Abstract
Oral squamous cell carcinomas (OSCCs) are a growing problem in the world. The various existing treatments have not markedly improved the survival rate of patients with OSCC during the past three decades. Novel treatment strategies are required. Sex determining region Y-box 2 (SOX2) is a transcription factor that is involved in the maintenance of embryonic stem cell pluripotency and in multiple developmental processes. SOX2 expression was indicated to act as a prognostic factor in various types of tumors, including breast, colorectal, gastric and lung cancer and glioblastoma, and as a link between malignancy and stemness. Cancer stem cells (CSCs) may be responsible for the genesis, growth and metastatic spread of tumors. The poor survival outcomes for OSCC patients may be attributable to a poor selection of target cells for treatment, as current oral cancer therapies are generally aimed at the global mass of tumor. Therefore, the consideration that novel approaches to oral cancer may be targeted using SOX2 and CSCs appears reasonable. In order to better understand the oncogenic roles and the corresponding signal transduction pathways of the SOX2 protein, the present study emphasizes the role of SOX2 in OSCC, including the proteins associated with OSCC, and reviews the literature regarding the role of SOX2 in lymph node metastasis. The aim of the present study is to provide a reference for future studies that engage in research on the aforementioned subject.
Keywords: oral cancer, lymph node metastasis, SOX2, cancer stem cell
1. Introduction
Sex determining region Y-box 2 (SOX2) is a transcription factor that is involved in the maintenance of embryonic stem cell pluripotency and in multiple developmental processes (1,2). Increasing numbers of studies regarding the association between SOX2 and malignant tumors have been reported. Numerous studies have indicated that SOX2 is involved in in tumorigenesis, including in skin squamous cell carcinoma, gastric cancer, glioblastoma, colorectal cancer, lung cancer and breast cancer (3–8). SOX2 has demonstrated a pro-oncogenic function in the majority of the various types of malignant tumor, including breast, colorectal and lung cancer and glioblastoma, but not gastric cancer. The expression of SOX2 in oral squamous cell carcinomas (OSCC) has also been reported, and SOX2 nuclear expression is closely associated with a poor prognosis in oral tongue squamous cell carcinoma (OTSCC) (9). In addition, the increased expression of SOX2 in OSCC is associated with lymph node metastasis (10).
Oral cancer is a growing problem in the world and demonstrates a high prevalence among men in Asia, particularly in India, as oral cancer is the most prevalent type of cancer in Indian men (11–13). The overall 5-year survival rate of patients following a surgical resection or other treatment has not markedly improved during the past three decades, and remains at ~50% (14). The presence of lymph node metastasis is considered to be an important indicator of predicting an adverse outcome (15,16). However, certain OSCC patients that do not possess node metastasis continue to suffer from tumor relapses subsequent to complete surgical resections, and then poor prognoses (9,17). Novel treatment strategies are required. In previous studies, SOX2 has been indicated to act as a prognostic factor in various types of tumors and as a link between malignancy and stemness (3,18,19). Novel studies indicate that cancer stem cells (CSCs) may be responsible for the genesis, growth and metastatic spread of the tumor (20,21). The poor survival outcomes for OSCC patients may be attributable to a poor selection of target cells for treatment, as current oral cancer therapies are generally aimed at the overall mass of the tumor. Therefore, the consideration that novel approaches to oral cancer may be targeted using SOX2 and CSCs appears reasonable.
In order to better understand the oncogenic roles and corresponding signal transduction pathways of the SOX2 protein, the present study emphasizes the role of SOX2 and the associated proteins in OSCC, and reviews the literature on the role of SOX2 in lymph node metastasis. The aim of the present study is to provide a reference for future studies that engage in research on the aforementioned subject.
2. Expression of SOX2 in OSCC
SOX2 is an amplified gene in OSCC, which is established as one of the hallmark participants throughout the developmental process in cancer. SOX2 is mainly expressed in CSCs. In order to study the role of SOX2 in OSCC, the concept and identification of CSC must be understood.
Concept and identification of CSCs
The major characteristic that defines stem cells is self-renewal, whereby stem cells result in numerous types of mature cells and lead to organogenesis (22). Similar cells that exist in cancer, termed CSCs, have been previously documented (22). In addition to the ability to self-renew, CSCs may result in phenotypically varied tumor cell populations through a process of aberrant differentiation. CSCs may, therefore, be responsible for driving tumorigenesis and tumor growth. The theory that tumorigenesis is based exclusively on the aberrant activity of CSCs derives from the heterogeneous nature of OSCCs and other tumors (23). The structural similarity of well-differentiated tumors with the epithelium of origin may also confirm the existence of CSCs. In particular, a well-differentiated OSCC may reproduce the proliferation pattern and histological appearance of the oral epithelium. Due to the lack of reliable specific markers of CSCs (24), there are numerous unresolved issues regarding OSCCs (25).
