Abstract
Background
As physical activity in people poststroke is low, devices that monitor and provide feedback of walking activity provide motivation to engage in exercise and may assist rehabilitation professionals in auditing walking activity. However, most feedback devices are not accurate at slow walking speeds.
Objective
This study assessed the accuracy of one accelerometer to measure walking steps of community-dwelling individuals poststroke.
Design
This was a cross-sectional study.
Methods
Two accelerometers were positioned on the nonparetic waist and ankle of participants (N=43), and walking steps from these devices were recorded at 7 speeds (0.3–0.9 m/s) and compared with video recordings (gold standard).
Results
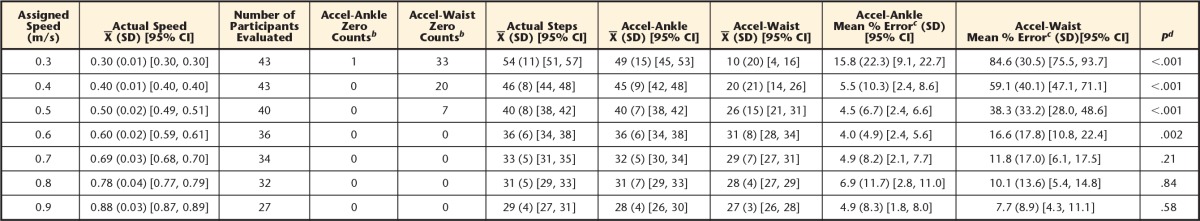
When positioned at the waist, the accelerometer had more than 10% error at all speeds, except 0.8 and 0.9 m/s, and numerous participants recorded zero steps at 0.3 to 0.5 m/s. The device had 10% or less error when positioned at the ankle for all speeds between 0.4 and 0.9 m/s.
Limitations
Some participants were unable to complete the faster walking speeds due to their walking impairments and inability to maintain the requested walking speed.
Conclusions
Although not recommended by the manufacturer, positioning the accelerometer at the ankle (compared with the waist) may fill a long-standing need for a readily available device that provides accurate feedback for the altered and slow walking patterns that occur with stroke.
Physical activity is extremely low in individuals poststroke compared with both healthy older adults and those with other chronic diseases.1 Devices, such as pedometers, that measure walking activity can be effective in motivating this population to exercise2 and may assist health care professionals to monitor and progress mobility during rehabilitation. However, to our knowledge, no pedometers accurately record steps in this population at slow walking speeds.3,4
With recent technological advances, activity monitors that incorporate accelerometers may provide an alternative to pedometers for accurately measuring walking steps. The Fitbit One (Fitbit) (Fitbit Inc, San Francisco, California) is an example of an activity monitor that includes an accelerometer and is readily available to consumers, providing instantaneous visual feedback of walking steps. Despite the accuracy of the Fitbit One in measuring walking steps in healthy adults at relatively normal walking speeds (0.9–1.78 m/s),5 it provides inaccurate step counts at slow walking speeds poststroke, particularly less than 0.6 m/s.6 This imprecision observed at slow walking speeds is consistent with that of another accelerometer, the Actical accelerometer (Philips Respironics, Bend, Oregon), which provides inaccurate step counts in healthy, older adults at walking speeds between 0.46 and 0.85 m/s.3 However, it is perhaps the accelerometer position on the body that may influence the step count accuracy at slow walking speeds. Although both the Fitbit One and Actical accelerometers are recommended by the manufacturers to be worn at the waist, one study in older in the acute postsurgical setting suggested that step count sensitivity with an accelerometer can be improved for slower walking speeds when positioned at the ankle.7 Furthermore, other accelerometers developed for research (not consumer) applications that do not provide instantaneous visual feedback have been found to be valid and reliable for mean walking speeds between 0.55 and 0.82 m/s when positioned at the ankle in individuals poststroke.8,9
Overall, measuring walking steps is one way to objectively assess an individual's walking activity and has been used in this regard in settings across the rehabilitation continuum (acute/subacute/community).2,7,8 However, a readily available device has not yet been identified that can accurately measure walking steps at a range of walking speeds (very slow to normal) in individuals poststroke with a variety of walking deficits. For this reason, it is necessary to establish how specific walking speeds and positioning on the body may influence the accuracy of activity monitors that provide instantaneous visual feedback for measuring walking steps in individuals poststroke. Knowledge of this information may assist both individuals poststroke and the health care professionals working with them to obtain accurate, objective data to assess and monitor walking during rehabilitation. Therefore, the objectives of this study were: (1) to examine the effect of walking speed on the accuracy of an accelerometer-based activity monitor in ambulatory individuals poststroke and (2) to compare the effect of position (waist versus ankle) on the accuracy of an accelerometer-based activity monitor. We hypothesized that: (1) step count accuracy would decline as walking speed decreased, and (2) step count measurements at the ankle would be more accurate than those at the waist for all walking speeds.
