Abstract
The growth and metastasis of tumors is dependent on angiogenesis; however, the association between tumor stem cells (TSCs) and tumor angiogenesis remains to be elucidated. The present study aimed to investigate the expression of the TSC markers aldehyde dehydrogenase 1 (ALDH1) and cluster of differentiation 133 (CD133) in invasive ductal breast carcinoma, and identify their correlation with tumor angiogenesis. Stem-like cells from the breast tissue of 120 patients, who were diagnosed with invasive ductal breast carcinoma at The First Affiliated Hospital of Zhengzhou University (Zhengzhou, Henan, China) between January 2009 and December 2010, were collected by surgical resection and analyzed using immunohistochemical double staining. The expression of the vascular markers CD34, CD105 and vascular endothelial growth factor (VEGF) were determined using single staining. Overall, 25.83% (31/120) of the specimens contained a large number of ALDH1+/CD133+ stem-like cells (ALDH1+/CD133+ tumor). ALDH1+/CD133+ expression is associated with microvessel density, VEGF-positive rate and estrogen receptor expression (P<0.05); however, ALDH1+/CD133+ expression was not associated with age, tumor diameter, lymph node metastasis, histological classification, progesterone receptor expression or human epidermal growth factor receptor 2 expression (P>0.05). The ALDH1+/CD133+ tumor phenotype and expression of VEGF were identified to be correlated in the present study (P=0.020). The present study revealed a close association between breast cancer TSC markers, including ALDH1 and CD133, and tumor angiogenesis. The results of the present study may provide a novel target and treatment strategy for future studies investigating tumor growth and metastasis.
Keywords: tumor stem cells, invasive ductal breast carcinoma, aldehyde dehydrogenase 1+/cluster of differentiation 133+ stem-like cells, tumor angiogenesis
Introduction
Breast cancer is one of the most commonly occurring malignant tumors in women. Approximately 1.38 million new breast cancer cases were estimated to be diagnosed in 2008 (23% of all cancers) worldwide, making it the second most common type of cancer (1). In China, rates of breast cancer in 2005 were 33 cases per 100,000 individuals in urban areas and 12.23 cases per 100,000 individuals in rural areas, with an increased prevalence in younger patients in rural areas (2). The incidence of ductal carcinoma was reportedly constant between 1987 and 1999 (3); however, another study reported that the occurrence of in situ ductal carcinoma of the breast had increased dramatically since 1983 4). Due to the increase in the prevalence of breast cancer, research has focused on the expression of various genes and proteins that are specific to invasive ductal breast carcinoma. Lee et al (5) identified four unique gene expression profiles that were able to regulate tumor progression. Similarly, Wojnar et al (6) reported that the expression of metallothionein may have a significant role in the proliferation of tumors.
Breast cancer is considered to be a disease that is associated with tumor stem cells (TSCs). Due to the potential for infinite proliferation and tumor development in vitro and in vivo, TCSs are considered to be the origin of tumor metastasis and recurrence. These cells may be useful markers for tumor-free survival in patients with cancer (7,8). TSCs are a small group of cells present in the tumor tissue that possess the properties of stem cells. They have the ability to self-replicate and proliferate, and are responsible for the formation, recurrence, metastasis and drug resistance of tumors (9). The growth and metastasis of a tumor is dependent on angiogenesis, however, the association between TSCs and tumor angiogenesis remains to be elucidated. A previous study reported that a cell subpopulation in breast cancer tissues that demonstrated aldehyde dehydrogenase (ALDH) activity may possess the properties of stem cells (10). Ginestier et al (10) identified ALDH1 as a marker of progenitor cells of normal breast and breast cancer tissues (10). By contrast, CD133, a TSC marker for various types of cancer, was demonstrated to promote angiogenesis in CD133+ glioblastoma stem cells (11). CD133 is a TSC marker that has a significant role in breast, liver and colon cancer (12–14), and is additionally a marker for the separation and identification of TSCs (15). Wright et al (14) identified the breast cancer stem cell properties of CD133.
The present study aimed to investigate the association between TSC-like cells in breast cancer and tumor angiogenesis. The results of the present study revealed that TSCs may be associated with tumor angiogenesis.
