Abstract
The peritumoral stroma and cancer-associated fibroblasts (CAFs) have been suggested to play an important role in breast tumorigenesis. The specific immunohistochemical characteristics of the stromal component according to the breast carcinoma subtype surrogates of molecular classes is poorly understood. In the present study, immunohistochemical staining was used to evaluate the expression of matrix metalloproteinase 2 (MMP2), which is one of the most important proteins considered to facilitate tumor invasion, in a series of invasive breast carcinomas according to subtype: Luminal A, luminal B, luminal-HER2, HER2-enriched and triple-negative. A significant increase in MMP2 expression was demonstrated in tumors known to exhibit a more aggressive metastatic behavior, such as luminal HER2 (37%), HER2-enriched (30%) and triple-negative tumors (17%), compared with the luminal A (6%) or luminal B (13%) subtypes. Our data indicated that the CAFs associated with different breast subtypes exhibit different specific properties to facilitate tumor invasion.
Keywords: breast carcinoma, cancer-associated fibroblasts, myofibroblasts, peritumoral stroma, matrix metalloproteinase-2, luminal, human epidermal growth factor receptor-2
Introduction
Over several years, the majority of studies on breast carcinoma have focused only on the epithelial component; however, the tumor-associated stroma and particularly the cancer-associated fibroblasts (CAFs) have been found to play a crucial role in cancer pathogenesis (1,2).
We previously demonstrated that the majority of these CAFs were smooth muscle actin (SMA)-positive, with a myofibroblastic-like phenotype, and that the presence of these peritumoral myofibroblasts (PMYs) is crucial for in situ and invasive breast carcinoma of no special type (NST), as well in metastatic disease (3,4). The origin of PMYs remains debatable, but we previously demonstrated that the resident CD34-positive breast fibroblasts are able to acquire SMA myofibroblastic characteristics under the control of the transforming growth factor β-1 pathway (3). It was suggested that these PMYs promote tumor invasion, growth and angiogenesis through paracrine factors and/or direct cell-cell crosstalk (5). Therefore, it was suggested that CAFs/PMYs potentially secrete various proteins, particularly matrix metalloproteinase 2 (MMP2), to facilitate tumor invasion (6,7). To elucidate this issue, we immunohistochemically analyzed the expression of MMP2 in normal breast stromal fibroblasts and in CAFs/PMYs present in invasive breast carcinoma of NST, according to clinicopathological variables. Our data were reviewed according to the highlights of the recent literature.
Materials and methods
Specimens
Formalin-fixed, paraffin-embedded postsurgical specimens from human breast carcinomas were retrieved from the archives of the Department of Pathology, Erasme University Hospital-Université Libre de Bruxelles (Brussels, Belgium). A total of 155 patients with invasive breast carcinoma of NST, who underwent surgery between 1997 and 2004, were randomly selected. A total of 20 specimens of normal breast tissue obtained from women who underwent resection for plastic surgery were also included in the study as controls. This study was approved by the Ethics Committee of Erasme University Hospital (reference no. P2014/418). The pathological stage and grade were defined according to the 2014 criteria of the World Heath Organization (8). A clinically positive test for estrogen and progesterone nuclear receptors (ER and PR, respectively) was defined as nuclear staining in ≥1% of the tumor cells, as previously described (9). Human epidermal growth factor receptor 2 (HER2) immunoreactivity was performed using the Oracle HER2 test (clone CB11; Leica Microsystems GmbH, Wetzlar, Germany) according to the manufacturer's instructions, and scoring was performed according to the recommendations of the American Society of Clinical Oncology (10). All HER2 scores of 2+ and 3+ were analyzed using the fluorescent in situ hybridization (FISH) PathVysion HER2 DNA test (Abbott Laboratories, Abbott Park, IL, USA) according to the manufacturer's instructions. Signal ratios (HER2/chromosome 17 centromere) of ≥2 were classified as amplified. In the present study, only 2+ and 3+ tumors with HER2 FISH amplification were considered as positive. A subtype immunohistochemical classification, as previously described with certain modifications (11), was adopted to characterize the tumors as follows: Luminal A (either one or both ER and PR present, HER2-negative and Ki-67 ≤14%); luminal B (one or both ER and PR present, HER2-negative and Ki-67 >14%); luminal-HER2 (one or both ER and PR present, HER2-positive, irrespective of the Ki-67 index); HER2-positive (ER and PR absent, HER2-positive, irrespective of the Ki-67 index); and triple-negative (ER and PR absent and HER2-negative).
