Abstract
Septic encephalopathy (SE) is a diffuse cerebral dysfunction resulting from a systemic inflammatory response, and is associated with an increased risk of mortality. The pathogenesis of SE is complex and multifactorial, but unregulated immune imbalance may be an important factor. The current retrospective study examined the clinical data of 86 patients with severe sepsis who were admitted to the Intensive Care Unit at Zhongshan Hospital, Xiamen University (Xiamen, China) from January, 2014 to January, 2015. The patients were assigned to SE and non-SE patient groups according to the presence or absence of SE. The proportion of T-lymphocyte subsets and natural killer (NK) cells in the immune cell population, representing the function of the immune system, were analyzed for their association with SE and compared with other clinical predictors and biomarkers. The incidence of SE in the patients was 39.5%, and this group demonstrated higher mortality rates (38 vs. 10% in non-SE patients; P=0.001). Univariate analysis revealed that the SE patients reported a lower percentage of cluster of differentiation 4+(CD4+) T-lymphocytes (51.67±7.12 vs. 60.72±3.70% in non-SE patients; P<0.01), a lower CD4+/cluster of differentiation 8+(CD8+) ratio (1.59±0.32 vs. 1.85±0.26% in non-SE patients; P<0.01) and a higher percentage of NK cells (11.80±1.44 vs. 9.19±2.36% in non-SE patients; P<0.01). Using a binary logistic regression model, the Acute Physiology and Chronic Health Evaluation II score and the percentage of CD4+ T-lymphocytes were demonstrated to be independently associated with SE (respectively, P=0.012 and OR, 4.763; P=0.005 and OR, 0.810). An area under the curve analysis of a receiver operating characteristic curve of the two indicators revealed that these were equally powerful measures in prediction of SE (Z=1.247, P>0.05). The present results confirm that SE leads to higher mortality in patients with severe sepsis, and demonstrate that immune imbalance is important in the development of SE. The proportion of CD4+ T-lymphocytes present were revealed in the current study to be a powerful predictor of SE in patients with severe sepsis.
Keywords: septic encephalopathy, mortality, immune imbalance, severe sepsis, CD4+ T-lymphocytes, natural killer cell, regression, predictor
Introduction
Septic encephalopathy (SE), defined as altered mental status and presenting with behavioral or cognitive abnormalities, is one of the most common complications in septic patients and is likely to be under-diagnosed (1). SE is associated with a higher mortality rate and is also a reliable indicator of a poor clinical outcome (2,3). Numerous animal and human studies have been performed to elucidate the etiology of SE (4–6) but, at present, the pathogenesis of SE is unknown. However, several potential mechanisms have been investigated, such as alterations to the blood-brain barrier (BBB), reduction in cerebral blood flow, the inflammatory response and activation of microglia and astrocytes and amino acid imbalance (7–10). The impact of an increased inflammatory response on the central nervous system (CNS) has been a key focus of investigation during the last two decades. Excessive inflammation, often termed a ‘cytokine storm’, characterizes early sepsis (11–14). As sepsis progresses, patients frequently develop multiple organ dysfunction and nosocomial infections by opportunistic pathogens (15–17) and the nervous system is particularly vulnerable to damage in response to systemic inflammation. Previous studies (11–13) suggest that patients with sepsis present with an immune factor imbalance, and there is a clear connection between immune imbalance and the occurrence of SE. Extensive previous evidence indicates that anti-inflammatory action in the brain and the resolution of neuroinflammation requires balance between the various branches of the immune system (18–20). An imbalance within the immune system and the systemic inflammation that results may promote CNS damage. The present retrospective study aimed to investigate the role of immune imbalance in SE, in addition to its effect on prognosis, using clinical data from patients with severe sepsis.
Subjects and methods
Ethics statement
The present study was approved by the Medical Ethics Committee of Zhongshan Hospital at Xiamen University (Xiamen, China). The present study did not increase the patient's medical expenses or pain and all research materials and results were used for research purposes. The requirement for informed consent was waived by the Medical Ethics Committee as the present study was an observational, respective study using a database from which the patients' identifying information had been removed.
Subjects
Severe sepsis was defined as sepsis combined with sepsis-induced organ dysfunction or tissue hypoperfusion, in accordance with criteria set out during the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference (21). Symptoms of SE include somnolence, stupor, coma, confusion, disorientation, agitation, irritability and a decreased score on the Glasgow Coma Scale. SE was defined as an altered mental status with behavioral or cognitive abnormalities, but there is no current unified standard for SE diagnoses (22,23). Patients suffering from the following underlying conditions that may affect brain and CNS function and symptomatic diagnosis were excluded: i) Intracranial organic diseases; ii) severe nutritional deficiency; iii) hypoglycemia; iv) hypernatremia; v) hepatic encephalopathy; and vi) a history of exposure to drugs, toxic substances, alcohol, industrial agents, heavy metals or any substance established to cause altered consciousness.
In the present study, 127 patients demonstrating severe sepsis were admitted to the Intensive Care Unit of Zhongshan Hospital at Xiamen University between January 2014 and January 2015. Of these patients, 41 patients were excluded due to the aforementioned exclusion criteria, predominantly linked to exposure to sedative drugs or intracranial organic disease, thus 86 patients were analyzed in the current study. In total, 57 men and 29 women were included, with an average age of 58.7 years. During their hospital stay, 34 patients developed SE although 52 patients did not, and patients were subsequently assigned to the SE and non-SE groups. Eighteen patients across the two groups succumbed to the disease during the 28-day study, representing a frequency of 20.93% (18/86 patients).