Materials and methods
Patients
Stem-like cells from the breast tissues of 120 patients with invasive ductal breast carcinoma were collected by surgical resection. Patient disease was diagnosed and confirmed histologically at The First Affiliated Hospital of Zhengzhou University (Zhengzhou, Henan, China) between January 2009 and December 2010. No patients received radiotherapy or chemotherapy prior to surgery. The protocol of the present study was approved by the Zhengzhou University Ethics Committee (Zhengzhou, Henan, China). Written informed consent was obtained from all patients prior to their enrollment in the current study.
Diagnosis criteria and parameters assessed
The histological classification of invasive breast cancer was based on the Nottingham modified Bloom and Richardson system (16). Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (Her-2) expression was determined prior to commencement of the present study. Antibodies and Streptavidin-Peroxidase (SP) Immunohistochemistry kits were purchased from Fuzhou Maixin Biotech Co., Ltd. (Fuzhou, Fujian, China), Beijing Zhongshanjinqiao Biological Technology Co., Ltd. (Beijing, China) and Epitomics (Burlingame, CA, USA), and tests were performed according to the manufacturer's protocol. The results of the histological diagnosis, classification and immunohistochemistry of all patients were examined by two independent, experienced pathologists, who were blinded to the clinical data.
Immunohistochemistry
The immunohistochemical detection of ALDH1 and CD133 utilized the double staining SP method (17). The Double Staining kit was obtained from Fuzhou Maixin Biotech Co., Ltd. Briefly, tissue samples were paraffin-embedded (Leica Biosystems, Beijing, China), cut into 4-µm thick slices, and gradually dewaxed and hydrated; the tissue sections were washed with xylene (twice for 10 min; Leica Biosystems), 100% ethanol (twice for 5 min; Leica Biosystems), 95% ethanol (2 min), 80% ethanol (2 min), 70% ethanol (2 min), distilled water (5 min) and phosphate-buffered saline (PBS; 3 times for 3 min; Fuzhou Maixin Biotech Co., Ltd). A high-pressure method was used for antigen retrieval in ethylenediaminetetraacetic acid solution (Leica Biosystems) at pH 8.0 for 15 min (18). The specimens were incubated with rabbit anti-ALDH1 monoclonal antibody (catalog no., ab52492; dilution, 1:150; Abcam, Cambridge, MA, USA) overnight at 4°C, and were additionally processed using the 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium color system (Fuzhou Maixin Biotech Co., Ltd.)to produce dark blue positive signals, according to the manufacturer's instructions. The specimens were washed for 5 min in PBS 3 times, incubated with double staining enhancement solution (Fuzhou Maixin Biotech Co., Ltd.) for 15 min and rabbit anti-CD133 monoclonal antibody (catalog no., ZA-0426; ready to use; Zhongshanjinqiao Biological Technology Co., Ltd.) at 37°C for 1 h, processed routinely and 3,3′-diaminobenzidine (DAB; Fuzhou Maixin Biotech Co., Ltd.) staining was applied to produce red positive signals.
The detection of CD34, CD105 and vascular endothelial growth factor (VEGF) was achieved using the single staining SP method, high-pressure antigen retrieval and DAB staining to produce brown positive signals. Mouse anti-CD105 monoclonal antibody (catalog no., ZM-0297; dilution, 1:100), murine anti-CD34 monoclonal antibody (catalog no., ZM-0046; dilution; 1:100) and rabbit anti-VEGF monoclonal antibody (catalog no., ZA-0580; ready to use) were purchased from Beijing Zhongshanjinqiao Biological Technology Co., Ltd.
Evaluation of immunohistochemical results
ALDH1-positive cells exhibited cytoplasm staining with a blue-black color, and CD133-positive cells possessed a red cell membrane and partially red cytoplasm. The cells were divided into the following four phenotypes at the cellular level, according to the staining of ALDH1 and CD133: i) ALDH1+/CD133+ phenotype with blue cytoplasm and red membrane; ii) ALDH1+/CD133- phenotype with blue cytoplasm and non-stained membrane; iii) ALDH1-/CD133+ phenotype with non-stained cytoplasm and red membrane; and iv) ALDH1-/CD133- phenotype with non-stained cytoplasm and membrane.
