes, a recent epidemiological study reported that vestibular dysfunction was 70% higher in people with diabetes than in people matched for age and serving as controls.7 The higher prevalence of diabetes and vestibular dysfunction was specifically seen in people with a longer duration of diabetes and with poor glucose control (ie, higher serum glycated hemoglobin [HbA1c] levels).8 In addition, animal and clinical studies reported structural and functional changes in the vestibular system due to diabetes.9–11
If the vestibular system is adversely affected by diabetes, it will be important for physical therapists to consider the broad influence of diabetes on the risk of falls in older adults and other populations. Although most clinicians may be aware of the role of some diabetic complications in increasing the risk of falls, the relationship between diabetes and vestibular dysfunction is not well known. The purpose of this perspective article is to describe the pathophysiology of diabetic complications and their influence on balance and falls, with specific attention to emerging evidence of vestibular dysfunction due to diabetes. The clinical implications for physical therapists are discussed, as a comprehensive evaluation of the vestibular system may be necessary in people who have diabetes and balance impairment and interventions that address all 3 sensory input systems (visual, vestibular, and somatosensory) may be essential for reducing the risk of falls.
Pathophysiology of Diabetic Complications
Diabetes mellitus is a chronic metabolic condition characterized by elevated blood glucose levels resulting from the body's inability to produce insulin, resist insulin action, or both.12 Chronic hyperglycemia plays a major role in the pathogenic mechanisms underlying microvascular (retinopathy, neuropathy, and nephropathy) and macrovascular (peripheral vascular disease, cardiovascular disease, and cerebrovascular disease) complications in diabetes.
One mechanism of microvascular damage in diabetes is the result of chemical reactions between by-products of sugars and proteins, which form irreversible cross-linked protein derivatives called advanced glycation end products.13 These derivatives affect the surrounding tissues, causing thickening of collagen and endothelium. Other mechanisms contributing to microvascular disease include the abnormal activation of signaling cascades, such as the protein kinase C pathway, which increases vascular permeability, and the polyol pathway, a mechanism by which sorbitol accumulation results in osmotic and oxidative stress damage to the endothelium14; the elevated production of reactive oxygen species, by which oxygen-containing molecules interact with other biomolecules and result in damage; and the abnormal stimulation of hemodynamic regulation systems, such as the renin-angiotensin system.4
Damage to the peripheral nerves may occur through irreversible changes to myelin protein caused by advanced glycation end products, which result in segmental demyelination of the peripheral nerves.13 In addition, the neuronal microvascular is affected, leading to impaired nerve perfusion.15 Diabetic peripheral neuropathy affects up to two-thirds of people with diabetes and is characterized by pain, paresthesia, and sensory loss.16
Similar pathophysiological changes occur in the retinal vasculature in people with diabetes. In the United States, about 40% of people with type 2 diabetes and 86% with type 1 diabetes develop retinopathy.17 Changes in the structure and cellular composition of the retinal vasculature are the hallmarks of early diabetic retinopathy. Damage to the endothelial cell lining causes an increase in vascular permeability, a breakdown of the inner blood-retinal barrier, and an accumulation of extracellular fluid in the macula.18 Damage to pericytes, which are responsible for maintaining capillary tone, leads to altered retinal hemodynamics and abnormal autoregulation of retinal blood flow, which cause the development of microaneurysms.19 Thickening of the capillary basement membrane and retinal leukostasis lead to capillary occlusions and nonperfusion in the retinal microcirculation.20 These changes, in turn, stimulate pathologic neovascularization, which results in proliferative diabetic retinopathy and ultimately leads to retinal detachment and blindness.21
People with diabetes, neuropathy, and retinopathy have significant physical limitations because of decreased proprioception and vision. Loss of light touch, visual acuity, contrast sensitivity, and depth perception may increase both the risk and the recurrence of falls in people with diabetes.5
Influence of Neuropathy and Retinopathy on Falls
The presence and severity of diabetic peripheral neuropathy (DPN) have been shown to increase postural instability.22,23 People with DPN have a larger range of sway in the anterior-posterior and medial-lateral directions and a higher sway speed than people matched for age and serving as controls.22 In quiet standing with eyes open, people with DPN have been shown to have 66% more sway than people who are of a similar age and healthy.6 The greatest decrease in postural stability in people with DPN has been observed with eyes closed; this finding reveals a reliance on vision to compensate for sensory deficits.6 In addition, decreased vibration sense and loss of pressure sensitivity have been shown to be associated with recurrent falls.24 Because of decreased proprioceptive feedback during walking, older adults with diabetes walk slower and have greater stride variability; these factors increase the risk of falls.25 Similarly, strong associations have been observed for diabetic retinopathy, the duration of diabetes, and the risk of falls, with severe cortical cataracts being significantly associated with fractures.26
Besides having a higher risk of falls, adults who are more than 70 years of age and have diabetes have been found to have a higher risk of sustaining more severe injuries after a fall.27 Both elderly men and elderly women with diabetes have a higher risk of fractures than adults without diabetes, despite similar bone mineral densities.28,29 This compromised bone quality, which may be due to higher concentrations of advanced glycation end products in the bones because of diabetes,30 increases the risk of fractures by 64% in people with diabetes compared with people who are healthy.29 Pijpers et al31 found that in people who were more than 65 years old and had diabetes, 30.6% fell recurrently, whereas 19.4% of people without diabetes fell recurrently; in that study, recurrent falls were defined as at least 2 falls within a 6-month period.
Although the diabetic complications of neuropathy and retinopathy clearly increase the risk of falls, other pathways may affect balance and gait in people with diabetes. For example, Chiles et al32 reported that after adjustment for age, sex, education, cognition, medications, and comorbidities (cardiovascular disease, hypertension, and kidney disease), people with diabetes had significantly decreased physical function (β=−0.99, P<.01) and decreased walking speed (β=−0.1 m/s, P<.01). Even after adjustment for impaired nerve function in the lower extremities (higher neuropathy scores), significant differences in physical function, balance, and gait speed between people with diabetes and people without diabetes persisted.32 This evidence suggests that other pathways may affect balance and gait in people with diabetes.