Treatment
The patients were treated with a standardized therapy based on the Severe Sepsis Campaign sepsis treatment guidelines (24). This therapy involved fluid resuscitation, antibiotic therapy, identification and control of infected tissue, mechanical ventilation, renal replacement, glucose control and supportive treatments, such as vasoactive drugs and steroid therapies.
Data collection
The medical record for each patient was reviewed. Patient demographics, mean arterial pressure, heart rate, duration of ventilator treatment, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score and outcome were recorded. APACHE II score and SOFA score at the time of admission to the Intensive Care Unit were also calculated.
Blood samples were obtained for routine examination of metrics, including liver and kidney function, such as alanine aminotransferase (ALT), bilirubin, activated partial thromboplastin time and serum creatinine levels; blood glucose; 6-h lactate clearance; B-type natriuretic peptide; blood gas analysis, including reporting of pH, arterial partial pressure of oxygen, arterial partial pressure of carbon dioxide and bicarbonate; brain injury markers, including levels of neuron specific enolase (NSE) and S-100β protein; and immune parameters, including white blood cell count, C-reactive protein, procalcitonin and the percentages of cluster of differentiation 4+ (CD4+) and cluster of differentiation 8+ (CD8+) T-lymphocytes and natural killer (NK) cells present. Flow cytometery (Elite XL4; Beckman Coulter, Inc., Brea, CA, USA) was used to detect the proportion of CD4+ and CD8+ T-lymphocytes, to calculate the CD4+/CD8+ ratio. Samples were collected in a test tube at 4°C containing tripotassium hydrogen ethylenediaminetetraacetate and analyzed within 30 min of collection.
The specimens were collected from the sputum in the lower respiratory tract using fiberbronchoscopy (Olympus BF P-30; Olympus Corporation, Tokyo, Japan), from wound excretions, from urine and from blood for cultivation and diagnosis of pathogenic bacteria in a 37°C incubator.
Statistical analysis
Statistical analysis was conducted using SPSS v. 19.0 software (IBM SPSS, Armonk, NY, USA). Measurement data were expressed as mean ± SD and compared using independent t-tests. Enumeration data were compared using a χ2 test or with Fishers exact test, as appropriate. For detection of correlation, Pearson's correlation analysis was performed. P<0.05 was considered to indicate a statistically significant difference. Statistically significant variables were subsequently analyzed using a binary logistic regression to identify the risk factors associated with SE. Only variables markedly associated with SE (P<0.05) were included in the final model. Receiver operating characteristic (ROC) curves and the area under the curves (AUCs) were examined in significant variables associated with SE, to determine a cut-off level and to predict mortality.
Results
Baseline data of the patients
During the period of the present study, 127 patients with severe sepsis were initially admitted, 86 of whom were included in the study. Patient characteristics of the two groups are provided in Table I. No significant differences were identified between the groups in age, gender, underlying diseases, mean arterial pressure or heart rate (P>0.05). However, duration of ventilator treatment and 28-day mortality were significantly higher in the SE group compared to the non-SE group (13.12±3.89 vs. 8.28±3.32 days, P<0.01; and 38 vs. 10%, P=0.001, respectively). With regard to the disease severity index, the SE group had higher APACHE II and SOFA scores than the non-SE group (21.74±2.96 vs. 16.25±2.62, P<0.01; and 8.21±1.45 vs. 5.38±1.84, P<0.01, respectively), indicating a greater degree of organ dysfunction.
Table I.
Baseline characteristics of the SE and non-SE groups.
| Characteristics | SE group (n=34) | Non-SE group (n=52) | P-value |
|---|---|---|---|
| Age in years, mean ± SD | 59.15±8.80 | 58.39±8.14 | 0.69 |
| Male/female, n | 24/10 | 33/19 | 0.49 |
| Underlying diseases, n (%) | |||
| Chronic lung disease | 9 (26) | 11 (21) | 0.57 |
| Hypertension | 8 (24) | 16 (31) | 0.46 |
| Hyperlipidemia | 10 (29) | 17 (68) | 0.75 |
| Coronary artery disease | 4 (12) | 8 (15) | 0.76 |
| Chronic liver disease | 3 (9) | 9 (17) | 0.35 |
| Chronic renal disease | 2 (6) | 2 (4) | 0.65 |
| Diabetes mellitus | 11 (32) | 19 (37) | 0.69 |
| Clinical presentation, mean ± SD | |||
| Mean arterial pressure, mmHg | 78.52±7.15 | 79.23±5.93 | 0.62 |
| Heart rate, beats/min | 104.71±15.79 | 109.21±15.03 | 0.19 |
| Ventilator treatment duration, days | 13.12±3.89 | 8.28±3.32 | <0.01a |
| 28-day mortality, n (% of cases) | 13 (38) | 5 (10) | 0.001a |
| Disease severity index, mean ± SD | |||
| APACHE II score | 21.74±2.96 | 16.25±2.62 | <0.01a |
| SOFA score | 8.21±1.45 | 5.38±1.84 | <0.01a |
Data presented as n (%) were analyzed by a χ2 test or Fisher's exact test when theoretical frequency <5.