Each specimen was classified for ALDH1+/CD133+ dominance at the tissue level by semiquantitative scoring, as described by Currie et al (19). Briefly, tumor cells were scored semiquantitatively according to the percentage of ALDH1+/CD133+ cells (0, 0%; 1, 1%; 2, 1–10%; 3, 10–33%; 4, 33–66%; 5, 66–100%) and intensity of staining of ALDH1+/CD133+ cells (0, negative; 1, weak; 2, moderate; and 3, strong). The composite scores (score range, 0–8) of the specimens were subsequently calculated as follows: composite score = percentage + intensity. Tumors with ALDH and CD133 composite scores of ≥6 were defined as ALDH1+/CD133+ tumors.
Microvessels were identified by monoclonal antibody staining, which revealed the localization of CD34 in the membrane and cytoplasm of endothelial cells and CD105 in the membrane only of endothelial cells. A single endothelial cell or cell cluster stained brown was considered to be a blood vessel, and blood vessels with a thick muscle layer or with a diameter >8 red blood cells were excluded from the vessel count. A total of five areas with high vascular density were localized on each slide following visualization under a low-power lens (magnification, ×40), and the blood vessels in each area were subsequently counted under a high-power lens (magnification, ×400) (Olympus CX31; Olympus Corporation China, Beijing, China). The microvessel density (MVD) was defined as the mean of the vessel count totals for the five areas of high vascular density.
Cytoplasm-localized VEGF staining, indicated by brown positive signals, was analyzed using the semiquantitative Shimizu scoring system (20). Percentage scores of 0, 1, 2 and 3 indicated the portion of positive cells stained of 0, <1/3, 1/3–2/3 and >2/3, respectively. Intensity scores of 0, 1, 2 and 3 indicated the conditions of no staining, yellow staining, light brown staining and dark brown staining, respectively. Composite scores (score range, 0–6) were used to evaluate VEGF staining as follows: 0–2, negative (−); 3, weak positive (+); 4–5; medium positive (++) and 6, strong positive (+++). A composite score >3 was considered to indicate VEGF expression.
Statistical analysis
SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA) was utilized for data processing and statistical analysis. Count data were presented as a percentage or proportion indicating the association between the ALDH1+/CD133+ tumor phenotype and other clinicopathological parameters, and this was analyzed using the χ2 test. Continuous variables were expressed as the mean ± standard deviation, and statistical analysis was performed using the independent samples t-test. Kendall rank correlation analysis was used for analysis of the co-expression of VEGF in ALDH1+/CD133+ phenotype tumor cells. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
All patients were women, with a mean age of 48.68±10.94 years (range, 24–72 years). The patients belonged to the following categories, according to the Nottingham modified Bloom and Richardson system (16): Grade I (n=21; 17.5%); grade II (n=78; 65%); and grade III (n=21; 17.5%). A total of 60 patients (50%) were found to have lymph node metastasis. In addition, 74 patients (61.7%) were ER+ and 46 (38.3%) were ER-, 43 patients (35.8%) were PR+ and 77 (64.2%) were PR-, and 22 patients (18.3%) were Her-2 (0), 36 (30%) were Her-2 1+, 28 (23.3%) were Her-2 2+ and 34 (28.3%) were Her-2 3+ (Table I).
Table I.
Correlation between ALDH1+/CD133+ stem-like cells in invasive ductal breast carcinoma and clinicopathological features.
