Abstract
Environmental and genetic factors interact in the process and treatment of colon cancer, although the underlying mechanisms remain elusive. The aim of the study was to examine the role of whether bone morphogenetic protein 7 (BMP7) is involved in the progression of colon cancer under local intratumoral infiltration lymphocytes. A total of 46 cases of pathologically confirmed specimens were obtained from patients with nodal invasion of colon cancer. The patients were subdivided into three groups based on the nodal invasion stages (N0, N1 and N2). Eleven cases without nodal invasion of colon cancer served as the control group (N0). The phenotype of CD45+, CD4+, CD8+, CD25+ and CD56+ cells and the expression of BMP7 were confirmed by immunofluorescence. The association between BMP7 expression and CD45+/CD4+CD25+/CD8+ cells infiltration was analyzed. The density of CD4+CD25+ T cells within the tumor was associated with nodal invasion in patients with colon cancer. More importantly, the expression of BMP7 was observed in the majority of the cancer tissues. The co-expression pattern of BMP7 in colon cancer cells and intratumor CD4+CD25+ T cells was associated with nodal invasion of colon cancer. In conclusion, the results have shown that the co-expression of BMP7 is inversely associated with the infiltration of CD4+CD25+ T cells of colon cancer. The results suggest the combination of adaptive immunotherapy and biological drugs impact the treatment strategy for colon cancer in distinct clinical settings.
Keywords: bone morphogenetic protein 7, CD4+CD25+ T cells, colon cancer, nodal invasion
Introduction
Inflammatory cells are present in the tumor microenvironment of most cancers and have been reported to affect tumor progression (1–3). The long-term survival of patients with colon cancer is dependent on the pathological stage as well as the complex interactions between tumor- and patient-associated factors. In particular, systemic and local host inflammatory responses are important determinants of cancer outcome. In contrast to the systemic response, local infiltration of inflammatory cells in the tumor microenvironment is associated with improved survival in patients with colon cancer. Tumor-infiltrating T cells may be an indicator of host immune response to tumor and an attractive target for immunotherapy (4–7). However, the specific role of individual leukocytic infiltrates in individual tumors remains to be elucidated. This diversity of immunologic response to malignancies renders the targeting of the immune system as part of anticancer therapies a challenge.
Treatment of advanced colon cancer has improved over the past 15 years. The combination of chemotherapy and biological drugs, such as anti-epidermal growth factor receptor (EGFR) or anti-vascular endothelial growth factor antibodies, as well as the sequencing of different active drugs as the disease progresses, can significantly improve outcomes (8,9). In particular, treatment with monoclonal antibodies (cetuximab or panitumumab) against the extracellular domain of the receptor has become a major therapeutic strategy in the treatment of metastatic colorectal cancer. However, the responses to EGFR-targeted antibodies are relatively low, with improvements in survival usually lasting only several months, and efficacy limited to certain patient subtypes (10). Nevertheless, despite these advances, the optimal treatment for patients with advanced colon cancer in clinical practice is not yet defined.
Overexpression of bone morphogenetic protein 7 (BMP7) promotes gene amplification and mutation consequence in cell proliferation, survival, invasion, metastasis, and tumor-induced neoangiogenesis (11–14). Thus targeting BMP7 constitutes an effective therapy in colon cancer. In the present study, the BMP7 expression in surgical specimens of colon cancer was examined to assess the association between this molecule and local immune response. The examination of individual cell types cannot predict outcomes, but it does suggest a prominent role at the level of nodal involvement and lymphatic invasion in these patients. Thus, the results support that the combination of adaptive immunotherapy and biological drugs impact the treatment strategy for colon cancer in distinct clinical settings.
Materials and methods
Clinical samples
Paraffin-embedded specimens of patients with colorectal cancer (stages I–III) were retrieved retrospectively from 46 patients who underwent surgery at the Department of Surgery, Siping Hospital of China Medical University (Siping China), between January 2005 and December 2014. The present study was approved by the Research Ethics Committee at Siping Hospital of China Medical University.
The patients were divided into 3 groups as per their nodal metastasis grade (N0, N1, or N2). The first group comprised 11 patients (N0), the second group 20 patients (N1), and the third group 15 patients (N2). The exclusion criteria for the study were: i) Clinical evidence of active infection; ii) the presence of a chronic inflammatory condition; and iii) preoperative chemoradiotherapy. The tumors were staged according to the fifth edition of the tumor, node and metastasis classification (15). Additional pathological data were obtained from reports issued at the time of resection. The clinicopathological characteristics of patients are shown in Table I.