Numerous distinct models attempt to explain the origin of CSCs. The most important hypothesis regarding the origin of CSCs is that they develop from the transformation of stem cells (SCs). This hypothesis is compelling for several reasons. The malignant transformation of a normal cell requires 3–6 oncogenic events, and the long life of SCs increases the risk of accumulating the multiple mutations (26). SCs and CSCs possessing the capacity for self-renewal is an additional reason to consider that the origin of CSCs is normal SCs, as the dysregulation of the self-renewal process is an early and important event in carcinogenesis. Although the hypothesis that CSCs derive from normal adult SCs appears to be the most plausible, other origins may also be possible. A CSC may originate from the fusion of a hemopoietic stem cell (HSC) with a mutated epithelial somatic cell. In 2004, Wagers and Weissman and Houghton et al demonstrated that the fusion of HSCs with epithelial cells has been shown in vitro and in vivo in animal models of stomach cancer (27,28). At present, the fusion of HSCs with epithelial cells has not been demonstrated in OSCC. A CSC may also result from the de-differentiation of a mature cell (29). Previous studies demonstrate that differentiated cancer cells may achieve a CSC-like state through epithelial mesenchymal transition (EMT). EMT is involved in the acquisition by differentiated cells of the properties of SCs, including in nasopharyngeal (30), breast (31) and head and neck squamous cell carcinomas (HNSCC) (32). A sparsely proliferative basal layer with highly proliferative parabasal cell layers in premalignant epithelia is another frequently observed proliferation pattern. The aforementioned observation may indicate that the tumor did not originate exclusively from a normal basal SC, but also from an amplifying transitory cell (33).
The scarcity of markers for identifying CSCs restricts the knowledge that studies may gain regarding the role of CSCs in carcinogenesis; therefore, the perfect identification technique does not yet exist. The most frequently applied method for identifying CSCs is flow cytometry, which detects cells with the ability to excrete the vital DNA dye Hoechst 33342 (34–38). A distinctive small non-dyed population of cells, termed the side population (SP), has been detected in numerous tumors, and is highly tumorigenic (39–44). SP cells express SCs markers, including cluster of differentiation (CD)44 (45), ATP-binding cassette sub-family G member 2 (44), octamer-binding transcription factor (Oct)4 (35) and B cell-specific Moloney murine leukemia virus integration site 1 proto-oncogene (45). The SP population ranges between 0.2–10.0% of the cancer cell population (35). Flow cytometry does not permit CSCs to be topographically localized in healthy or tumorous tissues for the assessment of proliferative activity or spatial associations with progeny. The transcription factors SOX2 (46,47), Oct3/4 (45), β-1 integrin (48), CD133 (49) and CD44 (50) demonstrate promise for the topographical localization of CSCs. The transcription factors SOX2 and Oct3/4 are essential for maintaining the self-renewal capacity and pluripotency of embryonic and adult SCs. CD133+ cells in OSCC form holoclones and possess self-renewal capacity (51), and CD44+ cells in HNSCC possess the capacity for self-renewal and differentiation (52). However, the usefulness of CD44 as a marker of CSCs in OSCC is questionable as CD44 is expressed by normal oral epithelial cells (53,54). The value of CD44 as a marker of OSCC progression and prognosis is also controversial. Certain studies in HNSCC have reported aldehyde dehydrogenase (ALDH)+ cells with typical CSC behavior, in particular the tumorigenic ability (55–58). The specificity of CD44 as a marker of CSCs in HNSCC is reported to be increased in combination with ALDH (55,56). Although a small number of ALDH+/CD44− CSCs exist (56). In addition, E-cadherin, CD97, CD117, CD147, CK19 and epithelial-specific antigen have been used in attempts to identify oral SCs/CSCs; however the antigens were not adequately specific (59–62). Notably, Miranda-Lorenzoan et al identified an intrinsic autoflorescent phenotype in CSCs from diverse epithelial cancers, and used the marker to isolate and characterize the CSCs (63).
SOX2 in OSCC
SOX2 is an important stem cell marker that is crucial for embryonic development and to maintain the differentiation potential of stem cells. SOX2 is one of the key transcription factors involved in inducing pluripotent stem cells. In the past decade, SOX2 has been established as one of the hallmark participants of the developmental process in cancer, including in skin squamous cell carcinoma, gastric cancer, glioblastoma, colorectal cancer, lung cancer, breast cancer and OSCC (3–9).