| Clinicopathological features | n | Positive, n (%) | Negative, n (%) | P-value |
|---|---|---|---|---|
| Age, years | 0.258 | |||
| ≤45 | 53 | 11 (20.8) | 42 (79.2) | |
| >45 | 67 | 20 (29.9) | 47 (70.1) | |
| Diameter, cm | 0.962 | |||
| ≤2 | 43 | 11 (25.6) | 32 (74.4) | |
| >2 | 77 | 20 (26.0) | 57 (74.0) | |
| Estrogen receptor | 0.028 | |||
| Positive | 74 | 14 (18.9) | 60 (81.1) | |
| Negative | 46 | 17 (37.0) | 29 (63.0) | |
| Progesterone receptor | 0.074 | |||
| Positive | 43 | 7 (16.3) | 36 (83.7) | |
| Negative | 77 | 24 (31.2) | 53 (68.8) | |
| Human epidermal growth factor receptor-2 | 0.632 | |||
| 0 | 22 | 6 (27.3) | 16 (72.7) | |
| 1+ | 36 | 9 (25.0) | 27 (75.0) | |
| 2+ | 28 | 5 (17.9) | 23 (82.1) | |
| 3+ | 34 | 11 (32.4) | 23 (67.6) | |
| Lymphatic metastasis | 0.835 | |||
| Positive | 60 | 16 (26.7) | 44 (73.3) | |
| Negative | 60 | 15 (25.0) | 45 (75.0) | |
| Gradea | 0.153 | |||
| I | 21 | 2 (9.5) | 19 (90.5) | |
| II | 78 | 22 (28.2) | 56 (71.8) | |
| III | 21 | 7 (33.3) | 14 (66.7) |
Histological grading of invasive breast cancer was performed according to the Nottingham modified Bloom and Richardson system. ALDH, aldehyde dehydrogense; CD, cluster of differentiation.
No significant associations were observed between ALDH1+/CD133+ tumor cells and clinicopathological features
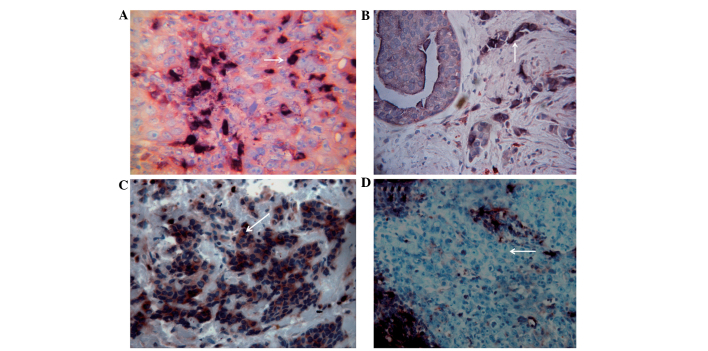
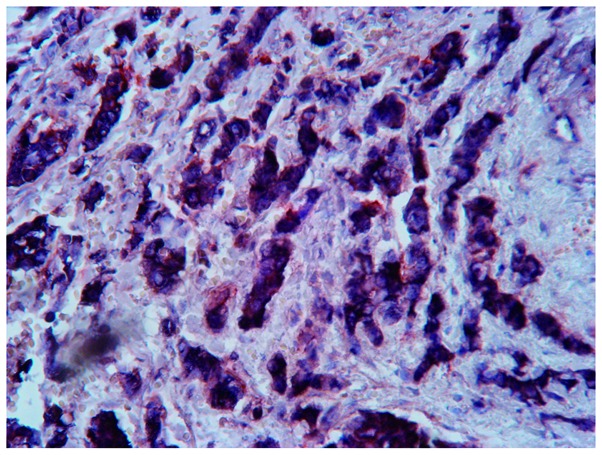
The 120 invasive ductal breast carcinoma tissue specimens demonstrated differential distribution patterns of the cells in the ALDH1+/CD133+, ALDH1+/CD133-, ALDH1-/CD133+ and ALDH1-/CD133- phenotypes. The ALDH1+/CD133+ phenotype was distributed in the center and towards the edge of the tumor nest and infiltrating strands, and appeared as randomly scattered cells or small clusters (Fig. 1A). The ALDH1+/CD133- phenotype demonstrated a scattered distribution (Fig. 1B), and the ALDH1-/CD133+ and ALDH1-/CD133- phenotypes had a sheet-like or nested distribution (Fig. 1C and D). ALDH1+/CD133+ tumor cells were identified in 31 specimens (Fig. 2). A total of 43 patients (35.8%) possessed ALDH1+/CD133+ stem-like cells with a diameter of ≤2 cm, and 77 patients (64.2%) had cells of >2 cm diameter (Table I).
Figure 1.