Table I.
Pathological characteristics of colon cancer patients.
| Characteristics | N0 | N1 | N2 |
|---|---|---|---|
| Median age (range), years | 62.36 (51–72) | 61.19 (53–69) | 60.72 (50–71) |
| Gender, no. (%) | |||
| Female | 5 (37.5) | 9 (46.9) | 6 (47.6) |
| Male | 6 (62.5) | 11 (53.1) | 9 (52.4) |
| T stage primary tumor, no. (%) | |||
| T0 | 0 | 0 | 0 |
| T1 | 0 | 0 | 0 |
| T2 | 2 | 0 | 0 |
| T3 | 9 | 20 | 15 |
| T4 | 0 | 0 | 0 |
| N stage primary tumor, no. (%) | |||
| Node-negative | 11 (100.0) | 0 | 0 |
| Node-positive | 0 | 20 (100.0) | 15 (100.0) |
| N1 | 0 | 20 (100.0) | 0 |
| N2 | 0 | 0 | 15 (100.0) |
| M stage primary tumor, no. (%) | |||
| M0 | 11 (100.0) | 14 (50.0) | 11 (100.0) |
| M1 | 0 | 6 (50.0) | 4 |
| Largest median diameter, cm (range) | 3.29 (2.11–7.36) | 5.56 (2.09–8.73) | 6.67 (2.13–9.84) |
Immunofluorescence of paraffin-embedded tissue sections and evaluation of staining
Paraffin-embedded sections (4 µm) were obtained to assess lymphocyte markers, including CD4 (rabbit, polyclonal, IgG, catalog no: ABIN671376 at a dilution of 1:1000), CD8 (rabbit, polyclonal, IgG, catalog no: ab85792 at a dilution of 1:1000), CD25 (mouse, monoclonal, catalog no: ABIN320392 at a dilution of 1:1000), CD45 (rabbit, polyclonal, IgG, catalog no: ab17553 at a dilution of 1:1000) and CD56 (mouse, monoclonal, catalog no: ABIN2658999 at a dilution of 1:1000). The primary antibodies were purchased from BD Biosciences (Wuhan, China). To detect CD4 and CD25, a general Treg cell antigen was used. CD45 was used as a common leukocyte antigen. CD8 was used as a cytotoxic T lymphocyte (CTL) marker. CD45 was used as a leukocyte common antigen and CD56 was used as an NK cell marker. BMP7 immunoreactivities were detected in the nucleus, and the data were evaluated as a labeling index (LI), as previously described (16). Fluorescent staining with anti-mouse and anti-rabbit Ig secondary antibodies [anti-rabbit IgG (H+L)-FITC: 211-095-109, Jackson ImmunoResearch, Düsseldorf, Germany] conjugated with Alexa dyes 488 and 568 (Invitrogen Life) conjugated with Alexa dyes 488 and 568 (Invitrogen Life Technologies, Paisley, UK) was used for double staining (CD4 and CD25; CD8 and CD56). Nuclear counterstaining in this case was performed with DAPI (Roche, Mannheim, Germany). Counting was performed under a microscope (Olympus, BX51TF, Tokyo, Japan) at a magnification of ×400. Stained immune cells were assessed without knowledge of the clinical parameters of each patient. The positive cells were evaluated in the nuclei of >1,000 tumor cells for each case, and LI was calculated as the percentage of each type of positive cell per 1,000 tumor cells counted at random in each section. The cells in each sample were examined blindly by three independent expert observers.