SOX2 is an amplified gene in OSCC, and the key role of SOX2 is to maintain the stemness of the cells. Nadja et al demonstrated that SOX2 amplifications are common in OSCC and the detection of SOX2 amplifications in the early stages of disease may be crucial for early disease detection and a more accurate prognosis (64). He et al revealed that the expression of SOX2 was amplified in OSCC and was significantly associated with the pathological grade (65). In addition, a significant difference in SOX2 staining was demonstrated between OSCC, oral epithelial dysplasia and normal oral mucosa. Previous studies showed that SOX2 was overexpressed in OSCCs, the expression of SOX2 was decreased in the CAL27 and UMSCC74A cell lines that were treated with cationic lipid nanoparticles to deliver pre-miR-107, and the tumorsphere formation efficiency and size were decreased (66–68). However, the mechanism by which miR-107 regulates SOX2 expression in HNSCC is unclear. Studies indicate that SOX2 is amplified in numerous types of tumors, which is associated with the indicators of a favorable prognosis (69). SOX2 overexpression has been demonstrated to deregulate genes in malignant processes, cellular migration and anchorage-independent growth (70).
SOX2 performs a similar role in CSCs to that in embryonic stem cells. In a previous study, SOX2 was preferentially expressed in cancer cells with a basal-like phenotype, and is, therefore, likely to contribute to defining the characteristics of less differentiated stem cell phenotypes (71). A similar phenomenon existed in studies on OSCCs and HNSCCs, as SOX2 was mainly expressed in the stratum basale, co-localizing with the region that contained stem cells (68,72). In addition to the application of SOX2 in identifying various subsets of tumor cells, SOX2 also maintains the stemness of tumor-initiating cells (TICs) and CSCs.
In a previous study, SOX2 knockdown in CSCs led to the inhibition of proliferation and loss of tumorigenicity in immunodeficient mice, indicating that SOX2 was crucial for maintaining the self-renewal capacity of CSCs (73). The role of SOX2 in maintaining the self-renewal capacity of CSCs in HNSCC and breast cancer has been previously confirmed (74,75). The findings of these studies indicate that SOX2 expression promotes and maintains the stemness of CSCs. Other important roles of SOX2 in cancer progression include the contribution to the physiological or pathophysiological process of cancer cells. The proliferation of HNSC CSCs was inhibited in vitro and in vivo, as SOX2 was suppressed by all-trans-retinoic acid (76). SOX2 expression has been demonstrated to improve the ability of invasion and migration of tumor cells in tongue squamous cell carcinoma (TSCC) (77). Other properties of cancer cells in OSCC or HNSCC that involve the SOX2 protein include apoptosis (74,78), chemoresistance (79), metastasis and tumorigenesis (10,79).
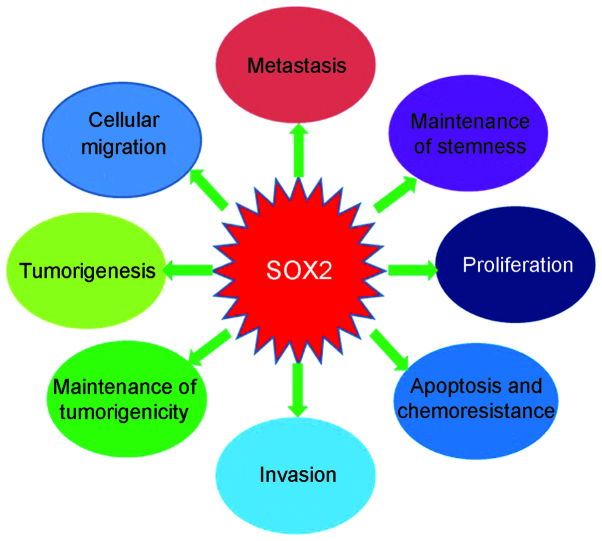
SOX2 is a key regulator of development and carcinogenesis and exhibited close associations with several microRNAs. In a previous study, the activities of SOX2 were indicated to be controlled by numerous microRNAs; therefore, certain microRNAs may also be regulated by SOX2, including microRNA-145 (80,81), microRNA-107 (67) and microRNA-302 (74). Bourguignon et al demonstrated that the stimulation of miR-302 expression by hyaluronan-CD44 is Oct4-SOX2-Nanog-dependent in HNSCC-specific CSCs, and that microRNA-302 expression was the underlying mechanism of self-renewal, clonal formation and cisplatin resistance in CSCs in HNSCC (74). The roles of SOX2 in cancers are summarized in Fig. 1.
Figure 1.