Various phenotypes of invasive breast cancer cells according to the expression of ALDH1 and CD133. Invasive breast cancer cells were stained by SP immunohistochemical double staining. ALDH1-positive cells exhibited dark blue stained cytoplasm, while CD133-positive cells exhibited a red stained cell membrane. (A) ALDH1+/CD133+ phenotype. Magnification, ×200. (B) ALDH1+/CD133- phenotype. Magnification, ×100. (C) ALDH1-/CD133+ phenotype. Magnification, ×200. (D) ALDH1-/CD133- phenotype. Magnification, ×100. White arrows indicate the corresponding phenotype cells. ALDH, aldehyde dehydrogenase; CD, cluster of differentiation; SP, streptavidin-peroxidase.
Figure 2.
ALDH1+/CD133+ tumor tissue (composite score of ALDH1 and CD133 >5) stained by SP immunohistochemical double staining. ALDH1-positive cells exhibited dark blue stained cytoplasm, while CD133-positive cells had a red stained cell membrane. Magnification, ×400. ALDH, aldehyde dehydrogenase; CD, cluster of differentiation; SP, streptavidin-peroxidase; VEGF, vascular endothelial growth factor.
A correlation was identified between ALDH1+/CD133 tumor cells and expression of VEGF and MVD
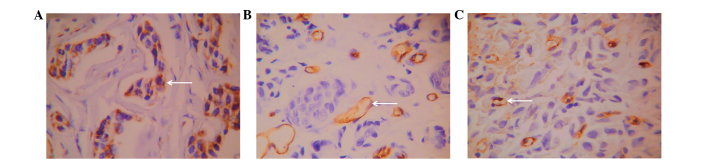
VEGF-positive cells demonstrated a sheet-like or nested distribution (Fig. 3A) and were observed in 68.3% (82/120) of patients. CD34 was expressed in microvessels and large vessels in normal and tumor tissues and demonstrated clear boundaries with other tissues (Fig. 3B). CD105 exhibited relatively high expression in single or small clusters of endothelial cells in microvessels, low expression in large vessels and no expression in normal tissues (Fig. 3C). The novel vascular endothelial cells exhibited an irregular arrangement, thin vessel walls, an unclear lumen and clear boundaries with other tissues. The mean microvessel density of the 120 patients with invasive ductal breast carcinoma marked by CD34 was 26.93±9.02, while that marked by CD105 was 17.63±8.59. Positive correlations were noted between ALDH1+/CD133+ tumor cells and VEGF expression, CD34-marked microvessel density and CD105-marked microvessel density (P<0.05; Table II).
Figure 3.
Expression of vessel-associated proteins. Expression of (A) VEGF, (B) CD34 and (C) CD105 in invasive breast cancer cells stained by SP immunohistochemistry. Magnification, ×200. Positive signals are yellow. White arrows indicate the corresponding positive expression. CD, cluster of differentiation; VEGF, vascular endothelial growth factor.
Table II.
Correlation between VEGF expression and vessel-associated factors in ALDH1+/CD133+ stem-like cells in invasive ductal breast carcinoma.
| Vessel-associated factors | Positive (n=31) | Negative (n=89) | P-value |
|---|---|---|---|
| Positive percentage of VEGF, n (%) | 26 (83.9) | 56 (62.9)c | <0.05 (χ2 test) |
| MVD (CD34)a, mean ± SD | 28.78±10.55 | 25.90±8.34 | <0.05 (t test) |
| MVD (CD105)b, mean ± SD | 21.29±9.06 | 16.35±8.10 | <0.01 (t test) |
MVD was assessed by IHC using monoclonal antibody against CD34.
MVD was assessed by IHC using monoclonal antibody against CD105.
The VEGF-positive rate was 68.33% (82/120) among the 120 patients, thus 56 VEGF-positive patients did not possess ALDH1+/CD133+ stem-like cells in invasive ductal breast carcinoma. VEGF, vascular endothelial growth factor; MVD, microvessel density; ALDH, aldehyde dehydrogenase, CD, cluster of differentiation; IHC, immunohistochemistry; SD, standard deviation.