Statistical analysis
Data are shown as mean ± standard deviation. Cross-tabulations variables were analyzed using Fisher's exact test. Statistical analyses were performed using SPSS software, version 19.0 (IBM SPSS, Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Evaluation of CD45+ cells at the site of colon cancer tissue
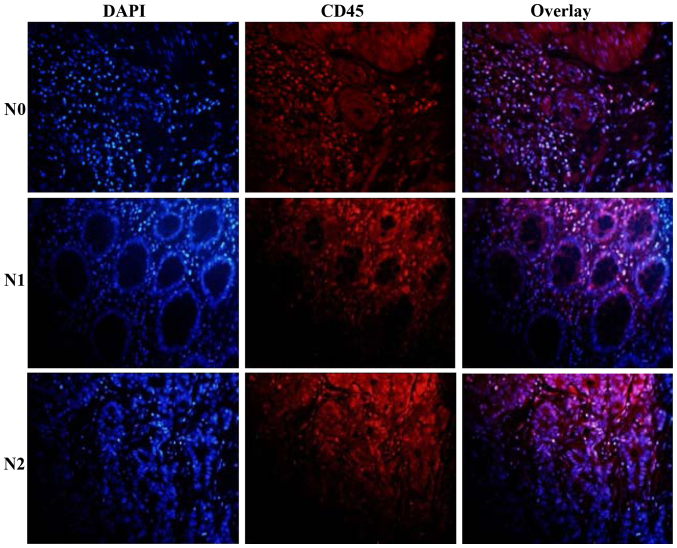
Gross examination revealed massive infiltration of CD45+ cells confined to nodal invasion (Fig. 1). Different patterns of immune cell accumulations were observed among the patients which potentially coexisted within the same specimen in colon cancer tissue as single cells and diffused cell aggregates (Table II, Fig. 1). CD45+ cells were ranged at a percentage of 46.32, 31.61 and 16.74% in the N0, N1 and N2 nodal invasion groups, respectively (Table II, Fig. 1).
Figure 1.
Expression of CD45+ lymphocytes in colon cancer tissue. CD45+ lymphocytes were observed in the N0, N1 and N2 nodal invasion groups.
Table II.
Associatoin between individual T lymphocyte and the nodal invasion of colon cancer.a
| Percentage of individual T lymphocyte of colon cancer | |||||
|---|---|---|---|---|---|
| Nodal invasion group | CD45+ | CD4+ | CD4+CD25+ | CD8+ | CD56+ |
| N0 | 46.32±6.08 | 14.63±3.47 | 8.11±3.26 | 6.23±3.13 | 4.41±1.13 |
| N1 | 31.61±4.65a,b | 21.19±6.12a | 15.42±5.09a | 2.14±2.08a | 1.98±0.69a |
| N2 | 16.74±3.37b,c | 28.63±11.35c | 21.37±11.33c | 0.21±0.36c | 0.19±1.01b |
In comparison to N1, parameters in N1 and N2 showed
P<0.05
P<0.01. In comparison to N1, parameters in N2, bP<0.05
P<0.01. Data are shown as mean ± standard deviation.
Patient-specific extent and organization pattern of CD4+CD25+ T lymphocytes in tumor tissue
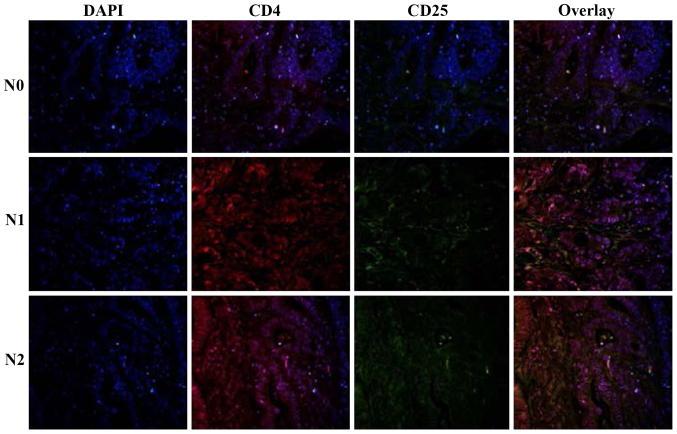
To characterize the T-cell lineages in tumor tissue, the tissue specimens were analyzed for CD4+CD25+ cells. The major proportion of cells within the large lymphocyte aggregates was CD4+CD25+ T cells in the N2 nodal invasion group. Quantitative evaluations of the three groups revealed the presence of CD4+CD25+ T cells in tumor tissue, ranging from 8.11, 15.42 to 21.37% in the N0, N1 and N2 nodal invasion groups (Table II, Fig. 2).
Figure 2.
Expression of CD4+CD25+ T cells in colon cancer tissue.
CD8+ T lymphocytes assemble within the metastatic colon cancer
An important observation of the current study was the presence of CD8+ T lymphocytes at the site of the tumor tissue. CD8+ T lymphocytes were detected in all of the patients in the N0 nodal invasion group. A comparison of the three groups regarding CD8+ T lymphocytes in the tumor tissue revealed statistically significant differences (Fig. 3, Table II), whereas no CD8+ T lymphocytes were typically present in the N2 nodal invasion group (Fig. 3, Table II).