Method
Participants
Volunteers were recruited from the community through a stroke research registry, flyers posted at recreational facilities, education sessions at stroke support groups, and word of mouth. Individuals satisfying the following inclusion criteria were recruited for the study: at least 3 months poststroke, able to follow 3-step commands, and able to walk independently for at least 30 m (orthotic/assistive devices permitted). Exclusion criteria for the study included having a major medical condition (ie, multiple sclerosis, Parkinson disease, angina) affecting the individual's ability to walk and having had major surgery (ie, hip replacement, heart surgery) in the previous 12 months. Ethics approval was obtained from the Institutional Review Board of the University of British Columbia, and informed consent was obtained from all participants.
Device
The Fitbit was used for the study. It is a small (4.8- × 1.9- × 1.0-cm), commercially available device containing a triaxial accelerometer that converts acceleration to step counts based on proprietary algorithms. The manufacturer suggests that the Fitbit One be worn on a waist belt or bra (attached by the clip on the back of the device) or put in a pant pocket.
Procedure
The accelerometer was positioned on each participant's nonparetic side on a waistband (lateral point [Accel-Waist]) and ankle strap (above the lateral malleolus [Accel-Ankle]). Participants walked a distance of 15 m for 8 walking trials: 1 trial at a self-selected walking speed and 7 trials from 0.3 m/s to 0.9 m/s in 0.1-m/s increments. Visual gait analysis of the participant's self-selected walking trial was conducted by a physical therapist or occupational therapist, and observed walking characteristics were selected from a checklist created by the study team of the most commonly observed gait deviations poststroke.
At the start of each walking trial, a “pace-setter” (trained assistant) instructed the participant to “walk directly beside him or her.” The “pace-setter” used visual (markings on the floor) and auditory (metronome beats via earbuds) feedback to maintain the selected speed. The step count on each accelerometer, obtained by pressing a button on the display, was recorded at the beginning and end of each walking trial. Participants self-determined the amount of sitting or standing rest needed between walking trials. The order of the 7 trials was randomized for each participant, and each trial was video recorded. Two independent viewers counted the actual number of steps from the video recordings of each trial (ie, considered gold standard). If there was greater than 1 step difference between the 2 viewers, a third viewer watched the video, and consensus was reached. All data from one viewer were randomly selected and used for the analysis.
Data Analysis
Descriptive statistics (means and standard deviations) were used to describe sample characteristics. Accuracy of the Fitbit device at each speed and location was assessed by examining the error between the Fitbit and observed steps. A linear mixed model was created with the restricted maximum-likelihood method to determine the effect of speed and location on the accuracy of the Fitbit (ie, error between the observed steps and the Fitbit steps). Error was calculated using the following calculation: (Fitbit steps − Observed steps)/Observed steps. Speed (continuous variable centered at its slowest speed [ie, 0.3 m/s]) and location (categorized into ankle and waist) were input as fixed variables. The interaction between speed and location also was explored. A participant variable was input as the random variable to account for the correlation between repeated measurements (ie, different speeds and locations). A varying intercept model was applied to allow the intercept for each participant to vary. A likelihood ratio test also was used to determine if the interaction between speed and location statistically improved the model fit and, therefore, should be retained in the final model. Post hoc paired t tests were performed to examine differences in error between the ankle and waist locations at each speed when the likelihood ratio test was statistically significant. Bonferroni-adjusted P values for multiple comparisons are reported. Finally, diagnostic plots of the final model were examined to investigate violations of regression assumptions. Statistical analyses were performed using R version 3.1.1 (R Development Core Team, Vienna, Austria).
Role of the Funding Source
This work was supported by the Canadian Institutes of Health Research and Heart and the Stroke Foundation of BC and Yukon.