VEGF and ALDH1+/CD133+ cancer cell phenotypes are co-expressed
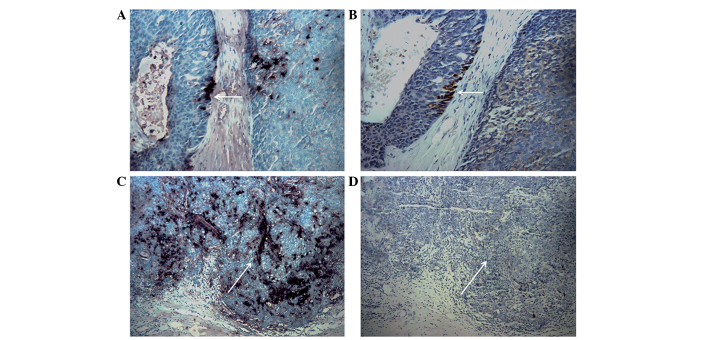
By comparing two consecutive sections of the same tissue, 16 specimens of invasive breast cancer with an invasive ALDH1+/CD133+ phenotype were revealed to exhibit VEGF expression, accounting for 51.6% (16/31) of the tumors with ALDH1+/CD133+ cells (Fig. 4). Statistical analysis suggested a correlation between the ALDH1+/CD133+ tumor phenotype and co-expression of VEGF (P=0.020; Table III).
Figure 4.
Co-expression of VEGF in ALDH1+/CD133+ tumor tissues. (A) Section stained by SP immunohistochemical double staining. ALDH1-positive cells exhibited dark blue stained cytoplasm, while CD133-positive cells had a red stained cell membrane. The white arrow indicates a double-positive area. (B) Section stained by SP immunohistochemistry. The white arrow indicates the yellow positive signals of VEGF expression. (C) Section stained by SP immunohistochemical double staining. ALDH1-positive cells exhibited dark blue stained cytoplasm, while CD133-positive cells had a red stained cell membrane. The white arrow indicates a double-positive area. (D) Section stained by SP immunohistochemistry. The white arrow indicates negative signals of VEGF expression. Magnification, ×40. A and B, and C and D show two consecutive slices of the same breast tissue. ALDH, aldehyde dehydrogenase; CD, cluster of differentiation; SP, streptavidin-peroxidase; VEGF, vascular endothelial growth factor.
Table III.
Expression of vascular endothelial growth factor in ALDH1+/CD133+ phenotype tumor cells.
| ALDH1+/CD133+ tumor | Positive (n=82), n (%) | Negative (n=38), n (%) | P-value |
|---|---|---|---|
| Positive (n=31) | 16 (51.6) | 15 (48.4) | 0.020 |
| Negative (n=89) | 66 (74.2) | 23 (25.8) |
The P-value was obtained using the Kendall correlation analysis. ALDH, aldehyde dehydrogenase; CD, cluster of differentiation.
Discussion
The present study investigated the correlation between TSC-like cells in breast cancer and tumor angiogenesis, and the results revealed that TSCs may be associated with tumor angiogenesis. The growth of tumor cells requires an adequate supply of oxygen and nutrients (21), and rapid growth leads to the development of hypoxia in the interior of tumors (22). Tumor angiogenesis is one of the compensating mechanisms that provide increased oxygen and nutrients to tumors (23). TSCs have been reported to have a significant role in the development of various types of cancer (24,25). As a marker for breast cancer stem cells, cell sorting based on ALDH1 is easier and more efficient compared with cell sorting based on CD44+/CD24-/low, offering a novel target and strategic direction for future studies investigating breast cancer stem cells (10). The expression of CD133 has been observed to increase during hypoxia (26). CD133+ glioma stem-like cells (GSCs) exhibit enhanced secretion of VEGF under hypoxic conditions, and these cells secrete increased levels of VEGF compared with CD133- GSCs under hypoxic and normal conditions (27). Previous studies have suggested that hypoxia may assist with maintenance of the phenotype of TSCs and may promote self-renewal of TSCs; however, hypoxia may additionally inhibit TSC differentiation into mature tumor cells (28,29). Ping et al (30) demonstrated that CD133+ GSCs were located close to capillaries and induced production of VEGF via activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway. The inhibition of PI3K/Akt or extracellular signal-regulated kinase 1/2 signaling reduced hypoxia-induced CD133 expression, indicating the significance of the aforementioned signaling pathways in the stem cell response to hypoxia (29). A total of 11 ALDH+/CD133+ ovarian cancer stem cells were required to form tumors in mice, which is markedly reduced compared with the 1,000 ALDH+/CD133- ovarian cancer stem cells required to generate a tumor, which indicates the increased tumorigenic capacity of ALDH+/CD133+ ovarian cancer stem cells (31). The correlation between ALDH1-positive cells and angiogenesis remains to be elucidated.