P<0.05, SE vs. non-SE group. SE, septic encephalopathy; SD, standard deviation; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment.
Table II reports the sources of infection and the causative microorganisms in the two groups of patients. No significant difference was identified in the infection source between the groups: the SE group did not report significantly different numbers of patients with gram-positive cocci, gram-negative bacilli or epiphyte infection compared with the non-SE group, nor any different frequency of each microorganism (P>0.05).
Table II.
Sources of infection in the SE and non-SE groups, expressed as n (%).
| Sources of infection | SE group (n=34) | Non-SE group (n=52) | P-value |
|---|---|---|---|
| Organ system infected | |||
| Respiratory system | 10 (29) | 17 (33) | 0.75 |
| Digestive system | 12 (35) | 20 (39) | 0.77 |
| Urinary system | 6 (18) | 8 (15) | 0.78 |
| Skin and soft tissue | 4 (12) | 5 (10) | 0.74 |
| Other | 2 (6) | 2 (4) | 0.65 |
| Concurrent bacteremia episodes | 6 (18) | 9 (17) | 0.97 |
| Causative pathogens | |||
| Gram-positive | 15 (44) | 25 (48) | 0.72 |
| Staphylococcus aureus | 8 (23) | 11 (21) | 0.80 |
| Streptococcus pneumonia | 1 (3) | 4 (8) | 0.64 |
| Enterococcus faecium | 5 (15) | 6 (12) | 0.75 |
| Enterococcus faecalis | 1 (3) | 4 (8) | 0.64 |
| Gram-negative | 24 (71) | 39 (75) | 0.65 |
| Acinetobacterbaum anni | 7 (21) | 13 (25) | 0.64 |
| Pseudomonas aeruginosa | 5 (15) | 10 (19) | 0.59 |
| Escherichia coli | 4 (12) | 8 (15) | 0.76 |
| Klebsiella pneumoniae | 4 (11.8) | 5 (9.6) | 0.74 |
| Proteus mirabilis | 2 (5.9) | 2 (3.8) | 0.65 |
| Entembacter cloacae | 2 (5.9) | 1 (1.9) | 0.56 |
| Epiphyte | 4 (11.8) | 5 (9.6) | 0.74 |
Data were analyzed by a χ2 test or Fisher's exact test when theoretical frequency<5. SE, septic encephalopathy.
Laboratory data are shown in Table III. A significant increase in the serum levels of ALT and S-100β protein in the SE group compared to the non-SE group was revealed using independent t-tests (156.79±33.57 vs. 98.69±38.12 U/l, P<0.01; and 1.21±0.15 vs. 0.98±0.20 µg/l, P<0.01, respectively), using the Karman-Worblewski method (25). In regard to inflammatory markers and immune parameters, the percentage of CD4+ T lymphocytes and the CD4+/CD8+ ratio were significantly lower in the SE group (51.67±7.12 vs. 60.72±3.70% in the non-SE group, P<0.01; and 1.59±0.32 vs. 1.85±0.26 in the non-SE group, P<0.01, respectively), while the percentage of NK cells was significantly higher in the SE group compared to the non-SE group (11.80±1.44 vs. 9.19±2.36%, P<0.01). No significant differences were identified in the other laboratory variables examined (P>0.05).
Table III.
Laboratory data of the SE and non-SE groups, presented as mean ± SD.
| Laboratory variable | SE group (n=34) | Non-SE group (n=53) | P-value |
|---|---|---|---|
| Biochemistry | |||
| Glucose, mmol/l | 9.63±3.21 | 9.20±2.94 | 0.52 |
| ALT, U/l | 156.79±33.57 | 98.69±38.12 | <0.01a |
| Creatinine, mmol/l | 86.49±24.62 | 79.28±22.04 | 0.16 |
| 6 h lactate clearance, % | 16.71±7.73 | 18.15±8.08 | 0.41 |
| Total bilirubin, mmol/l | 13.28±4.94 | 12.80±4.52 | 0.64 |
| BNP, pg/ml | 1224.20±586.99 | 1042.89±507.23 | 0.13 |
| PaO2, mmHg | 77.20±17.92 | 81.19±20.91 | 0.36 |
| APTT, sec | 38.56±6.87 | 40.08±7.59 | 0.36 |
| NSE, µg/l | 10.02±1.48 | 9.86±0.91 | 0.58 |
| S-100β, µg/l | 1.21±0.15 | 0.98±0.20 | <0.01a |
| Inflammatory markers | |||
| WBC, n × 109/l | 15.89±6.51 | 16.39±7.24 | 0.75 |
| CRP, mg/l | 230.99±67.59 | 205.99±102.81 | 0.18 |
| PCT, ng/ml | 10.69±5.41 | 9.97±4.94 | 0.53 |
| CD4+, % of total cells | 51.67±7.12 | 60.72±3.70 | <0.01a |
| CD8+, % of total cells | 32.92±2.48 | 33.26±2.71 | 0.56 |
| CD4+/CD8+ | 1.59±0.32 | 1.85±0.26 | <0.01a |
| NK, % of total cells | 11.80±1.44 | 9.19±2.36 | <0.01a |
SE, septic encephalopathy; SD, standard deviation; ALT, alanine aminotransferase; BNP, B-type natriuretic peptide; PaO2, arterial partial pressure of oxygen; APTT, activated partial thromboplastin time; NSE, neuron-specific enolase; WBC, white blood cells; CRP, C-reactive protein; PCT, procalcitonin; CD4+, cluster differentiation 4+ T helper cells; CD8+, cluster differentiation 8+ T helper cells; NK, natural killer cells.