Results
A total of 43 individuals participated in the study (Tab. 1). The majority of participants used an assistive device to ambulate and had an altered walking pattern (Tab. 2). The mean comfortable walking speed was 0.72 m/s (SD=0.3). Data were recorded for each walking trial only if it was within 10% of the assigned speed; therefore, some participants could not complete the faster speeds (Tab. 3). The pace-setter protocol resulted in participants being within 98% of the assigned speeds.
Table 1.
Participant Characteristics (N=43)
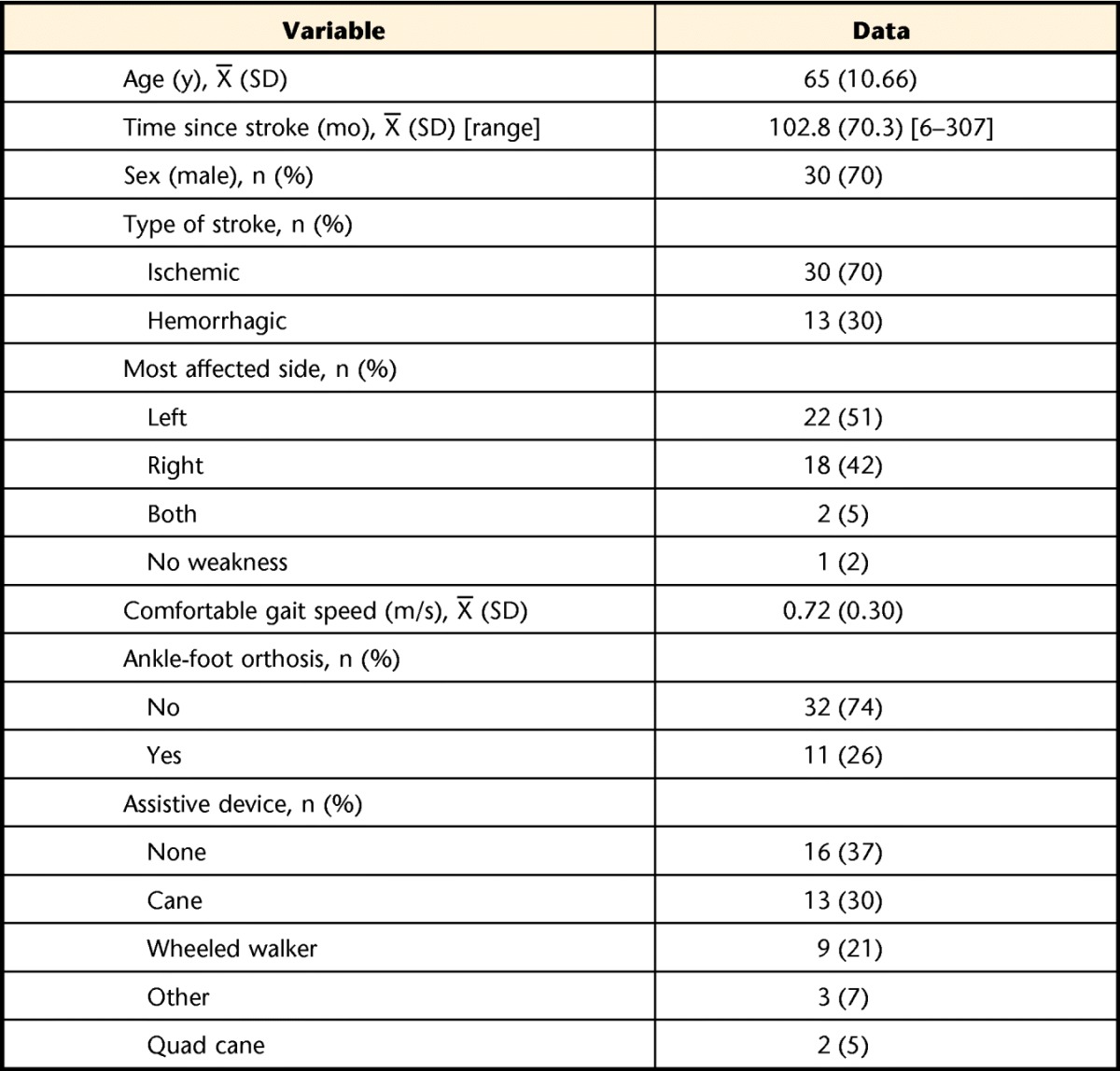
Table 2.