In order to investigate the correlation between TSC-like cells in invasive ductal breast carcinoma and tumor angiogenesis, the expression of VEGF was evaluated in various phenotypes of stem-like cells. The ALDH1+/CD133+ tumor phenotype and co-expression of VEGF were found to be correlated in the present study. CD34 and CD105 were selected as markers of tumor vessels in the present study. CD34 is a pan-endothelial marker, and its antibody is able to bind to vascular endothelial cells; however, it lacks the specificity to recognize tumor vascular endothelial cells (32). CD105 is a marker for neovascular endothelial cells that is extensively expressed in proliferating endothelial cells and demonstrates no or reduced expression in normal vascular endothelial cells, making it useful for tumor diagnosis, treatment and prognosis predictions (33). A previous study reported that CD105 possessed increased specificity compared with CD34 as a marker for neovascular tumors (34). The results of the present study revealed that ALDH1+/CD133+ stem-like cells were positively correlated with CD34 MVD and CD105 MVD.
The small sample size was one of the key limitations of the present study, with regard to the high prevalence of invasive ductal breast carcinoma worldwide. Therefore, the results of the present study require validation via studies with a larger patient cohort.
In conclusion, the present study revealed a correlation between breast cancer stem cells and tumor angiogenesis. However, the mechanisms involved have yet to be fully elucidated. The present results may provide a novel target and strategy for future studies investigating tumor growth and metastasis.
Acknowledgements
The present study was funded by the National Natural Science Foundation of China (grant no., 81172179; Beijing, China).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in: 2008 GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Curado MP. Breast cancer in the world: Incidence and mortality. Salud Publica Mex. 2011;53:372–384. [PubMed] [Google Scholar]
- 3.Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 4.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275:913–918. doi: 10.1001/jama.275.12.913. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Stewart S, Nagtegaal I, Luo J, Wu Y, Colditz G, Medina D, Allred DC. Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer. Cancer Res. 2012;72:4574–4586. doi: 10.1158/0008-5472.CAN-12-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wojnar A, Pula B, Piotrowska A, Jethon A, Kujawa K, Kobierzycki C, Rys J, Podhorska-Okolow M, Dziegiel P. Correlation of intensity of MT-I/II expression with Ki-67 and MCM-2 proteins in invasive ductal breast carcinoma. Anticancer Res. 2011;31:3027–3033. [PubMed] [Google Scholar]
- 7.Nanashima A, Hatachi G, Tsuchiya T, Matsumoto H, Arai J, Abo T, Murakami G, Tominaga T, Takagi K, Nagayasu T. Clinical significances of cancer stem cells markers in patients with intrahepatic cholangiocarcinoma who underwent hepatectomy. Anticancer Res. 2013;33:2107–2114. [PubMed] [Google Scholar]
- 8.Madjd Z, Ramezani B, Molanae S, Asadi-Lari M. High expression of stem cell marker ALDH1 is associated with reduced BRCA1 in invasive breast carcinomas. Asian Pac J Cancer Prev. 2012;13:2973–2978. doi: 10.7314/APJCP.2012.13.6.2973. [DOI] [PubMed] [Google Scholar]
- 9.Gil J, Stembalska A, Pesz KA, Sasiadek MM. Cancer stem cells: The theory and perspectives in cancer therapy. J Appl Genet. 2008;49:193–199. doi: 10.1007/BF03195612. [DOI] [PubMed] [Google Scholar]
- 10.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 13.Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau CK, Li ML, Tam KH, Lam CT, Poon RT, Fan ST. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47:919–928. doi: 10.1002/hep.22082. [DOI] [PubMed] [Google Scholar]
- 14.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Wu PY. CD133 as a marker for cancer stem cells: Progresses and concerns. Stem Cells Dev. 2009;18:1127–1134. doi: 10.1089/scd.2008.0338. [DOI] [PubMed] [Google Scholar]