Because the vestibular system plays a major role in maintaining balance in both static and dynamic conditions, it is important to consider this system as a potential mechanism by which balance and gait may be affected. Here, we provide a brief summary of the anatomy and physiology of the vestibular system and an overview of studies showing the relationship between diabetes and vestibular dysfunction.
Anatomy and Physiology of the Vestibular System
The vestibular system makes significant contributions to both sensory and motor systems. As a sensory system, it provides the central nervous system with information regarding motion of the head and its position in space. The central nervous system uses this information along with information from the visual and somatosensory systems to create an internal map of the position and movement of the entire body in relation to the environment. The vestibular system contributes directly to motor output by producing compensatory eye movements to maintain a stable gaze and to coordinate postural control during movement.
The peripheral sensory apparatus of the vestibular system comprises the semicircular canals and otolith organs. The 3 semicircular canals (anterior, posterior, and horizontal) function as rate sensors by providing input regarding angular head acceleration. The saccule and the utricle, which are the otolith organs, register forces related to linear acceleration and static tilt with respect to the gravitational axis.
Specialized hair cells in each ampulla and otolith organ are the biological sensors that convert head position to neural firing. Type 1 hair cells are sensitive to rotations during large head accelerations, and type 2 hair cells are most effective during low-frequency and small head accelerations. In addition, the maculae of the utricle and the saccule contain otoconia, which are calcium carbonate crystals, embedded in a gelatinous matrix. The otoconia move in response to gravity and other accelerative movements, making the macula very sensitive to linear acceleration.33
Information from the peripheral vestibular organs is processed in the central nervous system with input from the proprioceptive and visual systems. Motor output through the vestibular-ocular reflex (VOR) generates eye movements for gaze stability, and the vestibulospinal reflex generates muscle activity to maintain balance.
The maintenance of balance depends on information provided by the somatosensory, visual, and vestibular organs. These sensory systems provide information regarding body orientation within different frames of reference. Mirka and Black reported, “Vestibular input is referenced to gravity, while somatosensory and visual inputs are referenced to the support surface and visual surrounds. Hence, the vestibular system provides orientation to earth vertical, while the other senses provide relative orientation references.”34(p351) In people with impaired vision or somatosensory input, the vestibular system becomes very important for balance control.
Evidence for Vestibular Dysfunction in Diabetes
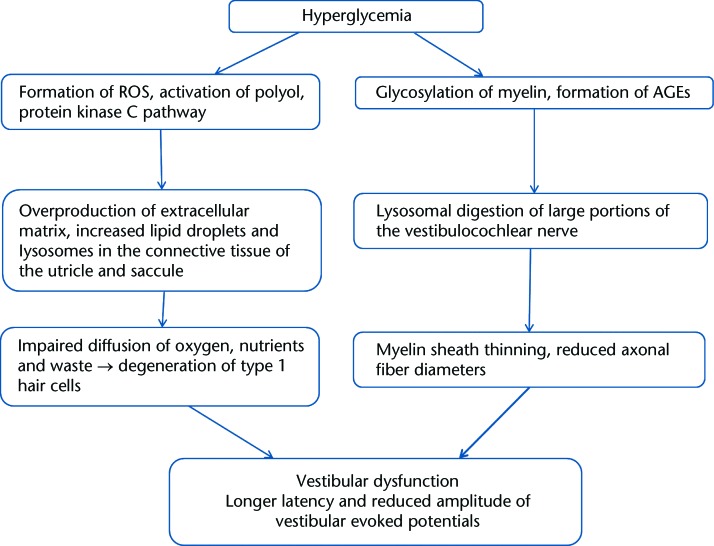
Morphological and physiological changes have been reported in the peripheral vestibular apparatus in animal models of diabetes. Myers and colleagues11,35 found morphological and structural changes in the peripheral vestibular system in animals with experimentally induced diabetes. These animals had an overproduction of extracellular matrix and a higher incidence of lysosomes and lipid droplets in the connective tissue of the utricle and the saccule.35 These findings were considered the consequences of metabolic stress. The accumulation of excess extracellular matrix leads to impaired diffusion of oxygen, nutrients, and waste products. Hair cell degeneration, noted in these animals, was suggested to be the result of impaired diffusion. The level of type 1 hair cell degeneration was higher in the saccule, suggesting that the saccule may be more susceptible to pathology in diabetes.35 The vestibulocochlear nerve of rats with diabetes was observed to have large portions of disrupted myelin sheath lamellae as well as thinning of the myelin sheath and smaller axonal fiber diameters.10,36 Physiological changes in the vestibular end organ due to diabetes were examined by Perez et al37 in mice with diet-induced type 2 diabetes. The latency of evoked potential responses was delayed, amplitudes were smaller, and thresholds were higher compared with those in control mice. Because short vestibular evoked potentials were recorded in response to linear and angular accelerations, damage due to diabetes was shown to affect the function of the vestibular system.37 The Figure illustrates the potential mechanisms contributing to vestibular dysfunction in diabetes.
Figure.
Potential mechanisms contributing to vestibular dysfunction in diabetes, as suggested from animal studies. Information was derived from Myers and colleagues,10,11,35,36 AGEs=advanced glycation end products, ROS=reactive oxygen species.
There is clinical evidence of the presence of vestibular dysfunction in people with diabetes. Clinical examinations with oculomotor tests, videonystagmography, and caloric testing have shown that children and young adults who are 6 to 28 years of age and have type 1 diabetes have central and peripheral vestibular dysfunction. Abnormal responses include impaired optokinetic responses and eye tracking9,38 and reduced responses to caloric stimulation and positional nystagmus.9,39,40 The frequency of abnormal responses was higher in the participants with a longer duration of diabetes, those with retinopathy and neuropathy, and those who had frequent hypoglycemic incidents.9,40 Despite the changes in central and peripheral structures, Biurrun et al reported no complaints of dizziness or imbalance40 and Gawron et al reported that only 6.3% of the participants had complaints of dizziness and imbalance.9 In neither study was balance function in the participants examined. The minimal complaints of dizziness could have been due to symmetrical impairment of function between the 2 inner ears.37 In people with type 1 diabetes, vestibular system dysfunction was seen more often than auditory dysfunction (60% versus 30%), indicating that metabolic disturbances may affect the homeostasis of the vestibular organ more quickly than the homeostasis of the cochlea.39
Studies in people with type 2 diabetes have shown significant abnormalities in the phase of the VOR and the optokinetic reflex compared with those in people matched for age and serving as controls.41 Because of VOR abnormalities and optokinetic reflex abnormalities, people with diabetes could have blurring of vision during head movements.41 In adults with multiple chronic disorders, such as diabetes, hypertension, and dyslipidemia, cochlear and vestibular dysfunction was significantly prevalent, particularly in people more than 60 years of age and having 2 or more comorbidities.42 Table 1 summarizes clinical studies examining vestibular dysfunction in people with type 1 and type 2 diabetes. On the basis of these studies, the strongest evidence presented is for VOR deficits, not balance problems—perhaps because direct assessment of the VOR is available during videonystagmography tests. Balance testing is more complex, and isolating the vestibular system is difficult because of proprioceptive feedback and inherent compensatory strategies that people use to avoid falling.