P<0.05, SE vs. non-SE group.
Correlation between immune parameters and disease severity
The percentage of CD4+ T lymphocytes, the CD4+/CD8+ ratio and the percentage of NK cells were significantly different between the SE and non-SE groups (Table III). Thus, additional correlation analysis between these immune parameters and disease severity was conducted. Based on the results of Pearson's correlation analysis (Tables IV and V), the percentage of CD4+ T-lymphocytes, the CD4+/CD8+ ratio and the percentage of NK cells demonstrated a marked correlation with APACHE II scores (r=−0.854, −0.824 and 0.816, respectively; P<0.01), SOFA scores (r=−0.878, −0.853 and 0.871, respectively; P<0.01), NSE levels (r=−0.738, −0.872 and 0.683, respectively; P<0.01) and S-100β protein levels (r=−0.696, −0.719 and 0.795, respectively; P<0.01). These analyses revealed that the percentage of CD4+ T lymphocytes and NK cells and the CD4+/CD8+ ratio were correlated with sepsis and brain injury severity. The results of the present analyses also indicated that the most marked correlation in sepsis severity was between the percentage of CD4+ T lymphocytes and SOFA score (R2=0.771) and, in brain injury severity, was between the CD4+/CD8+ ratio and NSE levels (R2=0.760).
Table IV.
Correlation between immune parameters and sepsis severity.
| APACHE II | SOFA | |||||||
|---|---|---|---|---|---|---|---|---|
| Immune parameter | r | P-value | R2 | 95% CI | r | P-value | R2 | 95% CI |
| CD4+ | −0.854 | <0.01 | 0.729 | −0.905 to −0.787 | −0.878 | <0.01 | 0.771 | −0.914 to −0.827 |
| CD4+/CD8+ | −0.824 | <0.01 | 0.679 | −0.883 to −0.741 | −0.853 | <0.01 | 0.728 | −0.909 to −0.783 |
| NK | 0.816 | <0.01 | 0.666 | 0.756 to 0.869 | 0.871 | <0.01 | 0.759 | 0.803 to 0.920 |
APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; r, correlation coefficient; R2, coefficient of determination; CI, confidence interval; CD4+, cluster differentiation 4+ T helper cells; CD8+, cluster differentiation 8+ T-helper cells; NK, natural killer cells.
Table V.
Correlation between immune parameters and brain injury severity.
| NSE | S-100β | |||||||
|---|---|---|---|---|---|---|---|---|
| Immune parameter | r | P-value | R2 | 95% CI | r | P-value | R2 | 95% CI |
| CD4+ | −0.738 | <0.01 | 0.545 | −0.828 to −0.613 | −0.696 | <0.01 | 0.484 | −0.863 to −0.457 |
| CD4+/CD8+ | −0.872 | <0.01 | 0.760 | −0.919 to −0.811 | −0.719 | <0.01 | 0.517 | −0.886 to −0.484 |
| NK | 0.683 | <0.01 | 0.466 | 0.555 to 0.778 | 0.795 | <0.01 | 0.632 | 0.607 to 0.930 |
NSE, neuron specific enolase; r, correlation coefficient; R2, coefficient of determination; CI, confidence interval; CD4+, cluster differentiation 4+ T helper cells; CD8+, cluster differentiation 8+ T-helper cells; NK, natural killer cells.
Prediction of SE
APACHE II and SOFA scores, serum ALT and S-100β protein levels, the percentage of CD4+ T lymphocytes and NK cells and the CD4+/CD8+ ratio were significantly different between the two groups and may be used as predictive factors. However, subsequent to use of a binary logistic regression analysis (using the forward conditional method), performed with ‘SE presence or absence’ as a dependent variable and all the predictors as independent variables, only the APACHE II score and the percentage of CD4+ T-lymphocytes (P=0.012 and OR, 4.763; P=0.005 and OR, 0.810, respectively) entered the final equation, demonstrating that they were independently associated with SE (Table VI).
Table VI.
Logistic regression analysis of risk factors of septic encephalopathy.
| Risk factor | B | SE | Wald | P-value | OR | 95% CI |
|---|---|---|---|---|---|---|
| APACHE II | 1.561 | 0.625 | 6.244 | 0.012 | 4.763 | 1.400–16.202 |
| CD4+ | −0.211 | 0.076 | 7.731 | 0.005 | 0.810 | 0.698–0.940 |
B, partial regression coefficient; SE, standard error; OR, odds ratio; CI, confidence interval; APACHE, Acute Physiology and Chronic Health Evaluation; CD4+, cluster differentiation 4+ T-helper cells.