Figure 3.
Expression of CD8+CD56+ T cells in colon cancer tissue. Compared to other groups, no CD8+ T lymphocytes were observed in the N2 nodal invasion group and few CD56+ T cells were observed in the N0 nodal invasion group.
To characterize the role of NK cells in tumor tissue, tissue sections were stained with anti-CD56 to define the fully active, intra-tumoral NK cells. Only trace numbers of CD56+ cells were observed in the N0 nodal invasion group (Fig. 3, Table II).
Expression of BMP7 in colon cancer
The expression patterns and cellular localization of BMP7 in 46 colon cancer tissues of three differentiation levels were assessed by immunofluorescence analysis. As shown in Table III and Fig. 4, BMP7 immunoreactivity was predominantly localized in the nuclei of CRC cells, whereas BMP7 was significantly higher in the poor nodal invasion group than in the negative nodal group (P<0.01) (Table III, Fig. 4).
Table III.
Analyze the expression of BMP7 intra-tumor of colon cancer.
In comparison to N0, parameters in N1 and N2 showed
P<0.05
P<0.01. In comparison to the N0 and N1 group, parameters in N2 showed bP<0.05
P<0.01. Data shown as mean ± standard deviation.
Figure 4.
The expression of bone morphogenetic protein 7 (BMP7) in colon cancer tissue.
Discussion
The clinical course of remission and relapse is commonly observed in patients undergoing therapy for colon cancer (17). In the present study, we detected the overexpression of BMP7 in colon cancer tissues in its advanced stage, in particular, its upregulation of BMP7 was closely associated with nodal metastasis. Thus, the combination of chemotherapy, radiation therapy, adaptive immunotherapy and biological drugs, such as anti-BMP7 antibodies for first-line treatment, can significantly improve outcomes.
The mechanisms by which a strong local adaptive immune response improves prognosis in patients with colon cancer remain to be elucidated. In the current study, we identified a highly significant and independent association of CD45+ lymphocyte infiltration in the preoperative biopsy with nodal invasion of colon cancer patients. The percentage of CD45+ lymphocytes decreased in the later stage of colon cancer tissue. Since the CD45 antigen was originally known as leukocyte common antigen (18), the findings strongly suggest that such patients mount a coordinated inflammatory response at a local level, mediated primarily by cells associated with adaptive immunity.
Immunotherapy was utilized for the treatment of colon cancers. To examine T-cell subsets, we evaluated the distribution of T lymphocytes with CD4, CD8 and CD25 phenotype and analyzed the association between different subsets of T lymphocytes and nodal metastasis in colon cancer tissue. For this study, CD4 and CD25 were selected as the regulatory markers for T cells, with CD8 being used as the effector CTLs. Using double immunoflourescent staining and confocal analysis, we confirmed that to a large extent the markers separate in the three different situations of nodal metastasis. We also evaluated CD56+ cells in colon cancer tissue, which is similar to CD8+ T cells, but decreased in the later stage of colon cancer. Of note, the mechanism of adaptive immunity limitation was of considerable interest and remains to be investigated.
Colon cancers resist certain therapies, such as chemotherapy and adaptive immunotherapy. To investigate the reason for this, the expression of BMP7 in colon cancer tissue was examined. BMP7 has been reported in a wide range of human cancers and has been associated with metastasis and poor prognosis (14,19,20). In the current study, the expression of BMP7 was 13.4, 28.1 and 40.6% in the N0, N1 and N2 stage, respectively. These findings suggest BMP7 overexpression tended to localize lymph node relapse. In contrast to cells associated with the adaptive immune response, an abundance of CD4+CD25+ T cells was associated with the abundance of BMP7 expression. These results suggest a protective host response, i.e., the collective effect of BMP7 expression and CD4+CD25+ T-cell infiltration in the tumor may favor tumor growth and dissemination. Thus, our results suggest monoclonal antibodies may be used under immune contexture.
The study has some limitations. The identification and classification of individual T-cell types should be investigated. Additionally, more patients should be examined for concrete evidence and restricting potential clinical application. The present study focused only on the intra-tumor tissue and did not assess the inflammatory cell invasive margin of the tumor, which is reported to constitute a critical interface between pro- and anti-tumor factors. Furthermore, examination of the prognostic value of intra-tumor inflammatory cells and tumor molecular features need to be verified.