The SOX2 protein affects numerous processes of cancer cells. SOX2, Sex determining region Y-box 2
At present, several studies have revealed that SOX2 overexpression in cancer cells exhibited a deleterious outcome, and resulted in lower survival rates of patients. Several studies have revealed that the SOX2 proteins were overexpressed in the TSCC, and demonstrated that SOX2, recurrence and distant metastasis were independent prognostic factors of overall survival in patients with TSCC. Therefore the studies concluded that the expression of SOX2 may be used as a prognostic indicator of TSCC (82). SOX2 was exclusively expressed in the CSCs of OSCC patients and the expression of SOX2 was significantly associated with a poor prognosis of OSCC and lymph node status, which indicates the potential prognostic value of SOX2 in OSCC. SOX2 may also act as a promising marker for directing OSCC diagnosis and therapy (65). The evidence that SOX2 overexpression was oncogenic was also demonstrated in HNSCC patients, and SOX2 was indicated to be associated with a worse prognosis. These findings are valuable in providing useful information for developing a novel classification system and therapeutic strategies for HNSCC (64). However, the opposite viewpoint from certain studies supported that increased levels of SOX2 was significantly associated with better prognosis in the patients of OSCC and squamous cell lung cancer (69,79).
SOX2 was amplified in numerous types of cancer, including OSCC. However, the role of SOX2 in cancer is controversial. The vast majority of studies show that SOX2 overexpression may promote cancer progression, hypothesizing that SOX2 may act as a potential target for cancer therapy. However, certain studies hypothesized that SOX2 may suppress tumors and that SOX2 overexpression inhibits the cell proliferation. In addition, the opposite views of the association between SOX2 and the survival rate of patient were stated in other studies. The vast majority of studies showed that the survival rate of OSCC patients possessing low levels of SOX2 is increased compared with those with high levels of SOX2. However, the opposite view was indicated by Züllig et al, who supported that low level SOX2 expression was significantly associated with worse survival (79). Additional studies are required in order to understand the conflicting opinions. In conclusion, the overexpression of SOX2 derived from amplification promotes cancer progression and tumor formation. SOX2 may provide a novel diagnostic marker for OSCC, be used as a therapeutic stratification marker, or target molecules for therapeutic interference; however, the underlying molecular mechanism remains unclear (83).
3. Association between SOX2 and lymph node metastasis
Tumor metastasis is the key factor that compromises the prognosis of tumor patients, which accounts for 90% of tumor-associated mortalities (84,85). Metastasis is a multistep process by which a percentage of primary tumor cells acquire the ability to spread between the initial site and secondary tissues or organs, or the surrounding normal tissues (86–88). Failure at any stage may restrict the entire metastatic process. Since metastasis is responsible for the majority of mortalities of cancer patients, an improved understanding of the molecular mechanism involved in the tumor spreading process is crucial for preventing tumor metastasis and improving the prognosis of patients.
SOX2 has recently been shown to be a putative CSC marker in several malignancies, including glioblastoma (5) and gastric (3), colorectal (6), breast (8), oral (9) and lung (7,89) cancers. In addition, the importance of SOX2 in cancer metastasis has also been addressed. Previously, several studies demonstrated that SOX2 expression was closely associated with lymph node metastasis, distant spread and poor prognosis in colorectal carcinoma patients (6,90,91). Certain data suggested that SOX2 knockdown may induce mesenchymal-epithelial transition (MET). MET is the reverse process of EMT. Epithelial cells gain polarity and motility during EMT, which are necessary for tumor invasion and metastasis in various types of epithelial carcinomas. Notably, studies have identified that knocking down SOX2 promoted MET and resulted in the translocation of β-catenin, which is critical in the WNT pathway (92). Studies indicated that all SOX2-positive primary tumors retained a SOX2-expressing phenotype during lymph node metastasis. This finding may indicate that SOX2-bearing cancer cells have an increased probability and ability to metastasize to the lymph node and supports the notion that SOX2 is crucial for breast cancer invasiveness and spread. Notably, a significantly increased SOX2 expression in lymph node metastasis compared with respective primary tumors was indicated (93). A similar finding was also reported in the study conducted by Lengerke et al, in which the data suggest that SOX2 is important in early breast carcinogenesis and that increased expression may promote metastatic potential (8). Downregulation of SOX2 significantly decreased angiogenesis and lymphomagenesis of breast cancer. Additionally, the promotion effect of SOX2 on tumor cell metastasis was observed in vitro and in the tumor bearing mice in vivo. SOX2 also promoted the EMT process in the tumor cells in breast cancer by regulating the WNT/β-catenin signal pathway (94,95). A previous study on lung cancer indicated that the overexpression of SOX2 was also positively associated with the tumor-node-metastasis stage and lymph node metastasis (96).
SOX2 protein expression was indicated to be significantly associated with lymph node metastasis and recurrence in HNSCC patients (97). However, in numerous HNSCC patients, SOX2 expression was not associated with disease stage, lymph node metastasis or distant metastasis (98,99). The contradictory results may be associated with methodological variations in the SOX2 immunopositivity scoring (99). Therefore, additional studies on the oncogenic function of SOX2 in HNSCC and the underlying molecular mechanisms accounting for the association of SOX2 with progression remain to be elucidated.