Table 1.
Summary of Clinical Studies Examining Vestibular Dysfunction in People With Diabetesa
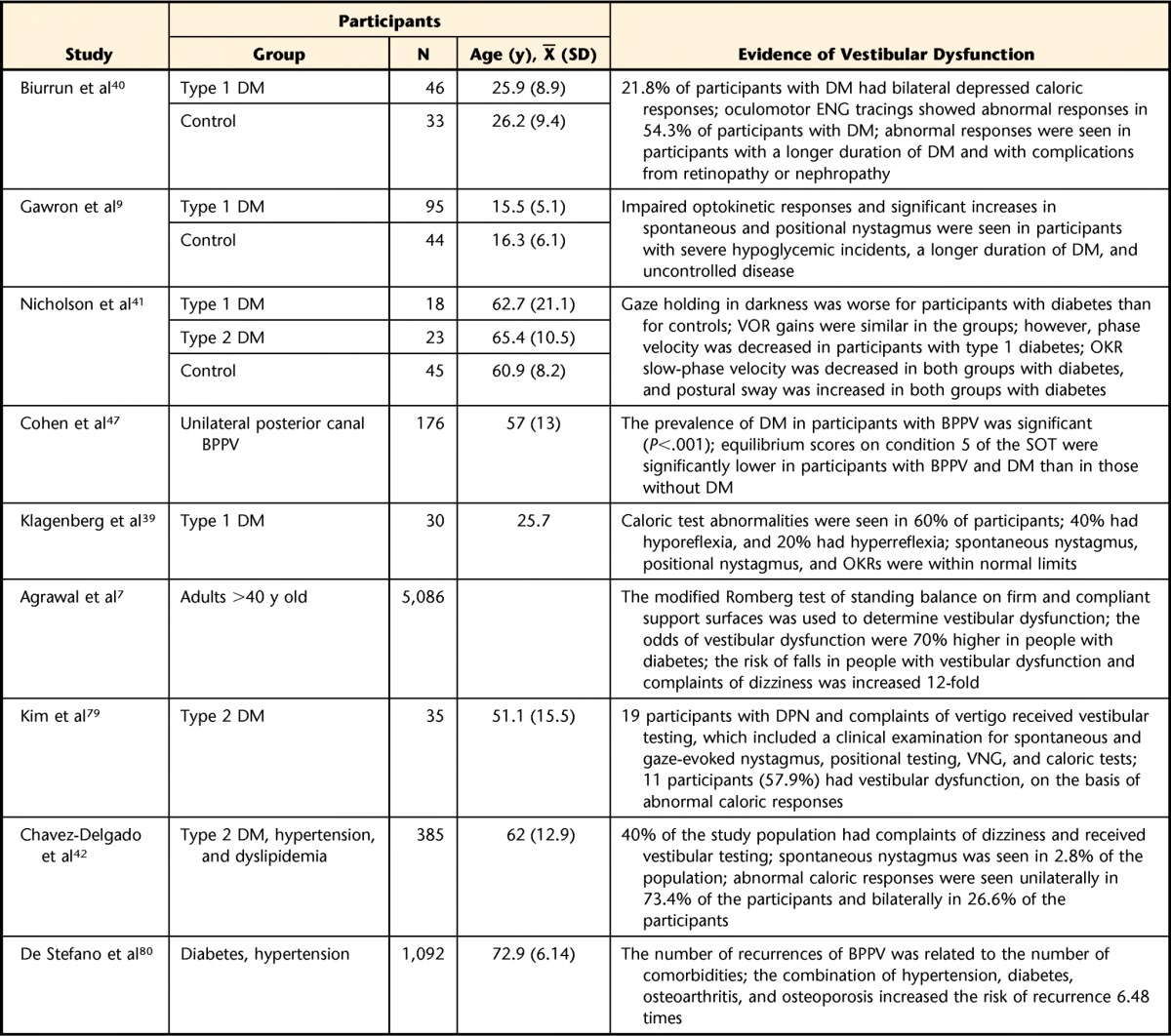
DM=diabetes mellitus, ENG=electronystagmography, VOR=vestibular-ocular reflex, OKR=optokinetic reflex, BPPV=benign paroxysmal positional vertigo, SOT=Sensory Organization Test, DPN=diabetic peripheral neuropathy, VNG=videonystagmography.
The odds of vestibular dysfunction have been shown to be 70% higher in adults with diabetes than in those without diabetes.7 In a large epidemiological study, Agrawal et al7 examined data from adults 40 years of age and older (N=5,086) by using the modified Romberg Test of Standing Balance. Participants had to stand unassisted for 30 seconds in each of the following 4 positions: on firm ground with eyes open, on firm ground with eyes closed, on a foam surface with eyes open, and on a foam surface with eyes closed. Fifty-four percent of the participants with diabetes were identified as having vestibular dysfunction because they had to take a step or open their eyes when standing on foam with eyes closed. The risk of falls was 12 times higher in the participants who had vestibular dysfunction and complaints of dizziness than in those without either complaint.7 Of interest, the participants with vestibular dysfunction but without complaints of dizziness also had a significantly higher risk of falls.7 The prevalence of vestibular dysfunction was significantly related to the duration of diabetes; participants with a history of diabetes of up to 5 years had a 41% prevalence of vestibular dysfunction, whereas a 61% prevalence of vestibular dysfunction was observed both in participants with a history of diabetes of 6 to 10 years and in those with a history of diabetes of more than 10 years. Higher serum HbA1c levels (≥7.0%) increased the odds of vestibular dysfunction by 60%.8 The prevalence of vestibular dysfunction was higher in participants with other diabetes-related complications, such as peripheral neuropathy (76% versus 49%) and retinopathy (71% versus 45%).8
The limitation of these studies7,8 was that vestibular dysfunction was assessed with the modified Romberg Test of Standing Balance. No direct assessment of the vestibular system with caloric or evoked potential studies was done. The modified Romberg Test of Standing Balance has been shown to have weak correlations with the Dizziness Handicap Inventory (r=.26); however, when compared with caloric or vestibular evoked potential testing, the modified Romberg Test of Standing Balance has a sensitivity and a specificity for the detection of peripheral vestibular dysfunction of 55% and 65%, respectively, making it a poor screening tool for vestibular dysfunction.43 Future studies of the relationship between direct assessment of the peripheral vestibular organs and balance and function in adults with diabetes are essential.