Immunostaining for MMP2 (clone 17B11, dilution 1:30; Leica Microsystems GmbH) was performed using a fully automated immunohistochemical system (Autostainer Link A48; Dako, Glostrup, Denmark).
A two-grade system was used to score the stromal expression of MMP2, which was classified as positive or negative according to a cut-off of 10%.
Statistical analysis
The Chi-square or Fisher's exact tests were used to statistically compare the clinicopathological variables described in the Table I. P<0.05 was considered to indicate a statistically significant difference.
Table I.
Clinicopathological chracateristics of the 155 patients included in the study.
| Characteristics | Total cases, no. (%) (n=155) |
|---|---|
| Age (years) | |
| <50 | 46 (30) |
| ≥50 | 109 (70) |
| Tumor size (mm) | |
| ≤20 | 90 (58) |
| >20 | 65 (42) |
| Lymph node status | |
| Negative | 87 (56) |
| Positive | 68 (44) |
| Histological grade | |
| 1 | 27 (17) |
| 2 | 63 (41) |
| 3 | 65 (42) |
| ER status | |
| Negative | 21 (14) |
| Positive | 134 (86) |
| PR status | |
| Negative | 37 (24) |
| Positive | 118 (76) |
| HER2 status | |
| Negative | 126 (81) |
| Positive | 29 (19) |
| Ki-67 | |
| <15% | 35 (23) |
| ≥15% | 120 (77) |
| Molecular subtypes | |
| Luminal A | 34 (22) |
| Luminal B | 86 (55) |
| Luminal-HER2 | 19 (12) |
| HER2-enriched | 10 (7) |
| Triple-negative | 6 (4) |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor.
Results
Stromal MMP2 expression
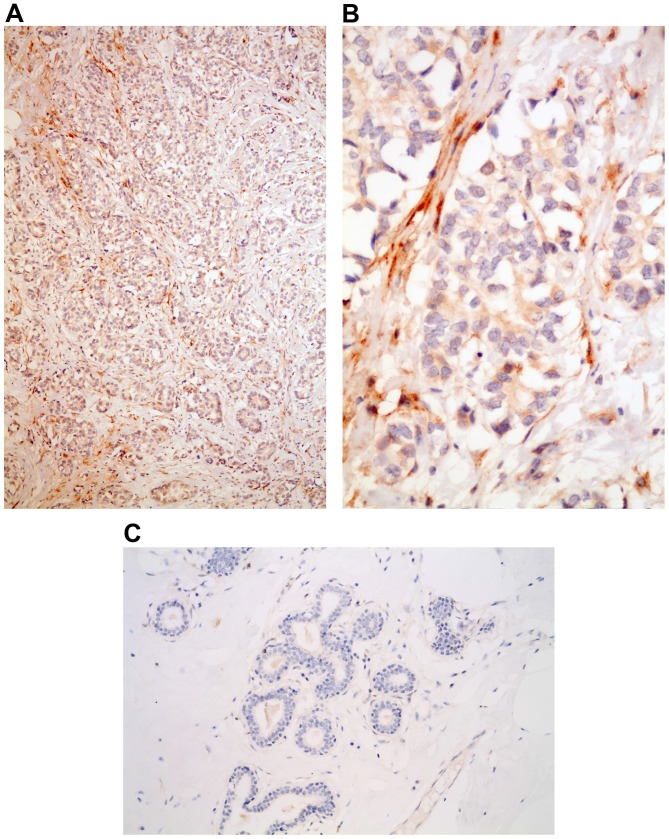
No MMP2 expression was observed in the stroma surrounding normal breast acini or ductal units (Fig. 1). By contrast, MMP2 expression was present in the peritumoral stroma in 24 of the 155 cases (15%) of invasive breast carcinoma (Fig. 1). According to the different clinical parameters, there was no correlation between MMP2 stromal expression and patient age, histological grade, lymph node involvement, ER/PR positivity, or Ki-67 index. Conversely, MMP2 stromal expression was statistically significantly different in tumors sized ≤20 mm (21% positivity) compared with tumors >20 mm (8% positivity) (P=0.02). In addition, stromal expression in HER2-positive carcinomas was more frequent compared with that in in HER2-negative tumors (35 vs. 12%, respectively; P=0.002). Finally, according to the subtype immunohistochemical classification surrogates of molecular classes, MMP2 stromal expression appeared more frequently by decreasing order in luminal-HER2 (37%), HER2-enriched (30%), triple-negative (17%), luminal B (13%) and luminal A (6%) tumors (P=0.025) (Table II).