Similarly to HNSCC, the studies on the association between SOX2 and lymph node metastasis in OSCC are inadequate. Certain data showed that the SOX2 may be classified into diffuse staining patterns and peripheral staining patterns, and that the SOX2 diffuse staining pattern was associated with lymph node metastasis of OSCC (10). Even in OTSCCs without lymph node metastasis, SOX2 expression has been demonstrated to be involved in tumor progression (9). An additional study indicated that the poor prognosis associated with lymph node metastasis was significantly associated with the high expression of SOX2 in the primary sites of OSCCs (10). Qiao et al supported the finding that SOX2 expression was significantly associated with lymph node metastasis (100). However, the study conducted by Züllig et al showed that increased expression levels of SOX2 were significantly associated with a lack of lymph node metastasis, which implies a good prognosis in OSCC patients, and indicated that the heterogeneity of primary tumors may be one of the reasons for controversial results (79). This result is consistent with certain findings in lung cancer (101). According to the data in the study by Züllig et al, SOX2 is a potential predictive marker for the lack of metastasis to the sentinel lymph nodes of the neck in early SCC of the oral cavity (79).
Overall, based on the phenomenon of overexpression of SOX2 in several types of cancer and the role of SOX2 in promoting cancer progression, the application of SOX2 is hypothesized to aid decisions on cancer diagnosis and therapy, and may even predict the prognosis of patients. However, underlying molecular mechanisms of SOX2 in OSCC remain to be elucidated. The association between the expression of SOX2 in OSCC and lymph node metastasis is remains unclear. The molecular mechanisms underlying the association between SOX2 and lymph node metastasis require additional studies.
References
- 1.Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 3.Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 4.Power DG, Kelsen DP, Shah MA. Advanced gastric cancer - slow but steady progress. Cancer Treat Rev. 2010;36:384–392. doi: 10.1016/j.ctrv.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Koyama-Nasu R, Haruta R, Nasu-Nishimura Y, Taniue K, Katou Y, Shirahige K, Todo T, Ino Y, Mukasa A, Saito N, et al. The pleiotrophin-ALK axis is required for tumorigenicity of glioblastoma stem cells. Oncogene. 2014;33:2236–2244. doi: 10.1038/onc.2013.168. [DOI] [PubMed] [Google Scholar]
- 6.Neumann J, Bahr F, Horst D, Kriegl L, Engel J, Luque RM, Gerhard M, Kirchner T, Jung A. SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer. 2011;11:518. doi: 10.1186/1471-2407-11-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drilon A, Rekhtman N, Ladanyi M, Paik P. Squamous-cell carcinomas of the lung: Emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol. 2012;13:e418–e426. doi: 10.1016/S1470-2045(12)70291-7. [DOI] [PubMed] [Google Scholar]
- 8.Lengerke C, Fehm T, Kurth R, Neubauer H, Scheble V, Müller F, Schneider F, Petersen K, Wallwiener D, Kanz L, et al. Expression of the embryonic stem cell marker SOX2 in early-stage breast carcinoma. BMC Cancer. 2011;11:42. doi: 10.1186/1471-2407-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du L, Yang Y, Xiao X, Wang C, Zhang X, Wang L, Zhang X, Li W, Zheng G, Wang S, Dong Z. SOX2 nuclear expression is closely associated with poor prognosis in patients with histologically node-negative oral tongue squamous cell carcinoma. Oral Oncol. 2011;47:709–713. doi: 10.1016/j.oraloncology.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Michifuri Y, Hirohashi Y, Torigoe T, Miyazaki A, Kobayashi J, Sasaki T, Fujino J, Asanuma H, Tamura Y, Nakamori K, et al. High expression of ALDH1 and SOX2 diffuse staining pattern of oral squamous cell carcinomas correlates to lymph node metastasis. Pathol Int. 2012;62:684–689. doi: 10.1111/j.1440-1827.2012.02851.x. [DOI] [PubMed] [Google Scholar]