Degerman et al44 examined the human saccule and identified the insulin receptor, insulin receptor substrate 1, protein kinase B, and insulin-sensitive glucose transporter (GLUT4) in the sensory epithelium of the human saccule. These signaling mechanisms maintain ion and water homeostasis in the saccule and are important for oto-protection and neuronal survival. Chronic hyperglycemia and insulin resistance can affect these signaling pathways,45 resulting in impaired inner ear function.
One particularly common vestibular condition, benign paroxysmal positional vertigo (BPPV), has been studied in adults with diabetes. In a histopathology study, Yoda et al46 examined 28 temporal bones of people with type 1 diabetes and compared them with the temporal bones of 56 people who were matched for age (healthy controls). None of the people with type 1 diabetes had a documented medical history of vestibular or balance problems. Each temporal bone was sectioned at a thickness of 20 μm, stained with hematoxylin and eosin, and studied under light microscopy. The cupulae were observed by light microscopy at a magnification of ×400. A significantly higher prevalence of deposits of otoconia was seen in the posterior and lateral semicircular canals as well as attached to the cupula in people with type 1 diabetes than in the controls.46 Cohen et al47 reported a significantly higher prevalence of diabetes in people with BPPV. In adults who were 65 to 74 years of age, 20% of those with BPPV had diabetes; in comparison, 9.2% of people in the general population have diabetes. In addition, comorbidities such as diabetes and hypertension have been shown to increase the rate of recurrence of BPPV.48 The combination of hypertension, diabetes, and osteoarthritis increases the risk of recurrence of BPPV 4.55 times.48 Once diagnosed, BPPV can be effectively treated by appropriate repositioning maneuvers.49 These results suggest that vestibular dysfunction could be considered an underreported complication of diabetes. Vestibular dysfunction has been shown to independently increase the risk of falls and could be an independent mediator of the association between diabetes and falls.8
On the basis of the literature presented, there appears to be a complex relationship between diabetes and vestibular dysfunction in people with both type 1 diabetes and type 2 diabetes. Damage to the inner ear may cause people with diabetes to have impaired gaze stability. In addition, there may be decreased sensory feedback from the peripheral vestibular organs due to hypofunction of the otolith organs and semicircular canals. Hence, the high risk of postural instability and falls may be further increased because of vestibular dysfunction. The prevalence of BPPV also may be increased in people with diabetes. This information may be important to physical therapists who treat patients with diabetes.
Clinical Implications for Examination and Treatment of People with Diabetes
Physical activity and exercise are essential components for managing diabetes and preventing complications,50,51 and clinical guidelines have been published to provide recommendations for starting and advancing an exercise program for people with type 2 diabetes.52 However, the vestibular system is not included in these guidelines because vestibular dysfunction is not yet a widely recognized complication of diabetes. Here we present recommendations for physical therapy examinations and interventions that include an awareness of the possible role of vestibular dysfunction in people with type 2 diabetes.
Recommendations for Examination
For a clear understanding of the impact of diabetes on the vestibular system, a comprehensive examination of the vestibular system is necessary. The use of appropriate outcome measures can help identify deficits, provide direction for treatment, and improve outcomes for patients. Function and participation in daily activities can be assessed with standardized questionnaires.
The Dizziness Handicap Inventory is a measure of self-reported activity limitations and participation restrictions due to either dizziness or unsteadiness.53 It is a 25-item questionnaire with 3 subscales: functional, emotional, and physical. The maximum score is 100 points (32 points for the functional dimension, 40 points for the emotional dimension, and 28 points for the physical dimension). Higher scores indicate greater levels of self-perceived disability. People with scores of more than 60 points on the Dizziness Handicap Inventory have been shown to have impaired functional mobility and a higher risk of falls.54
The Activities-specific Balance Confidence Scale is a 16-item measure of self-efficacy on a 10-point ordinal scale; people rate their confidence in maintaining their balance during various activities of daily living. Scores range from 0 to 100 points, where 0 indicates no confidence. Scores of more than 80 points indicate high functioning observed in physically active older adults55; scores of less than or equal to 67 points indicate an increased risk of falls.56 The Activities-specific Balance Confidence Scale may be a useful tool for assessing people's level of confidence as a treatment plan is developed. Symptom severity can be assessed with a visual analog scale and the Vertigo Symptoms Scale, both of which can be used to monitor people's progress during the course of treatment.57