Figure 1.
MMP2 expression in the stroma of an HER2-positive tumor; magnification, (A) ×40 and (B) ×400. (C) Absence of stromal MMP2 positivity in the stroma of normal breast tissue; magnification, ×200. MMP2, matrix metalloproteinase 2; HER2, human epidermal growth factor receptor 2.
Table II.
MMP2 stromal expression according to the different clinicopathological characteristics.
| MMP2 expression, no. | |||
|---|---|---|---|
| Characteristics | Positive | Negative | P-value |
| Age (years) | 0.95 | ||
| ≤50 | 7 | 39 | |
| >50 | 17 | 92 | |
| Tumor size (mm) | 0.02 | ||
| ≤20 | 19 | 71 | |
| >20 | 5 | 60 | |
| Lymph node status | 0.11 | ||
| Negative | 17 | 70 | |
| Positive | 7 | 61 | |
| Histological grade | 0.17 | ||
| 1 | 2 | 25 | |
| 2 | 8 | 55 | |
| 3 | 14 | 51 | |
| ER status | 0.075 | ||
| Negative | 6 | 15 | |
| Positive | 18 | 116 | |
| PR status | 0.09 | ||
| Negative | 9 | 28 | |
| Positive | 15 | 103 | |
| HER2 status | 0.002 | ||
| Negative | 14 | 112 | |
| Positive | 10 | 19 | |
| Ki-67 | 0.45 | ||
| <15% | 4 | 31 | |
| ≥15% | 20 | 100 | |
| Molecular subtypes | 0.025 | ||
| Luminal A | 2 | 32 | |
| Luminal B | 11 | 75 | |
| Luminal-HER2 | 7 | 12 | |
| HER2-enriched | 3 | 7 | |
| Triple-negative | 1 | 5 | |
MMP2, matrix metalloproteinase 2; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor.
Discussion
Several recent studies support the hypothesis that, in invasive breast carcinoma, the gene expression profile of the epithelial component and, therefore, the immunohistochemical profile surrogates of molecular classes, represent biologically distinct diseases with different response to therapy and clinical outcome (12,13). In addition to the epithelial cell autonomous processes, it has been hypothesized that the tumor microenvironment, and particularly CAFs, are able to promote tumor cell proliferation, angiogenesis and metastasis (3,4,14). Certain breast CAFs, which are characterized by SMA expression and are referred to as PMYs, appear to play an important role in metastasis, including lymph node metastasis (3,4). Therefore, similar to the epithelial counterpart, it was hypothesized that the tumor's aggressiveness may be affected by the stromal composition, as well as the stroma's own biological properties (‘stromal signature’) (15,16). In the present study, we demonstrated that the stromal expression of MMP2, which is known to promote cancer invasion and metastasis by degrading various components of the extracellular matrix, varies according to the different tumor subtypes. In particular, in HER2-positive (luminal-HER2 and HER2-enriched) and triple-negative tumors, stromal expression of MMP2 was more frequently detected compared with the luminal subtypes (Table II). Of note, it was recently indicated that, on multivariate analysis, luminal-HER2, HER2-enriched and triple-negative tumors are associated with a higher rate of distant metastasis, including brain, liver and lung metastases (13,17). Therefore, the metastatic potential may be determined by the intrinsic properties of the epithelial component of the different breast tumor subtypes, as well as by the stromal properties of the microenvironment, as in the present case, by expressing different MMP2 levels, which have been implicated in the degradation of extracellular matrix and the enhancement of tumor cell motility (18,19,7,20). In conclusion, different stromal properties, such as MMP2 expression, may predispose the different histological breast tumor subtypes to different metastatic outcomes. Further studies are in progress, with the aim to accurately characterize stromal properties based on breast cancer subtype classification.