- 11.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 12.Ren ZH, Wu HJ, Zhang S, Wang K, Gong ZJ, He ZJ, Peng J. A new surgical strategy for treatment of tongue squamous cell carcinoma based on anatomic study with preliminary clinical evaluation. J Craniomaxillofac Surg. 2015;43:1577–1582. doi: 10.1016/j.jcms.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Ren ZH, Wu HJ, Wang K, Zhang S, Tan HY, Gong ZJ. Anterolateral thigh myocutaneous flaps as the preferred flaps for reconstruction of oral and maxillofacial defects. J Craniomaxillofac Surg. 2014;42:1583–1589. doi: 10.1016/j.jcms.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Brinkman BM, Wong DT. Disease mechanism and biomarkers of oral squamous cell carcinoma. Curr Opin Oncol. 2006;18:228–233. doi: 10.1097/01.cco.0000219250.15041.f8. [DOI] [PubMed] [Google Scholar]
- 15.Guo BH, Feng Y, Zhang R, Xu LH, Li MZ, Kung HF, Song LB, Zeng MS. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol Cancer. 2011;10:10. doi: 10.1186/1476-4598-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren ZH, Wu HJ. Extracapsular spread in cervical lymph nodes. Shiyong Kou Qiang Yi Xue Za Zhi. 2012;28:514–517. (In Chinese) [Google Scholar]
- 17.El-Naaj IA, Leiser Y, Shveis M, Sabo E, Peled M. Incidence of oral cancer occult metastasis and survival of T1-T2N0 oral cancer patients. J Oral Maxillofac Surg. 2011;69:2674–2679. doi: 10.1016/j.joms.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitamura H, Torigoe T, Hirohashi Y, Asanuma H, Inoue R, Nishida S, Tanaka T, Fukuta F, Masumori N, Sato N, Tsukamoto T. Prognostic impact of the expression of ALDH1 and SOX2 in urothelial cancer of the upper urinary tract. Mod Pathol. 2013;26:117–124. doi: 10.1038/modpathol.2012.139. [DOI] [PubMed] [Google Scholar]
- 20.Teodorczyk M, Kleber S, Wollny D, Sefrin JP, Aykut B, Mateos A, Herhaus P, Sancho-Martinez I, Hill O, Gieffers C, et al. CD95 promotes metastatic spread via Sck in pancreatic ductal adenocarcinoma. Cell Death Differ. 2015;22:1192–1202. doi: 10.1038/cdd.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel HL, Gritsko T, Pursell B, Chang C, Shultz LD, Greiner DL, Norum JH, Toftgard R, Shaw LM, Mercurio AM. Regulated splicing of the α6 integrin cytoplasmic domain determines the fate of breastcancer stem cells. Cell Rep. 2014;7:747–761. doi: 10.1016/j.celrep.2014.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 23.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 24.González-Moles MA, Scully C, Ruiz-Ávila I, Plaza-Campillo JJ. The cancer stem cell hypothesis applied to oral carcinoma. Oral Oncol. 2013;49:738–746. doi: 10.1016/j.oraloncology.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Boman BM, Wicha MS. Cancer stem cells: A step toward the cure. J Clin Oncol. 2008;26:2795–2799. doi: 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- 26.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 27.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/S0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 28.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 29.Zhu AJ, Watt FM. Beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Y, Cheung AK, Ko JM, Phoon YP, Chiu PM, Lo PH, Waterman ML, Lung ML. Physiological β-catenin signaling controls self-renewal networks and generation of stem-like cells from nasopharyngeal carcinoma. BMC Cell Biol. 2013;14:44. doi: 10.1186/1471-2121-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Filho MS, Nör JE. The biology of head and neck cancer stem cells. Oral Oncol. 2012;48:1–9. doi: 10.1016/j.oraloncology.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González-Moles MA, Bravo M, Ruiz-Avila I, Acebal F, Gil-Montoya JA, Brener S, Esteban F. Ki-67 expression in non-tumour epithelium adjacent to oral cancer as risk marker for multiple oral tumours. Oral Dis. 2010;16:68–75. doi: 10.1111/j.1601-0825.2009.01611.x. [DOI] [PubMed] [Google Scholar]
- 34.Yu S, Zhang R, Liu F, Wang H, Wu J, Wang Y. Notch inhibition suppresses nasopharyngeal carcinoma by depleting cancer stem-like side population cells. Oncol Rep. 2012;28:561–566. doi: 10.3892/or.2012.1830. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, Zhang Y, Mao L, Zhang Z, Chen W. Side population in oral squamous cell carcinoma possesses tumor stem cell phenotypes. Cancer Lett. 2009;277:227–234. doi: 10.1016/j.canlet.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Song J, Chang I, Chen Z, Kang M, Wang CY. Characterization of side populations in HNSCC Highly invasive, chemoresistant and abnormal Wnt signaling. PLoS One. 2010;5:e11456. doi: 10.1371/journal.pone.0011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanamoto S, Kawasaki G, Yamada S, Yoshitomi I, Kawano T, Yonezawa H, Rokutanda S, Naruse T, Umeda M. Isolation and characterization of cancer stem-like side population cells in human oral cancer cells. Oral Oncol. 2011;47:855–860. doi: 10.1016/j.oraloncology.2011.06.501. [DOI] [PubMed] [Google Scholar]