Because damage to the vestibular system is associated with increased postural sway and balance impairment,58,59 it is necessary to examine static balance as well as balance during dynamic activities. The modified Clinical Test of Sensory Integration of Balance is a useful test that can be performed easily in the clinic setting to challenge the vestibular system while altering proprioceptive and visual feedback.60 The Sharpened Romberg Test is particularly valuable in the clinic setting because people with type 2 diabetes have been shown to have higher medial-lateral sway.23 In addition, the risk of falls can be assessed with reliable tests that have been validated in people with DPN. With modified cutoff scores of greater than or equal to 10.7 seconds on the Timed “Up & Go” Test, scores of less than or equal to 22 on the Dynamic Gait Index, and scores of less than or equal to 52 on the Berg Balance Scale, the accuracy with which fallers were identified was greatest with the Timed “Up & Go” Test; the Dynamic Gait Index and the Berg Balance Scale were less accurate.61 Even though the Functional Gait Assessment has not been specifically validated in people with diabetes, it may be useful for identifying the risk of falls. People with vestibular dysfunction and scores of less than or equal to 22 on the Functional Gait Assessment have been shown to report recurrent falls.62
Oculomotor and gaze-holding deficits are seen in people with diabetes; hence, assessment of the vestibular system should include gaze stability testing. The Dynamic Visual Acuity test provides information to clinicians regarding the degradation of visual acuity with rapid head movements. A patient reads a wall eye chart with the head stationary, followed by head oscillations at 2 Hz. A loss of the ability to read 3 or 4 lines on the chart suggests decreased gaze stability.63 Making a diagnosis of BPPV in older adults with dizziness, multiple comorbidities, and coexisting cardiovascular disease is difficult, resulting in delayed interventions.64 Hence, performing the Dix-Hallpike test to assess anterior and posterior canal BPPV and the roll test to assess the horizontal canal in patients complaining of dizziness or vertigo may help identify the presence of BPPV.65 It is necessary to examine patients for diabetic complications such as peripheral neuropathy and lower extremity pain. The Michigan Neuropathy Screening Instrument can be easily completed in the clinic setting; scores of greater than or equal to 2 on the physical examination section indicate the presence of neuropathy.66,67 Pain due to neuropathy can be assessed with the Brief Pain Inventory for DPN, a scale validated for people with DPN; it assesses pain severity and how the pain may affect aspects of daily life.68
Suggestions for Interventions
Gaze stability exercises have been shown to be effective in reducing the risk of falls in adults with vestibular dysfunction.69 However, the role of gaze stability exercises in people with type 2 diabetes has not been explored. In people with diabetes, deficits in the VOR and the optokinetic reflex may make maintaining a stable gaze during dynamic activities difficult41; hence, we recommend the use of gaze stability exercises for maintaining a stable gaze and reducing the risk of falls in this population. Recommended gaze stability exercises include ×1 viewing (gaze stability exercise where a visual target is stationary and the patient moves his or her head from side to side or up and down while fixating on the target), ×2 viewing (head and target are moving in the same direction), or both.63
Although studies have shown that BPPV may be more prevalent and recurrent in adults with diabetes,47,48 no studies have examined the response to repositioning maneuvers in patients with type 2 diabetes and concurrent BPPV. In patients with BPPV, providing appropriate repositioning maneuvers is essential for reducing patient complaints of vertigo and disequilibrium.49,70
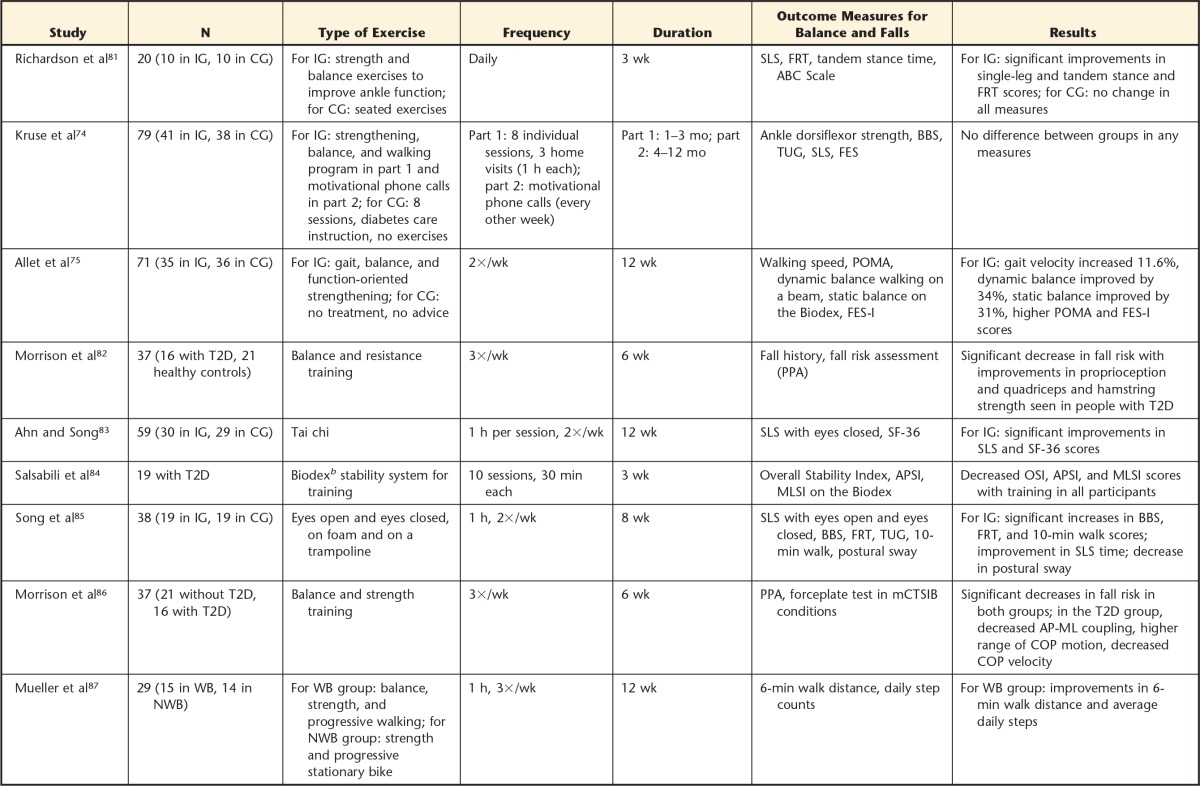
The roles of aerobic exercise, resistance exercise, and a combination of these exercises have been well established; these exercises have been shown to improve glycemic control, overall physical function, balance, and gait speed in people with diabetes.32,51,71–73 Balance training has been shown to improve postural control and clinical measures of balance; however, the intensity and duration of balance training as well as the presence of diabetic complications may affect outcomes. Kruse et al74 found no improvement in balance after a 12-month training program that included balance training exercises; however, Allet et al75 reported significant improvement in balance with similar exercises. The 2 studies differed in the frequency and mode of delivery of the training program as well as the severity of neuropathy. For patients with diagnosed DPN, Kruse et al74 prescribed balance training with a physical therapist once per week for 11 weeks. After this initial training period, patients received regular phone calls encouraging them to continue the exercise program. Allet et al75 prescribed training 2 times per week for 12 weeks, for 60 minutes per session, in a physical therapy clinic; however, their patients had minimal neuropathy. Table 2 summarizes a few studies in which balance training programs were effectively used to improve balance and function in people with type 2 diabetes.