References
- 1.Luo H, Tu G, Liu Z, Liu M. Cancer-associated fibroblasts: A multifaceted driver of breast cancer progression. Cancer Lett. 2015;361:155–163. doi: 10.1016/j.canlet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Han Y, Zhang Y, Jia T, Sun Y. Molecular mechanism underlying the tumor-promoting functions of carcinoma-associated fibroblasts. Tumour Biol. 2015;36:1385–1394. doi: 10.1007/s13277-015-3230-8. [DOI] [PubMed] [Google Scholar]
- 3.Catteau X, Simon P, Noël JC. Myofibroblastic stromal reaction and lymph node status in invasive breast carcinoma: Possible role of the TGF-β1/TGF-βR1 pathway. BMC Cancer. 2014;14:499. doi: 10.1186/1471-2407-14-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catteau X, Simon P, Noël JC. Myofibroblastic reaction is a common event in metastatic disease of breast carcinoma: A descriptive study. Diagn Pathol. 2014;9:196. doi: 10.1186/s13000-014-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohlhapp FJ, Mitra AK, Lengyel E, Peter ME. MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment. Oncogene April. 2015;13 doi: 10.1038/onc.2015.89. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassona Y, Cirillo N, Heesom K, Parkinson EK, Prime SS. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br J Cancer. 2014;111:1230–1237. doi: 10.1038/bjc.2014.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med Sci Monit. 2009;15:RA32–RA40. [PubMed] [Google Scholar]
- 8.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors. WHO Classification of Tumours. 4th. Vol. 4. Lyon: IARC Press; 2012. Invasive breast carcinoma: Introduction and general features; pp. 19–22. [Google Scholar]
- 9.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 11.Preat F, Simon P, Noel JC. Differences in breast carcinoma immunohistochemical subtypes between immigrant Arab and European women. Diagn Pathol. 2014;9:26. doi: 10.1186/1746-1596-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sihto H, Lundin J, Lundin M, Lehtimäki T, Ristimäki A, Holli K, Sailas L, Kataja V, Turpeenniemi-Hujanen T, Isola J, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: A nationwide cohort study. Breast Cancer Res. 2011;13:R87. doi: 10.1186/bcr2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 14.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 15.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 16.Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, André S, Piccart M, Campone M, Brain E, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 17.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Duffy MJ, Maguire TM, Hill A, McDermott E, O'Higgins N. Metalloproteinases: Role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones JL, Glynn P, Walker RA. Expression of MMP-2 and MMP-9, their inhibitors and the activator MT1-MMP in primary breast carcinomas. J Pathol. 1999;189:161–168. doi: 10.1002/(SICI)1096-9896(199910)189:2<161::AID-PATH406>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Thompson EW, Yu M, Bueno J, Jin L, Maiti SN, Palao-Marco FL, Pulyaeva H, Tamborlane JW, Tirgari R, Wapnir I. Collagen induced MMP-2 activation in human breast cancer. Breast Cancer Res Treat. 1994;31:357–370. doi: 10.1007/BF00666168. [DOI] [PubMed] [Google Scholar]