- 16.Frierson HF, Jr, Wolber RA, Berean KW, Franquemont DW, Gaffey MJ, Boyd JC, Wilbur DC. Interobserver reproducibility of the Nottingham modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinoma. Am J Clin Pathol. 1995;103:195–198. doi: 10.1093/ajcp/103.2.195. [DOI] [PubMed] [Google Scholar]
- 17.Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, Nakopoulou L. The clinicopathologic and prognostic significance of CD44+/CD24(−/low) and CD44-/CD24+ tumor cells in invasive breast carcinomas. Human Pathol. 2008;39:1096–1102. doi: 10.1016/j.humpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Jasani B, Rhodes A. The role and mechanism of high-temperature antigen retrieval in diagnostic pathology. Curr Diag Pathol. 2001;7:153–160. doi: 10.1054/cdip.2001.0076. [DOI] [Google Scholar]
- 19.Currie MJ, Beardsley BE, Harris GC, Gunningham SP, Dachs GU, Dijkstra B, Morrin HR, Wells JE, Robinson BA. Immunohistochemical analysis of cancer stem cell markers in invasive breast carcinoma and associated ductal carcinoma in situ: Relationships with markers of tumor hypoxia and microvascularity. Human Pathol. 2013;44:402–411. doi: 10.1016/j.humpath.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu M, Saitoh Y, Itoh H. Immunohistochemical staining of Ha-ras oncogene product in normal, benign, and malignant human pancreatic tissues. Hum Pathol. 1990;21:607–612. doi: 10.1016/S0046-8177(96)90006-4. [DOI] [PubMed] [Google Scholar]
- 21.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer cell. 2004;5:429–441. doi: 10.1016/S1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 22.Horbinski C, Mojesky C, Kyprianou N. Live free or die: Tales of homeless (cells) in cancer. Am J Pathol. 2010;177:1044–1052. doi: 10.2353/ajpath.2010.091270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy JP, Eibl G, Reber HA, Hines OJ. Influence of hypoxia and neoangiogenesis on the growth of pancreatic cancer. Mol Cancer. 2003;2:12. doi: 10.1186/1476-4598-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei B, Han XY, Qi CL, Zhang S, Zheng ZH, Huang Y, Chen TF, Wei HB. Coaction of spheroid-derived stem-like cells and endothelial progenitor cells promotes development of colon cancer. PloS One. 2012;7:e39069. doi: 10.1371/journal.pone.0039069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin TA, Jiang WG. Evaluation of the expression of stem cell markers in human breast cancer reveals a correlation with clinical progression and metastatic disease in ductal carcinoma. Oncol Rep. 2014;31:262–272. doi: 10.3892/or.2013.2813. [DOI] [PubMed] [Google Scholar]
- 26.Platet N, Liu SY, Atifi ME, Oliver L, Vallette FM, Berger F, Wion D. Influence of oxygen tension on CD133 phenotype in human glioma cell cultures. Cancer Lett. 2007;258:286–290. doi: 10.1016/j.canlet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 28.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 29.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 30.Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC, Jiang T, Lin MC, Chen JH, Wang B, Zhang R, et al. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J Pathol. 2011;224:344–354. doi: 10.1002/path.2908. [DOI] [PubMed] [Google Scholar]
- 31.Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds RK, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fanelli M, Locopo N, Gattuso D, Gasparini G. Assessment of tumor vascularization: Immunohistochemical and non-invasive methods. Int J Biol Markers. 1999;14:218–231. doi: 10.1177/172460089901400405. [DOI] [PubMed] [Google Scholar]
- 33.Pufe T, Harde V, Petersen W, Goldring MB, Tillmann B, Mentlein R. Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J Pathol. 2004;202:367–374. doi: 10.1002/path.1527. [DOI] [PubMed] [Google Scholar]
- 34.Takase Y, Kai K, Masuda M, Akashi M, Tokunaga O. Endoglin (CD105) expression and angiogenesis status in small cell lung cancer. Pathol Res Pract. 2010;206:725–730. doi: 10.1016/j.prp.2010.05.015. [DOI] [PubMed] [Google Scholar]