Table 2.
Summary of Balance Training Interventions in People With Type 2 Diabetesa
IG=intervention group, CG=control group, SLS=single-leg stance, FRT=Functional Reach Test, ABC=Activities-specific Balance Confidence, BBS=Berg Balance Scale, TUG=Timed “Up & Go” Test, FES=Falls Efficacy Scale, POMA=Performance-Oriented Mobility Assessment, FES-I=Falls Efficacy Scale International, PPA=Physiological Profile Assessment, SF-36=Medical Outcomes Study 36-Item Health Survey Questionnaire (version 2), APSI=Anterior-Posterior Stability Index, MLSI=Medial-Lateral Stability Index, OSI=Overall Stability Index, T2D=type 2 diabetes, mCTSIB=Modified Clinical Test of Sensory Integration of Balance, AP-ML=anteroposterior-mediolateral, COP=center of pressure, WB=weight bearing, NWB=non–weight bearing.
b Biodex Stability System, Biodex Medical Systems Inc, Shirley, New York.
Beside weakness and imbalance, many factors contribute to falls in people with type 2 diabetes; these include glycemic control, fatigue, executive function, inappropriate footwear, polypharmacy, proprioceptive loss, and retinopathy.32,76–78 The American Diabetes Association provides a comprehensive understanding of these risk factors and their management.52
Because of somatosensory and visual disturbances, adults with diabetes are limited in their ability to reweigh sensory information. Evaluation for an assistive device may be necessary, particularly in people who are not confident with walking outdoors or are limited in participation due to dizziness. The added stability of an assistive device may encourage older adults with diabetes to participate in a walking program.
Conclusion
The relationship among diabetes, vestibular function, and the risk of falls is complex. People with diabetes have many deficits, including neuropathy, retinopathy, and polypharmacy, all of which compromise their activity and daily functional status. Vestibular dysfunction is another possible complication of diabetes and may increase the risk of falls. Understanding this relationship, identifying and treating BPPV, and working toward integrating all systems—visual, vestibular, and somatosensory—to improve balance may be ways in which physical therapists can prevent falls.
Footnotes
Mrs D'Silva, Dr Lin, Dr Staecker, and Dr Kluding provided concept/idea. Mrs D'Silva, Dr Whitney, and Dr Kluding provided writing. All authors provided consultation (including review of manuscript before submission).
This work was supported by Award Number T32HD057850 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1. Centers for Disease Control and Prevention. Diabetes fact sheet. Published 2014. Available at: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf Accessed December 25, 2014.
- 2. Boyle JP, Thompson TJ, Gregg EW, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36:1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Diabetes-related complications, glycemic control, and falls in older adults [erratum in: Diabetes Care. 2008;31:1089]. Diabetes Care. 2008;31:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simoneau GG, Ulbrecht JS, Derr JA, et al. Postural instability in patients with diabetic sensory neuropathy. Diabetes Care. 1994;17:1411–1421. [DOI] [PubMed] [Google Scholar]
- 7. Agrawal Y, Carey JP, Della Santina CC, et al. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004 [erratum in: Arch Intern Med. 2009;169:1419]. Arch Intern Med. 2009;169:938–944. [DOI] [PubMed] [Google Scholar]
- 8. Agrawal Y, Carey JP, Della Santina CC, et al. Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. Otol Neurotol. 2010;31:1445–1450. [DOI] [PubMed] [Google Scholar]
- 9. Gawron W, Pospiech L, Orendorz-Fraczkowska K, Noczynska A. Are there any disturbances in vestibular organ of children and young adults with Type I diabetes? Diabetologia. 2002;45:728–734. [DOI] [PubMed] [Google Scholar]
- 10. Myers SF. Myelin-sheath abnormalities in the vestibular nerves of chronically diabetic rats. Otolaryngol Head Neck Surg. 1998;119:432–438. [DOI] [PubMed] [Google Scholar]
- 11. Myers SF, Ross MD, Jokelainen P, et al. Morphological evidence of vestibular pathology in long-term experimental diabetes mellitus; I: microvascular changes. Acta Otolaryngol. 1985;100:351–364. [DOI] [PubMed] [Google Scholar]
- 12. Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30-31, 2006 Endocr Pract. 2006;12(suppl 3):3–111. [PubMed] [Google Scholar]
- 13. Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. [DOI] [PubMed] [Google Scholar]
- 14. Kinoshita JH, Nishimura C. The involvement of aldose reductase in diabetic complications. Diabetes Metab Rev. 1988;4:323–337. [DOI] [PubMed] [Google Scholar]
- 15. Malik RA, Tesfaye S, Thompson SD, et al. Endoneurial localisation of microvascular damage in human diabetic neuropathy. Diabetologia. 1993;36:454–459. [DOI] [PubMed] [Google Scholar]
- 16. Pasnoor M, Dimachkie MM, Kluding P, Barohn RJ. Diabetic neuropathy, part 1: overview and symmetric phenotypes. Neurol Clin. 2013;31:425–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kempen JH, O'Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. [DOI] [PubMed] [Google Scholar]
- 18. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. 1999;14:223–232. [DOI] [PubMed] [Google Scholar]
- 19. Speiser P, Gittelsohn AM, Patz A. Studies on diabetic retinopathy, 3: influence of diabetes on intramural pericytes. Arch Ophthalmol. 1968;80:332–337. [DOI] [PubMed] [Google Scholar]
- 20. Miyamoto K, Ogura Y. Pathogenetic potential of leukocytes in diabetic retinopathy. Semin Ophthalmol. 1999;14:233–239. [DOI] [PubMed] [Google Scholar]
- 21. Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev. 1997;13:37–50. [DOI] [PubMed] [Google Scholar]
- 22. Boucher P, Teasdale N, Courtemanche R, et al. Postural stability in diabetic polyneuropathy. Diabetes Care. 1995;18:638–645. [DOI] [PubMed] [Google Scholar]