Walking Impairments Observed During Self-selected Walking Speed Trial
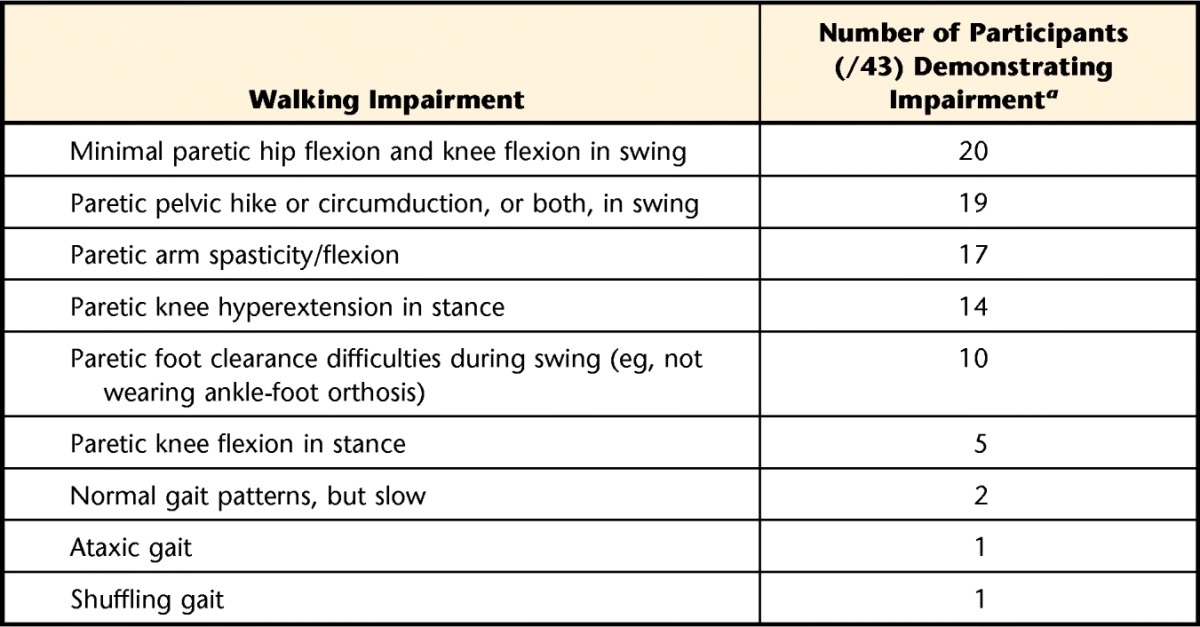
Participants may exhibit several impairments.
Table 3.
Accuracy of the Accelerometer at Varying Walking Speedsa
CI=confidence interval.
b Number of participants for whom the accelerometer counted zero steps for the walking trial. The accelerometer was positioned on each participant's nonparetic side on a waistband (lateral point [Accel-Waist]) and ankle strap (above the lateral malleolus [Accel-Ankle]).
c Absolute error, calculated as: [(Accelerometer steps – Actual steps)/Actual steps) × 100]. Calculation performed on individual data, so may not equate if calculation completed on group data presented above.
d Paired t test between Accel-Ankle and Accel-Waist; Bonferroni-corrected P values reported.