In conclusion, our results have demonstrated the co-expression of CD4+CD25+ T cells and BMP7 in a considerable percentage of patients with nodal metastatic colon cancer. Overexpression of BMP7 is a potential predictor of nodal invasion, while the molecular marker for high-risk patients may be useful in individualizing patient therapy, such as anti-BMP7 antibodies for first-line treatment, can significantly improve outcomes. In particular, the results suggest the combination of adaptive immunotherapy and biological drugs impact the treatment strategy for colon cancer in distinct clinical settings.
Acknowledgements
This study received a grant from the Department of Science and Technology of Jilin Province (Jilin, China) (no. 201105100), and special funds for Innovation and Industrial Development of the Independent Core Area (Beiijng, China) (2014).
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, Sica A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. 2010;29:243–248. doi: 10.1007/s10555-010-9227-2. [DOI] [PubMed] [Google Scholar]
- 4.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 5.Speetjens FM, Lauwen MM, Franken KL, van Janssen Rhijn CM, van Duikeren S, Bres SA, van de Velde CJ, Melief CJ, Kuppen PJ, van der Burg SH, et al. Prediction of the immunogenic potential of frameshift-mutated antigens in microsatellite instable cancer. Int J Cancer. 2008;123:838–845. doi: 10.1002/ijc.23570. [DOI] [PubMed] [Google Scholar]
- 6.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373:673–683. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 9.Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, et al. Jean-Nicolas Vauthey of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group: The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–1239. doi: 10.1634/theoncologist.2012-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101:1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alarmo EL, Rauta J, Kauraniemi P, Karhu R, Kuukasjärvi T, Kallioniemi A. Bone morphogenetic protein 7 is widely overexpressed in primary breast cancer. Genes Chromosomes Cancer. 2006;45:411–419. doi: 10.1002/gcc.20307. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki H, Watabe T, Kitamura T, Miyazono K. BMP signals inhibit proliferation and in vivo tumor growth of androgen-insensitive prostate carcinoma cells. Oncogene. 2004;23:9326–9335. doi: 10.1038/sj.onc.1208127. [DOI] [PubMed] [Google Scholar]
- 13.Beck SE, Jung BH, Del Rosario E, Gomez J, Carethers JM. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal. 2007;19:1465–1472. doi: 10.1016/j.cellsig.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki M, Ishigami S, Uenosono Y, Arigami T, Uchikado Y, Kita Y, Kurahara H, Matsumoto M, Ueno S, Natsugoe S. Expression of BMP-7 in human gastric cancer and its clinical significance. Br J Cancer. 2011;104:714–718. doi: 10.1038/sj.bjc.6606075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming ID, Cooper J, Henson DE, Hutter VPR, Kennedy BJ, Murphy GP, O'Sullivan B, Sobin LH, Yarbro JW, et al., editors. AJCC cancer staging manual. 5th. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- 16.Rodriguez-Martinez A, Alarmo EL, Saarinen L, Ketolainen J, Nousiainen K, Hautaniemi S, Kallioniemi A. Analysis of BMP4 and BMP7 signaling in breast cancer cells unveils time-dependent transcription patterns and highlights a common synexpression group of genes. BMC Med Genomics. 2011;4:80. doi: 10.1186/1755-8794-4-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huh JW, Park YA, Jung EJ, Lee KY, Kwon JE, Sohn SK. Complete remission of unresectable colon cancer after preoperative chemotherapy selected by adenosine triphosphate-based chemotherapy response assay. J Korean Med Sci. 2008;23:916–919. doi: 10.3346/jkms.2008.23.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorbye SW, Kilvaer T, Valkov A, Donnem T, Smeland E, Al-Shibli K, Bremnes RM, Busund LT. Prognostic Impact of Lymphocytes in Soft Tissue Sarcomas. PLoS One. 2011;6:e14611. doi: 10.1371/journal.pone.0014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motoyama K, Tanaka F, Kosaka Y, Mimori K, Uetake H, Inoue H, Sugihara K, Mori M. Clinical significance of BMP7 in human colorectal cancer. Ann Surg Oncol. 2008;15:1530–1537. doi: 10.1245/s10434-007-9746-4. [DOI] [PubMed] [Google Scholar]
- 20.Megumi K, Ishigami S, Uchikado Y, Kita Y, Okumura H, Matsumoto M, Uenosono Y, Arigami T, Kijima Y, Kitazono M, et al. Clinicopathological significance of BMP7 expression in esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19:2066–2071. doi: 10.1245/s10434-011-2024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]