- 23. Corriveau H, Prince F, Hebert R, et al. Evaluation of postural stability in elderly with diabetic neuropathy. Diabetes Care. 2000;23:1187–1191. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749–1754. [DOI] [PubMed] [Google Scholar]
- 25. Roman de Mettelinge T, Cambier D, Calders P, et al. Understanding the relationship between type 2 diabetes mellitus and falls in older adults: a prospective cohort study. PloS One. 2013;8:e67055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ivers RQ, Cumming RG, Mitchell P, et al. Diabetes and risk of fracture: the Blue Mountains Eye Study. Diabetes Care. 2001;24:1198–1203. [DOI] [PubMed] [Google Scholar]
- 27. Yau RK, Strotmeyer ES, Resnick HE, et al. Diabetes and risk of hospitalized fall injury among older adults. Diabetes Care. 2013;36:3985–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Napoli N, Strotmeyer ES, Ensrud KE, et al. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia. 2014;57:2057–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strotmeyer ES, Cauley JA, Schwartz AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med. 2005;165:1612–1617. [DOI] [PubMed] [Google Scholar]
- 30. Yamaguchi T, Sugimoto T. Bone metabolism and fracture risk in type 2 diabetes mellitus. Endocr J. 2011;58:613–624. [DOI] [PubMed] [Google Scholar]
- 31. Pijpers E, Ferreira I, de Jongh RT, et al. Older individuals with diabetes have an increased risk of recurrent falls: analysis of potential mediating factors, the Longitudinal Ageing Study Amsterdam. Age Ageing. 2012;41:358–365. [DOI] [PubMed] [Google Scholar]
- 32. Chiles NS, Phillips CL, Volpato S, et al. Diabetes, peripheral neuropathy, and lower-extremity function. J Diabetes Complications. 2014;28:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fife TD. Benign paroxysmal positional vertigo. Semin Neurol. 2009;29:500–508. [DOI] [PubMed] [Google Scholar]
- 34. Mirka A, Black FO. Clinical application of dynamic posturography for evaluating sensory integration and vestibular dysfunction. Neurol Clin. 1990;8:351–359. [PubMed] [Google Scholar]
- 35. Myers SF, Ross MD. Morphological evidence of vestibular pathology in long-term experimental diabetes mellitus, II: connective tissue and neuroepithelial pathology. Acta Otolaryngol. 1987;104:40–49. [DOI] [PubMed] [Google Scholar]
- 36. Myers SF, Tormey MC, Akl S. Morphometric analysis of horizontal canal nerves of chronically diabetic rats. Otolaryngol Head Neck Surg. 1999;120:174–179. [DOI] [PubMed] [Google Scholar]
- 37. Perez R, Ziv E, Freeman S, et al. Vestibular end-organ impairment in an animal model of type 2 diabetes mellitus. Laryngoscope. 2001;111:110–113. [DOI] [PubMed] [Google Scholar]
- 38. Rybak LP. Metabolic disorders of the vestibular system. Otolaryngol Head Neck Surg. 1995;112:128–132. [DOI] [PubMed] [Google Scholar]
- 39. Klagenberg KF, Zeigelboim BS, Jurkiewicz AL, Martins-Bassetto J. Vestibulocochlear manifestations in patients with type I diabetes mellitus. Braz J Otorhinolaryngol. 2007;73:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Biurrun O, Ferrer JP, Lorente J, et al. Asymptomatic electronystagmographic abnormalities in patients with type I diabetes mellitus. ORL J Otorhinolaryngol Relat Spec. 1991;53:335–338. [DOI] [PubMed] [Google Scholar]
- 41. Nicholson M, King J, Smith PF, Darlington CL. Vestibulo-ocular, optokinetic and postural function in diabetes mellitus. Neuroreport. 2002;13:153–157. [DOI] [PubMed] [Google Scholar]
- 42. Chavez-Delgado ME, Vazquez-Granados I, Rosales-Cortes M, Velasco-Rodriguez V. Cochleovestibular dysfunction in patients with diabetes mellitus, hypertension and dyslipidemia [article in Spanish]. Acta Otorrinolaringol Esp. 2012;63:93–101. [DOI] [PubMed] [Google Scholar]
- 43. Jacobson GP, McCaslin DL, Piker EG, et al. Insensitivity of the “Romberg test of standing balance on firm and compliant support surfaces” to the results of caloric and VEMP tests. Ear Hear. 2011;32:e1–e5. [DOI] [PubMed] [Google Scholar]
- 44. Degerman E, Rauch U, Lindberg S, et al. Expression of insulin signalling components in the sensory epithelium of the human saccule. Cell Tissue Res. 2013;352:469–478. [DOI] [PubMed] [Google Scholar]
- 45. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. [DOI] [PubMed] [Google Scholar]
- 46. Yoda S, Cureoglu S, Yildirim-Baylan M, et al. Association between type 1 diabetes mellitus and deposits in the semicircular canals. Otolaryngol Head Neck Surg. 2011;145:458–462. [DOI] [PubMed] [Google Scholar]
- 47. Cohen HS, Kimball KT, Stewart MG. Benign paroxysmal positional vertigo and comorbid conditions. ORL J Otorhinolaryngol Relat Spec. 2004;66:11–15. [DOI] [PubMed] [Google Scholar]
- 48. De Stefano A, Dispenza F, Suarez H, et al. A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo [erratum in: Auris Nasus Larynx. 2014;41:325]. Auris Nasus Larynx. 2014;41:31–36. [DOI] [PubMed] [Google Scholar]
- 49. Prokopakis E, Vlastos IM, Tsagournisakis M, et al. Canalith repositioning procedures among 965 patients with benign paroxysmal positional vertigo. Audiol Neurootol. 2013;18:83–88. [DOI] [PubMed] [Google Scholar]
- 50. Boule NG, Haddad E, Kenny GP, et al. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–1227. [DOI] [PubMed] [Google Scholar]
- 51. Sigal RJ, Kenny GP, Wasserman DH, et al. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–1438. [DOI] [PubMed] [Google Scholar]
- 52. American Diabetes Association. Standards of medical care in diabetes: 2015 abridged for primary care providers. Diabetes Care. 2015;33:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–427. [DOI] [PubMed] [Google Scholar]
- 54. Whitney SL, Wrisley DM, Brown KE, Furman JM. Is perception of handicap related to functional performance in persons with vestibular dysfunction? Otol Neurotol. 2004;25:139–143. [DOI] [PubMed] [Google Scholar]