The effectiveness of these variables in predicting SE was evaluated by assessing the AUC of each ROC curve (Table VII; Fig. 1). The AUCs for the percentage of CD4+ T lymphocytes and APACHE II score were 0.919 and 0.855, respectively (P<0.001), reflecting good discrimination. A Z-test was subsequently used to compare the predictive ability of these variables, identifying no significant difference between the AUCs of the percentage of CD4+ T lymphocytes and APACHE II score (Z=1.247, P=0.212), revealing that these were equally powerful measures in the prediction of SE (P>0.05). Based on the ROC curve and the maximum Youden's index, the most appropriate cut-off value was selected. With regard to the percentage of CD4+ T lymphocytes, the most appropriate cut-off value for predicting SE was 55.655%, corresponding to the sensitivity and specificity values of 67.6 and 90.4%, respectively. With regard to APACHE II score, the most appropriate cut-off value for predicting SE was 18.500, corresponding to the sensitivity and specificity values of 88.2 and 75.0%, respectively (Table VIII).
Table VII.
Receiver operating characteristic curve analysis of independent risk factors in diagnosing septic encephalopathy.
| Risk factor | AUC | SE | 95% CI | P-value |
|---|---|---|---|---|
| APACHE II | 0.919 | 0.028 | 0.864–0.973 | <0.001 |
| CD4+ | 0.855 | 0.043 | 0.771–0.939 | <0.001 |
AUC, area under the curve; SE, standard error; CI, confidence interval; APACHE, Acute Physiology and Chronic Health Evaluation; CD4+, cluster differentiation 4+ T-helper cells.
Figure 1.
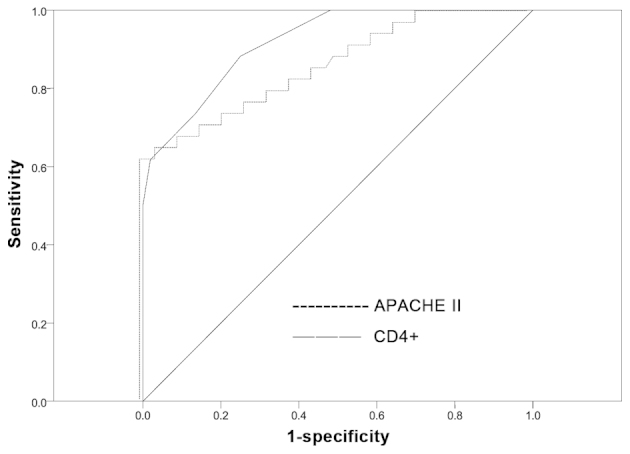
Comparison receiver operating characteristic curve of CD4+ with APACHE II score for predicting septic encephalopathy. APACHE, Acute Physiology and Chronic Health Evaluation; CD4+, cluster differentiation 4+ T-helper cells.
Table VIII.
Prediction of septic encephalopathy.
| Risk factor | Maximum Youden's index | Best cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| APACHE II | 1.632 | 18.500 | 88.2 | 75.0 |
| CD4+ | 1.580 | 55.655 | 67.6 | 90.4 |
APACHE, Acute Physiology and Chronic Health Evaluation; CD4+, cluster differentiation 4+ T-helper cells.
Discussion
SE is an acute neurological dysfunction that results from sepsis and is associated with high morbidity and mortality. During sepsis, the brain is vulnerable, and encephalopathy frequently results but is not commonly identified (26,27). According to previous studies, the incidence of SE following severe sepsis varies from 9–71% with a mortality frequency of ~50% (2,28), dependent on the method used to grade altered mental status (3,29). In the present study, SE resulted in 40% of severe sepsis cases, with a mortality of 38%. Although the incidence and mortality are inconsistent across studies, the brain is sensitive to sepsis, and SE often has severe consequences (2,28). SE should therefore be recognized as an indicator of poor prognosis in patients with sepsis, inducing prompt and aggressive therapy.
APACHE II and SOFA scores have been applied to critically ill patients to evaluate the severity of SE and clinical outcomes. Previous findings have shown that the severity of encephalopathy was associated with the global severity of disease, as assessed by APACHE II score or SOFA, and mortality rates (1,2). In the present study, the mortality of septic patients significantly increased with increased APACHE II and SOFA scores, which is consistent with previous reports (30), supporting an association of SE with an increased mortality risk in patients with severe sepsis.
The present study indicated that patients with SE required a greater duration of ventilator treatment, revealing more severe respiratory failure. In clinical practice, patients with disturbance of consciousness are prone to respiratory failure due to an inability to protect the airway and respiratory drive. During a period of hypoxemia that occurs prior to respiratory failure, a greater degree of brain injury may be generated. The present analyses demonstrated a significantly increased level of ALT in patients with SE. Sepsis is often associated with multiple organ failure and numerous abnormal biochemical indicators, which indicates there may be a complex inherent association between individual organ failure and an amplification process that hastens injury to other organs. This could be seen to explain the increased mortality in the SE group.
The pathophysiology of SE is complex, and may include activation of the inflammatory response, microglia and astrocytes, alteration in the BBB, amino acid disruption, brain hypoperfusion/ischemia and translocation of neurotoxic molecules (7–10). An upregulated inflammatory response is recognized as an integral contributor to SE. Previous studies have comprehensively investigated the effect of infection on CNS. However, the severity of SE is reportedly not associated with infection by specific microorganisms, nor groups thereof (31). Furthermore, inflammatory mediators, including interleukin-1 and tumor necrosis factor-α and oxidative stress have a critical role in the abnormal neurotransmitter composition and impaired neuronal and microglia function (32–34). Reduced hepatic clearance and increased neurotoxic amino acids in sepsis associated with muscle proteolysis also contribute to the development of brain dysfunction (35,36). The S-100β protein has been previously employed as an indicator of astrocyte activation and injury, and as a marker for brain injury in SE (37,38); however, not all studies concur with this finding (39,40). In the present study, S-100β protein was significantly higher in SE patients, but regression analysis indicated that this is not the most reliable indicator for predicting SE, a finding that was consistent with previously conflicting findings (37–40). Additional evaluation of the direct effect of brain injury to SE is required to clarify the most effective markers.