- 38.Richard V, Nair MG, Santhosh Kumar TR, Pillai MR. Side population cells as prototype of chemoresistant, tumor-initiating cells. Biomed Res Int. 2013;2013:517237. doi: 10.1155/2013/517237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhai JM, Yin XY, Hou X, Hao XY, Cai JP, Liang LJ, Zhang LJ. Analysis of the genome-wide DNA methylation profile of side population cells in hepatocellular carcinoma. Dig Dis Sci. 2013;58:1934–1947. doi: 10.1007/s10620-013-2663-4. [DOI] [PubMed] [Google Scholar]
- 40.Broadley KW, Hunn MK, Farrand KJ, Price KM, Grasso C, Miller RJ, Hermans IF, McConnell MJ. Side population is not necessary or sufficient for a cancer stem cell phenotype in glioblastoma multiforme. Stem Cells. 2011;29:452–461. doi: 10.1002/stem.582. [DOI] [PubMed] [Google Scholar]
- 41.Guo D, Xu BL, Zhang XH, Dong MM. Cancer stem-like side population cells in the human nasopharyngeal carcinoma cell line cne-2 possess epithelial mesenchymal transition properties in association with metastasis. Oncol Rep. 2012;28:241–247. doi: 10.3892/or.2012.1781. [DOI] [PubMed] [Google Scholar]
- 42.Wei X, Dombkowski D, Meirelles K, Pieretti-Vanmarcke R, Szotek PP, Chang HL, Preffer FI, Mueller PR, Teixeira J, MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance preferentially inhibits stem/progenitors in human ovarian cancer cell lines compared with chemotherapeutics. Proc Natl Acad Sci USA. 2010;107:18874–18879. doi: 10.1073/pnas.1012667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CP, Zhou L, Xie M, Du HD, Tian J, Sun S, Li JY. Identification of cancer stem-like side population cells in purified primary cultured human laryngeal squamous cell carcinoma epithelia. PLoS One. 2013;8:e65750. doi: 10.1371/journal.pone.0065750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabor MH, Clay MR, Owen JH, Bradford CR, Carey TE, Wolf GT, Prince ME. Head and neck cancer stem cells: The side population. Laryngoscope. 2011;121:527–533. doi: 10.1002/lary.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mack B, Gires O. CD44s and CD44v6 expression in head and neck epithelia. PLoS One. 2008;3:e3360. doi: 10.1371/journal.pone.0003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keramari M, Razavi J, Ingman KA, Patsch C, Edenhofer F, Ward CM, Kimber SJ. SOX2 is essential for formation of trophectoderm in the preimplantation embryo. PLoS One. 2010;5:e13952. doi: 10.1371/journal.pone.0013952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okumura-Nakanishi S, Saito M, Niwa H, Ishikawa F. Oct-3/4 and SOX2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem. 2005;280:5307–5317. doi: 10.1074/jbc.M410015200. [DOI] [PubMed] [Google Scholar]
- 48.Evans RD, Perkins VC, Henry A, Stephens PE, Robinson MK, Watt FM. A tumor-associated beta 1 integrin mutation that abrogates epithelial dfferentiation control. J Cell Biol. 2003;160:589–596. doi: 10.1083/jcb.200209016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 50.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-A. [DOI] [PubMed] [Google Scholar]
- 51.Felthaus O, Ettl T, Gosau M, Driemel O, Brockhoff G, Reck A, Zeitler K, Hautmann M, Reichert TE, Schmalz G, Morsczeck C. Cancer stem cell-like cells from a single cell of oral squamous carcinoma cell lines. Biochem Biophys Res Commun. 2011;407:28–33. doi: 10.1016/j.bbrc.2011.02.084. [DOI] [PubMed] [Google Scholar]
- 52.Faber A, Barth C, Hörmann K, Kassner S, Schultz JD, Sommer U, Stern-Straeter J, Thorn C, Goessler UR. CD44 as a stem cell marker in head and neck squamous cell carcinoma. Oncol Rep. 2011;26:321–326. doi: 10.3892/or.2011.1322. [DOI] [PubMed] [Google Scholar]
- 53.Oliveira LR, Oliveira-Costa JP, Araujo IM, Soave DF, Zanetti JS, Soares FA, Zucoloto S, Ribeiro-Silva A. Cancer stem cell immunophenotypes in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:135–142. doi: 10.1111/j.1600-0714.2010.00967.x. [DOI] [PubMed] [Google Scholar]
- 54.Grimm M, Alexander D, Munz A, Hoffmann J, Reinert S. Is 1,25-dihydroxyvitamin D3 receptor expression a potential Achilles' heel of CD44+ oral squamous cell carcinoma cells? Target Oncol. 2013;8:189–201. doi: 10.1007/s11523-013-0255-z. [DOI] [PubMed] [Google Scholar]