- 55. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the Activities-specific Balance Confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1998;53:M287–M294. [DOI] [PubMed] [Google Scholar]
- 56. Lajoie Y, Gallagher SP. Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch Gerontol Geriatr. 2004;38:11–26. [DOI] [PubMed] [Google Scholar]
- 57. Yardley L, Masson E, Verschuur C, et al. Symptoms, anxiety and handicap in dizzy patients: development of the vertigo symptom scale. J Psychosom Res. 1992;36:731–741. [DOI] [PubMed] [Google Scholar]
- 58. Murray KJ, Hill KD, Phillips B, Waterston J. The influence of otolith dysfunction on the clinical presentation of people with a peripheral vestibular disorder. Phys Ther. 2007;87:143–152. [DOI] [PubMed] [Google Scholar]
- 59. McCaslin DL, Jacobson GP, Grantham SL, et al. The influence of unilateral saccular impairment on functional balance performance and self-report dizziness. J Am Acad Audiol. 2011;22:542–561. [DOI] [PubMed] [Google Scholar]
- 60. Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance: suggestion from the field. Phys Ther. 1986;66:1548–1550. [DOI] [PubMed] [Google Scholar]
- 61. Jernigan SD, Pohl PS, Mahnken JD, Kluding PM. Diagnostic accuracy of fall risk assessment tools in people with diabetic peripheral neuropathy. Phys Ther. 2012;92:1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wrisley DM, Kumar NA. Functional gait assessment: concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys Ther. 2010;90:761–773. [DOI] [PubMed] [Google Scholar]
- 63. Herdman S. Vestibular Rehabilitation. 2nd ed Philadelphia, PA: Lippincott, Williams & Wilkins; 2000. [Google Scholar]
- 64. Oghalai JS, Manolidis S, Barth JL, et al. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol Head Neck Surg. 2000;122:630–634. [DOI] [PubMed] [Google Scholar]
- 65. Bhattacharyya N, Baugh RF, Orvidas L, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(5 suppl 4):S47–S81. [DOI] [PubMed] [Google Scholar]
- 66. Feldman EL, Stevens MJ. Clinical testing in diabetic peripheral neuropathy. Can J Neuro Sci. 1994;21:S3–S7. [DOI] [PubMed] [Google Scholar]
- 67. Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108:477–481. [DOI] [PubMed] [Google Scholar]
- 68. Zelman DC, Gore M, Dukes E, et al. Validation of a modified version of the Brief Pain Inventory for painful diabetic peripheral neuropathy. J Pain Symptom Manage. 2005;29:401–410. [DOI] [PubMed] [Google Scholar]
- 69. Hall CD, Schubert MC, Herdman SJ. Prediction of fall risk reduction as measured by Dynamic Gait Index in individuals with unilateral vestibular hypofunction. Otol Neurotol. 2004;25:746–751. [DOI] [PubMed] [Google Scholar]
- 70. Korres S, Balatsouras DG, Ferekidis E. Prognosis of patients with benign paroxysmal positional vertigo treated with repositioning manoeuvres. J Laryngol Otol. 2006;120:528–533. [DOI] [PubMed] [Google Scholar]
- 71. Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morrison S, Colberg SR, Parson HK, Vinik AI. Exercise improves gait, reaction time and postural stability in older adults with type 2 diabetes and neuropathy. J Diabetes Complications. 2014;28:715–722. [DOI] [PubMed] [Google Scholar]
- 73. Reid RD, Tulloch HE, Sigal RJ, et al. Effects of aerobic exercise, resistance exercise or both, on patient-reported health status and well-being in type 2 diabetes mellitus: a randomised trial. Diabetologia. 2010;53:632–640. [DOI] [PubMed] [Google Scholar]
- 74. Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90:1568–1579. [DOI] [PubMed] [Google Scholar]
- 75. Allet L, Armand S, de Bie RA, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rucker JL, Jernigan SD, McDowd JM, Kluding PM. Adults with diabetic peripheral neuropathy exhibit impairments in multitasking and other executive functions. J Neurol Phys Ther. 2014;38:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tilling LM, Darawil K, Britton M. Falls as a complication of diabetes mellitus in older people. J Diabetes Complications. 2006;20:158–162. [DOI] [PubMed] [Google Scholar]
- 78. Wallace C, Reiber GE, LeMaster J, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25:1983–1986. [DOI] [PubMed] [Google Scholar]
- 79. Kim SK, Lee KJ, Hahm JR, et al. Clinical significance of the presence of autonomic and vestibular dysfunction in diabetic patients with peripheral neuropathy. Diabetes Metab J. 2012;36:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. De Stefano A, Dispenza F, Suarez H, et al. A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo [erratum in: Auris Nasus Larynx. 2014;41:325]. Auris Nasus Larynx. 2014;41:31–36. [DOI] [PubMed] [Google Scholar]
- 81. Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:205–209. [DOI] [PubMed] [Google Scholar]
- 82. Morrison S, Colberg SR, Mariano M, et al. Balance training reduces falls risk in older individuals with type 2 diabetes. Diabetes Care. 2010;33:748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ahn S, Song R. Effects of Tai Chi exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy. J Altern Complement Med. 2012;18:1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Salsabili H, Bahrpeyma F, Forogh B, Rajabali S. Dynamic stability training improves standing balance control in neuropathic patients with type 2 diabetes. J Rehabil Res Dev. 2011;48:775–786. [DOI] [PubMed] [Google Scholar]
- 85. Song CH, Petrofsky JS, Lee SW, et al. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol Ther. 2011;13:803–811. [DOI] [PubMed] [Google Scholar]
- 86. Morrison S, Colberg SR, Parson HK, Vinik AI. Relation between risk of falling and postural sway complexity in diabetes. Gait Posture. 2012;35:662–668. [DOI] [PubMed] [Google Scholar]
- 87. Mueller MJ, Tuttle LJ, Lemaster JW, et al. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]