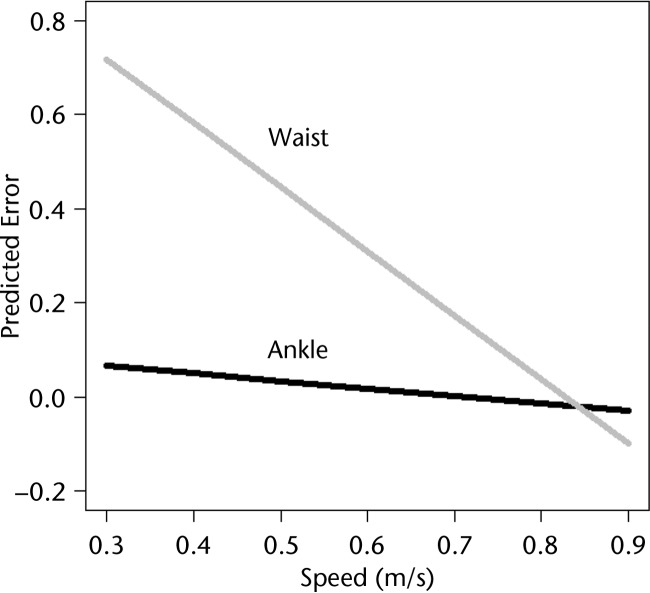
The final linear mixed model resulted in significant effects of speed and location on Fitbit error (Tab. 4). The interaction between speed and location significantly increased the model fit and, therefore, was retained in the final model. Review of the diagnostic plots did not reveal any violations of the regression assumptions. Figure 1 displays the relationship between speed and error at the ankle and waist placements. An increase in speed was associated with a decrease in error when the attachment location was held constant. Post hoc paired t tests revealed statistically lower mean error at the ankle compared with the waist at speeds ranging from 0.3 to 0.6 m/s (Tab. 3). No statistically significant differences in mean error were observed between the ankle and waist locations at the 3 highest speeds (ie, 0.7–0.9 m/s) (Tab. 3). At the slower walking speeds (0.3–0.5 m/s), the Accel-Waist placement recorded a value of zero for 33, 20, and 7 participants, respectively. The Accel-Ankle placement only recorded a value of zero for one participant at the slowest walking speed of 0.3 m/s (Tab. 3).
Table 4.
Final Linear Mixed Model Predicting Fitbit Error
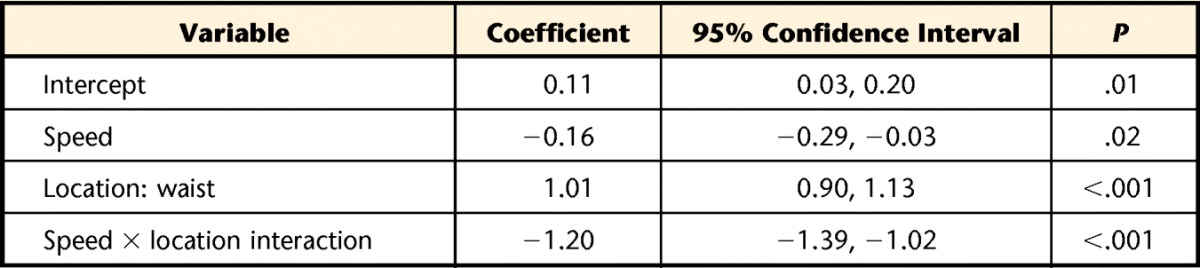
Figure 1.
Estimated relationship between speed and error for each accelerometer location (waist, ankle) based on the linear mixed model.
Discussion
This study examined the effect of walking speed on the accuracy of an accelerometer-based monitor in ambulatory individuals poststroke and compared the effect of the position (waist versus ankle) on the accuracy of the monitor. Our findings suggest that the accelerometer is more accurate as walking speed increases from 0.3 to 0.9 m/s. In addition, the accelerometer is more accurate when placed at the ankle, versus the waist, at the slower speeds (0.3–0.6 m/s) (Fig. 2).