Lipopolysaccharide stimulation has previously been reported to induce the release of proinflammatory and anti-inflammatory cytokines, in addition to their receptors, from a number of nervous system-associated cells, including neurones, astrocytes and microglia (41,42). This coexpression of proinflammatory and anti-inflammatory cytokines indicates that the immune system is highly regulated within the brain. Concordantly, in sepsis survivors, the initial proinflammatory burst often develops into immune suppression, characterized by T-cell dysfunction and adaptive immune suppression accompanied by innate immune system activation (43–45) This immune imbalance develops throughout sepsis and study in this area has made significant progress (46). Reduction in the number of circulating CD4+ T lymphocytes and their shift to a Th2 phenotype are indicative of aspects of sepsis-induced adaptive immunosuppression (47). The association between clinical course of contradiction and poor prognosis of patients with sepsis and the decline of peripheral blood CD4+ T lymphocytes has been established in a majority of patients with sepsis (48,49). Furthermore, NK cells, a type of cytotoxic lymphocyte, are likely to be involved in the antibacterial response of the innate immune system due to their ability to recognize pathogen-associated molecular patterns (50,51). Findings of a previous study have shown that patients with the highest NK cell number have the lowest probability of survival (52). In the present study, the percentage of CD4+ T lymphocytes and the CD4+/CD8+ ratio were significantly lower and the percentage of NK cells was significantly higher in the SE group than in the non-SE group, suggesting adaptive immune suppression and innate immune activation of patients. The present results are comparable with previous studies and indicate a highly significant functional imbalance of immune cells in patients with SE, which may be crucial in the development of encephalopathy.
CD4+ T lymphocytes are particularly vulnerable to apoptotic death in polymicrobial sepsis models, according to previous studies (43,53). In addition, the response of T lymphocytes to continuously elevated serum levels of anti-inflammatory cytokines was an improved predictor of mortality than proinflammatory cytokines in patients with severe sepsis (54), which are typically used. In the current study, only the percentage of CD4+ T lymphocytes and APACHE II score were determined to be similarly accurate predictors of clinical outcome, based on the regression analysis, when compared with the CD4+/CD8+ ratio and percentage of NK cells. These results indicate that the percentage of CD4+ T lymphocytes is a promising biomarker in predicting SE occurrence among patients with severe sepsis. The recirculation of CD4+ T lymphocytes may be responsible for the percentage of CD4+ T lymphocytes decreasing in the peripheral blood of patients with sepsis. This recirculation may be associated with the generation of stress hormones, cytokines and other humoral factors, including prostaglandin E2-α, cortisol and interleukin-10 (55,56), which is supported by previous studies revealing a rapid decrease in circulating CD4+ T lymphocytes following the experimental administration of endotoxin and an observed increase in the concentration of these cells in the thoracic ducts of patients with systemic inflammatory response syndrome (57,58).
The present study provides novel insights into the role of CD4+ T lymphocytes during SE, but has several limitations. Poor nutritional status was typical and varied among patients with severe sepsis. Malnutrition has marked consequences on the immune response that may affect results. Furthermore, the diagnosis of SE may have been affected by a negative mood in patients. In conclusion, the present study provides a unique insight into the status of the immune system in SE. SE leads to higher mortality rates in patients with severe sepsis, and immune imbalance has an important role in this increase in mortality rates. The current study indicates that the proportion of CD4+ T lymphocytes present in the blood of patients with severe sepsis is a powerful predictor of SE. However, additional investigation is required to elucidate the pathogenesis of SE.
Acknowledgements
The authors of the current study thank all biotechnicians of the clinical laboratories in Zhongshan Hospital Xiamen University for their technical support. The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81071529 and 81272105).