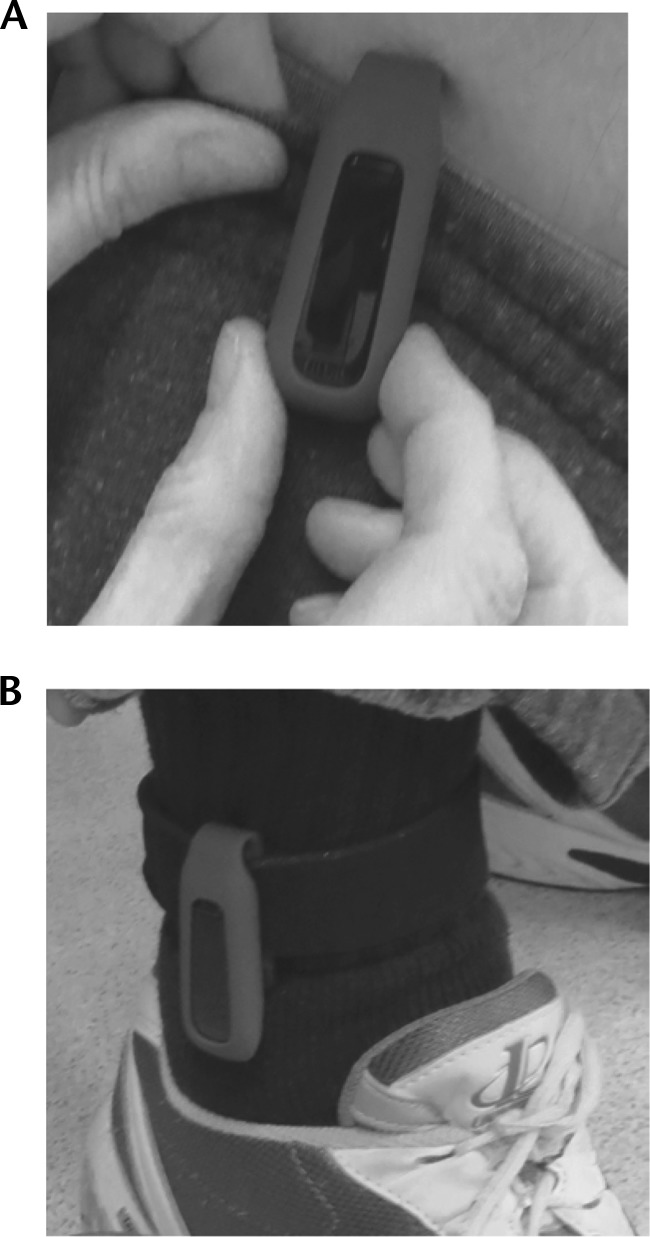
Figure 2.
The Fitbit One positioned on a participant's (A) waist and (B) ankle.
The walking speeds selected for this study (0.3–0.9 m/s) are representative of the typical gait speeds observed in individuals poststroke.10 Designing a novel “pace-setter” paradigm allowed us to systematically test the accuracy of the accelerometer at a wide range of walking speeds without constraining step length or cadence while simulating a natural task of walking with a companion. Previous literature with individuals poststroke has only captured a single comfortable speed and reported accuracy of step count devices at walking speeds greater than 0.6 m/s.4,6,11 Our study population had a variety of walking impairments, and although all participants were able to complete the slower walking speeds (0.3–0.4 m/s), the study population decreased as the walking speed increased. This finding is consistent with the heterogeneity observed in the poststroke population and signifies the importance of identifying a monitor that is accurate at recording step counts at a wide range of walking speeds.
The device step count accuracy also was dependent upon where it was positioned on the body. Although manufacturers commonly recommend that monitoring devices be positioned at the waist, the results supported our hypothesis that the accelerometer positioned at the ankle (versus the waist) was more accurate for speeds between 0.3 and 0.6 m/s. Most importantly, with a modified placement at the ankle, the accelerometer was able to accurately capture steps at speeds as slow as 0.3 m/s. At the slowest walking speeds (0.3–0.5 m/s), the waist-positioned accelerometer recorded 0 steps for 60/126 walking trials (48%), whereas the ankle-positioned accelerometer recorded 0 steps for only 1/126 trials (0.01%). The improved sensitivity and accuracy are likely due to the higher accelerations at the ankle compared with the hip. In individuals poststroke, propulsion of the nonparetic limb, particularly at the toe-off phase of the gait cycle, was shown to be exaggerated in the attempt to limit single-leg stance time on the paretic, or weak, limb.12 At very slow walking speeds, the ankle-positioned accelerometer is able to consistently detect the limb acceleration, whereas the waist-positioned device cannot accurately respond to the limb accelerations until much faster walking speeds (0.8–0.9 m/s). Therefore, to accurately monitor walking activity across a range of walking speeds, our results suggest that positioning the accelerometer at the ankle versus the waist yields more accurate results.
Overall, our results fill a long-standing need for identifying a readily available monitoring device that can provide accurate, instantaneous, visual feedback for the altered and slow walking patterns that occur with stroke. Accurate devices to audit walking activity are imperative to progress rehabilitation research and clinical practice to assess dose-response relationships13 and provide objective outcome measures14 with individuals poststroke. By accurately assessing a wide range of walking speeds (from very slow to normal), this device would enable health professionals to evaluate patients' progress from the acute to community settings and provide an objective measure to monitor and progress activity.
Limitations
Not all participants could complete the faster walking speeds due to their stroke impairments; however, all could complete the slowest speeds, which was the primary focus of the study. Also, the speed of each walking trial may not have been representative of the participant's self-selected walking speed. We assessed a wide range of walking speeds for each participant to capture what typically may be experienced within the course of a day, including slow, comfortable, and fast walking speeds. With respect to device application, we did not require the participants to put on and take off the device or view the data independently. Although these processes can be successfully completed in a sitting position, using only one upper extremity, some individuals poststroke with significant visual, perceptual, or sensorimotor deficits may have challenges independently donning the device and observing the data. These challenges may be met by having assistance from another person, or the device data can be viewed wirelessly through a compatible mobile device (ie, phone/tablet).
Footnotes
Ms Klassen, Ms Simpson, Ms Lim, Ms Parappilly, and Dr Eng provided concept/idea/research design. All authors provided writing. Ms Klassen, Ms Simpson, Ms Lim, Mr Louie, Ms Parappilly, Dr Sakakibara, and Mr Zbogar provided data collection. Ms Klassen, Ms Simpson, Mr Louie, Ms Parappilly, and Dr Eng provided data analysis. Ms Klassen and Dr Eng provided project management. Dr Eng provided fund procurement. Ms Klassen provided participants and administrative support. Ms Klassen, Ms Parappilly, and Dr Sakakibara provided consultation (including review of manuscript before submission). The authors thank Jaymi Booth, Christie Chan, Zoe Hassall, Chihya Hung, Amanda Mow, and Caryne Torkia for their assistance.
This work was supported by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of BC and Yukon.
References
- 1. Ashe MC, Miller WC, Eng JJ, Noreau L. Older adults, chronic disease and leisure-time physical activity. Gerontology. 2009;55:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sullivan JE, Espe LE, Kelly AM, et al. Feasibility and outcomes of a community-based, pedometer-monitored walking program in chronic stroke: a pilot study. Top Stroke Rehabil. 2014;21:101–110. [DOI] [PubMed] [Google Scholar]
- 3. Martin JB, Krč KM, Mitchell EA, et al. Pedometer accuracy in slow walking older adults. Int J Ther Rehabil. 2012;19:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carroll SL, Greig CA, Lewis SJ, et al. The use of pedometers in stroke survivors: are they feasible and how well do they detect steps? Arch Phys Med Rehabil. 2012;93:466–470. [DOI] [PubMed] [Google Scholar]
- 5. Takacs J, Pollock CL, Guenther JR, et al. Validation of the Fitbit One activity monitor device during treadmill walking. J Sci Med Sport. 2014;17:496–500. [DOI] [PubMed] [Google Scholar]
- 6. Fulk GD, Combs SA, Danks KA, et al. Accuracy of two activity monitors in detecting steps in people with stroke and traumatic brain injury. Phys Ther. 2014;94:222–229. [DOI] [PubMed] [Google Scholar]
- 7. Cook DJ, Thompson JE, Prinsen SK, et al. Functional recovery in the elderly after major surgery: assessment of mobility recovery using wireless technology. Ann Thorac Surg. 2013;96:1057–1061. [DOI] [PubMed] [Google Scholar]
- 8. Dobkin BH, Xu X, Batalin M, et al. Reliability and validity of bilateral ankle accelerometer algorithms for activity recognition and walking speed after stroke. Stroke. 2011;42:2246–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haeuber E, Shaughnessy M, Forrester LW, et al. Accelerometer monitoring of home and community-based ambulatory activity after stroke. Arch Phys Med Rehabil. 2004;85:1997–2001. [DOI] [PubMed] [Google Scholar]
- 10. Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. [DOI] [PubMed] [Google Scholar]
- 11. Elsworth C, Dawes H, Winward C, et al. Pedometer step counts in individuals with neurological conditions. Clin Rehabil. 2009;23:171–175. [DOI] [PubMed] [Google Scholar]
- 12. Chen C, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22:51–56. [DOI] [PubMed] [Google Scholar]
- 13. Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. 2014;45:2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dorsch AK, Thomas S, Xu X, et al. ; on behalf of the SIRRACT investigators. SIRRACT: an international randomized clinical trial of activity feedback during inpatient stroke rehabilitation enabled by wireless sensing. Neurorehabil Neural Repair. 2015;29:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]