References
- 1.Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, Hinshaw LB. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990;18:801–806. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Eidelman LA, Putterman D, Putterman C, Sprung CL. The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities. JAMA. 1996;275:470–473. doi: 10.1001/jama.275.6.470. [DOI] [PubMed] [Google Scholar]
- 3.Straver JS, Keunen RW, Stam CJ, Tavy DL, de Ruiter GR, Smith SJ, Thijs LG, Schellens RG, Gielen G. Nonlinear analysis of EEG in septic encephalopathy. Neurol Res. 1998;20:100–106. doi: 10.1080/01616412.1998.11740490. [DOI] [PubMed] [Google Scholar]
- 4.Schraag S. Studying septic encephalopathy: What animal models can predict. Intensive Care Med. 2003;29:667–668. doi: 10.1007/s00134-003-1718-y. [DOI] [PubMed] [Google Scholar]
- 5.Eggers V, Fügener K, Hein OV, Rommelspacher H, Heyes MP, Kox WJ, Spies CD. Antibiotic-mediated release of tumour necrosis factor alpha and norharman in patients with hospital-acquired pneumonia and septic encephalopathy. Intensive Care Med. 2004;30:1544–1551. doi: 10.1007/s00134-004-2285-6. [DOI] [PubMed] [Google Scholar]
- 6.Angel MJ, Young GB. Metabolic encephalopathies. Neurol Clin. 2011;29:837–882. doi: 10.1016/j.ncl.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Koo DJ, Jackman D, Chaudry IH, Wang P. Adrenal insufficiency during the late stage of polymicrobial sepsis. Crit Care Med. 2001;29:618–622. doi: 10.1097/00003246-200103000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Tsao N, Hsu HP, Wu CM, Liu CC, Lei HY. Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J Med Microbiol. 2001;50:812–821. doi: 10.1099/0022-1317-50-9-812. [DOI] [PubMed] [Google Scholar]
- 9.Basler T, Meier-Hellmann A, Bredle D, Reinhart K. Amino acid imbalance early in septic encephalopathy. Intensive Care Med. 2002;28:293–298. doi: 10.1007/s00134-002-1217-6. [DOI] [PubMed] [Google Scholar]
- 10.Deng YY, Fang M, Zhu GF, Zhou Y, Zeng HK. Role of microglia in the pathogenesis of sepsis-associated encephalopathy. CNS Neurol Disord Drug Targets. 2013;12:720–725. doi: 10.2174/18715273113126660178. [DOI] [PubMed] [Google Scholar]
- 11.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 12.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: Understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 13.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. doi: 10.1097/01.CCM.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 14.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, Capron F, Agut H, Gibert C, Chastre J. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 16.Kollef KE, Schramm GE, Wills AR, Reichley RM, Micek ST, Kollef MH. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134:281–287. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- 17.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Hayes Santo TK, Corey L, Boeckh M. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: Leukocyte recruitment via the choroid plexus. EMBO J. 2014;33:7–22. doi: 10.1002/embj.201386609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian L, Ma L, Kaarela T, Li Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J Neuroinflammation. 2012;9:155. doi: 10.1186/1742-2094-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neumann H. Control of glial immune function by neurons. Glia. 2001;36:191–199. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- 21.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. International Sepsis Definitions Conference: 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 22.Cotena S, Piazza O. Sepsis-associated encephalopathy. Transl Med UniSa. 2012;2:20–27. [PMC free article] [PubMed] [Google Scholar]
- 23.Dal-Pizzol F, Tomasi CD, Ritter C. Septic encephalopathy: Does inflammation drive the brain crazy? Rev Bras Psiquiatr. 2014;36:251–258. doi: 10.1590/1516-4446-2013-1233. [DOI] [PubMed] [Google Scholar]
- 24.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup: Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 25.Karmen A, Worblewski F, Landue JS. Transaminases activity in human blood. J Clin Invest. 1955;34:126–131. doi: 10.1172/JCI103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milbrandt EB, Angus DC. Bench-to-bedside review: Critical illness-associated cognitive dysfunction - mechanisms, markers, and emerging therapeutics. Crit Care. 2006;10:238. doi: 10.1186/cc5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med. 2007;33:941–950. doi: 10.1007/s00134-007-0622-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhang LN, Wang XT, Ai YH, Guo QL, Huang L, Liu ZY, Yao B. Epidemiological features and risk factors of sepsis-associated encephalopathy in intensive care unit patients: 2008–2011. Chin Med J (Engl) 2012;125:828–831. [PubMed] [Google Scholar]
- 29.Zauner C, Gendo A, Kramer L, Kranz A, Grimm G, Madl C. Metabolic encephalopathy in critically ill patients suffering from septic or nonseptic multiple organ failure. Crit Care Med. 2000;28:1310–1315. doi: 10.1097/00003246-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Taylor SL, Morgan DL, Denson KD, Lane MM, Pennington LR. A comparison of the Ranson, Glasgow, and APACHE II scoring systems to a multiple organ system score in predicting patient outcome in pancreatitis. Am J Surg. 2005;189:219–222. doi: 10.1016/j.amjsurg.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Bone RC, Sprung CL, Sibbald WJ. Definitions for sepsis and organ failure. Crit Care Med. 1992;20:724–726. doi: 10.1097/00003246-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Babior BM. NADPH oxidase: An update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 33.Wang CX, Shuaib A. Involvement of inflammatory cytokines in central system injury. Prog Neurobiol. 2002;67:161–172. doi: 10.1016/S0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 34.Choi SH, Lee DY, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: Role of microglial NADPH oxidase. J Neurosci. 2005;25:4082–4090. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprung CL, Cerra FB, Freund HR, Schein RM, Konstantinides FN, Marcial EH, Pena M. Amino acid alterations and encephalopathy in the sepsis syndrome. Crit Care Med. 1991;19:753–757. doi: 10.1097/00003246-199106000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kadoi Y, Saito S. An alteration in the gamma-aminobutyric acid receptor system in experimentally induced septic shock in rats. Crit Care Med. 1996;24:298–305. doi: 10.1097/00003246-199602000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, Huyghens L. Elevated serum levels of S-100beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med. 2006;34:1967–1974. doi: 10.1097/01.CCM.0000217218.51381.49. [DOI] [PubMed] [Google Scholar]
- 38.Piazza O, Cotena S, De Robertis E, Caranci F, Tufano R. Sepsis associated encephalopathy studied by MRI and cerebral spinal fluid S100B measurement. Neurochem Res. 2009;34:1289–1292. doi: 10.1007/s11064-008-9907-2. [DOI] [PubMed] [Google Scholar]
- 39.Piazza O, Russo E, Cotena S, Esposito G, Tufano R. Elevated S100B levels do not correlate with the severity of encephalopathy during sepsis. Br J Anaesth. 2007;99:518–521. doi: 10.1093/bja/aem201. [DOI] [PubMed] [Google Scholar]
- 40.van den Boogaard M, Ramakers BP, van Alfen N, van der Werf SP, Fick WF, Hoedemaekers CW, Verbeek MM, Schoonhoven L, van der Hoeven JG, Pickkers P. Endotoxemia-induced inflammation and the effect on the human brain. Crit Care. 2010;14:R81. doi: 10.1186/cc9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omari KM, Dorovini-Zis K. CD40 expressed by human brain endothelial cells regulates CD4+ T cell adhesion to endothelium. J Neuroimmunol. 2003;134:166–178. doi: 10.1016/S0165-5728(02)00423-X. [DOI] [PubMed] [Google Scholar]
- 42.Sankowski R, Mader S, Valdés-Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci. 2015;9:28. doi: 10.3389/fncel.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 44.Roth G, Moser B, Krenn C, Brunner M, Haisjackl M, Almer G, Gerlitz S, Wolner E, Boltz-Nitulescu G, Ankersmit HJ. Susceptibility to programmed cell death in T-lymphocytes from septic patients: A mechanism for lymphopenia and Th2 predominance. Biochem Biophys Res Commun. 2003;308:840–846. doi: 10.1016/S0006-291X(03)01482-7. [DOI] [PubMed] [Google Scholar]
- 45.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, II, Kreisel D, Krupnick AS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sunkara B, Bheemreddy S, Lorber B, Lephart PR, Hayakawa K, Sobel JD, Kaye KS, Marchaim D. Group B Streptococcus infections in non-pregnant adults: The role of immunosuppression. Int J Infect Dis. 2012;16:e182–e186. doi: 10.1016/j.ijid.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Ferguson NR, Galley HF, Webster NR. T helper cell subset ratios in patients with severe sepsis. Intensive Care Med. 1999;25:106–109. doi: 10.1007/s001340050795. [DOI] [PubMed] [Google Scholar]
- 48.Cheadle WG, Pemberton RM, Robinson D, Livingston DH, Rodriguez JL, Polk HC., Jr Lymphocyte subset responses to trauma and sepsis. J Trauma. 1993;35:844–849. doi: 10.1097/00005373-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Wakefield CH, Carey PD, Foulds S, Monson JR, Guillou PJ. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg. 1993;80:205–209. doi: 10.1002/bjs.1800800224. [DOI] [PubMed] [Google Scholar]
- 50.Chalifour A, Jeannin P, Gauchat JF, Blaecke A, Malissard M, N'Guyen T, Thieblemont N, Delneste Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104:1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 51.Andaluz-Ojeda D, Iglesias V, Bobillo F, Almansa R, Rico L, Gandía F, Loma AM, Nieto C, Diego R, Ramos E, et al. Early natural killer cell counts in blood predict mortality in severe sepsis. Crit Care. 2011;15:R243. doi: 10.1186/cc10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anduluz-Ojeda D, Iglesias V, Bobillo F, Almansa R, Rico L, Gandía F, Loma AM, Nieto C, Diego R, Ramos E, et al. Early natural killer cell counts in blood predict mortality in severe sepsis. Crit Care. 2011;15:R243. doi: 10.1186/cc10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markwart R, Condotta SA, Requardt RP, Borken F, Schubert K, Weigel C, Bauer M, Griffith TS, Förster M, Brunkhorst FM, et al. Immunosuppression after sepsis: Systemic inflammation and sepsis induce a loss of naïve T-cells but no enduring cell-autonomous defects in T-cell function. PLoS One. 2014;9:e115094. doi: 10.1371/journal.pone.0115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Pablo R, Monserrat J, Reyes E, Diaz-Martin D, Zapata Rodriguez M, Carballo F, de la Hera A, Prieto A, Alvarez-Mon M. Mortality in patients with septic shock correlates with anti-inflammatory but not proinflammatory immunomodulatory molecules. J Intensive Care Med. 2011;26:125–132. doi: 10.1177/0885066610384465. [DOI] [PubMed] [Google Scholar]
- 55.Menges T, Engel J, Welters I, Wagner RM, Little S, Ruwoldt R, Wollbrueck M, Hempelmann G. Changes in blood lymphocyte populations after multiple trauma: Association with posttraumatic complications. Crit Care Med. 1999;27:733–740. doi: 10.1097/00003246-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 56.Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocrinol Metab. 2000;85:42–7. doi: 10.1210/jc.85.1.42. [DOI] [PubMed] [Google Scholar]
- 57.Toft P, Hokland M, Hansen TG, Tønnesen E. Changes in lymphocyte subpopulations and adhesion/activation molecules following endotoxemia and major surgery. APMIS. 1995;103:261–266. doi: 10.1111/j.1699-0463.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 58.Lemaire LC, van Deventer SJ, van Lanschot JJ, Meenan J, Gouma DJ. Phenotypical characterization of cells in the thoracic duct of patients with and without systemic inflammatory response syndrome and multiple organ failure. Scand J Immunol. 1998;47:69–75. doi: 10.1046/j.1365-3083.1998.00265.x. [DOI] [PubMed] [Google Scholar]