- 55.Nishikawa S, Konno M, Hamabe A, Hasegawa S, Kano Y, Ohta K, Fukusumi T, Sakai D, Kudo T, Haraguchi N, et al. Aldehyde dehydrogenase high gastric cancer stem cells are resistant to chemotherapy. Int J Oncol. 2013;42:1437–1442. doi: 10.3892/ijo.2013.1837. [DOI] [PubMed] [Google Scholar]
- 56.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nör JE. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70:9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z, Dong Z, Lauxen IS, Filho MS, Nör JE. Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Res. 2014;74:2869–2881. doi: 10.1158/0008-5472.CAN-13-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richard V, Pillai MR. The stem cell code in oral epithelial tumorigenesis: 'The cancer stem cell shift hypothesis'. Biochim Biophys Acta. 2010;1806:146–162. doi: 10.1016/j.bbcan.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Allegra E, Trapasso S, Pisani D, Puzzo L. The role of BMI1 as a biomarker of cancer stem cells in head and neck cancer: A review. Oncology. 2014;86:199–205. doi: 10.1159/000358598. [DOI] [PubMed] [Google Scholar]
- 61.Zhou ZT, Jiang WW. Cancer stem cell model in oral squamous cell carcinoma. Curr Stem Cell Res Ther. 2008;3:17–20. doi: 10.2174/157488808783489426. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi S, Tanaka J, Okada S, Isobe T, Yamamoto G, Yasuhara R, Irie T, Akiyama C, Kohno Y, Tachikawa T, Mishima K. Lin28a is a putative factor in regulating cancer stem cell-like properties in side population cells of oral squamous cell carcinoma. Exp Cell Res. 2013;319:1220–1228. doi: 10.1016/j.yexcr.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Miranda-Lorenzo I, Dorado J, Lonardo E, Alcala S, Serrano AG, Clausell-Tormos J, Cioffi M, Megias D, Zagorac S, Balic A, et al. Intracellular autofluorescence: A biomarker for epithelial cancer stem cells. Nat Methods. 2014;11:1161–1169. doi: 10.1038/nmeth.3112. [DOI] [PubMed] [Google Scholar]
- 64.Kokalj Vokač N, Cizmarević B, Zagorac A, Zagradišnik B, Lanišnik B. An evaluation of SOX2 and hTERC gene amplifications as screening markers in oral and oropharyngeal squamous cell carcinomas. Mol Cytogenet. 2014;7:5. doi: 10.1186/1755-8166-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He KF, Zhang L, Huang CF, Ma SR, Wang YF, Wang WM, Zhao ZL, Liu B, Zhao YF, Zhang WF, Sun ZJ. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed Res Int. 2014;2014:838632. doi: 10.1155/2014/838632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Albers AE. Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One. 2011;6:e16466. doi: 10.1371/journal.pone.0016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piao L, Zhang M, Datta J, Xie X, Su T, Li H, Teknos TN, Pan Q. Lipid-based nanoparticle delivery of Pre-miR-107 inhibits the tumorigenicity of head and neck squamous cell carcinoma. Mol Ther. 2012;20:1261–1269. doi: 10.1038/mt.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walter V, Yin X, Wilkerson MD, Cabanski CR, Zhao N, Du Y, Ang MK, Hayward MC, Salazar AH, Hoadley KA, et al. Correction: Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS One. 2014;8:e56823. doi: 10.1371/journal.pone.0056823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilbertz T, Wagner P, Petersen K, Stiedl AC, Scheble VJ, Maier S, Reischl M, Mikut R, Altorki NK, Moch H, et al. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol. 2011;24:944–953. doi: 10.1038/modpathol.2011.49. [DOI] [PubMed] [Google Scholar]
- 70.Hussenet T, Dali S, Exinger J, et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One. 2010;5:e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez-Pinilla SM, Sarrio D, Moreno-Bueno G, Rodriguez-Gil Y, Martinez MA, Hernandez L, Hardisson D, Reis-Filho JS, Palacios J. Sox2: A possible driver of the basal-like phenotype in sporadic breast cancer. Mod Pathol. 2007;20:474–481. doi: 10.1038/modpathol.3800760. [DOI] [PubMed] [Google Scholar]
- 72.Lu W, Feng F, Xu J, et al. QKI impairs self-renewal and tumorigenicity of oral cancer cells via repression of SOX2. Cancer Biol Ther. 2014;15:1174–1184. doi: 10.4161/cbt.29502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hägerstrand D, He X, Bradic Lindh M, Hoefs S, Hesselager G, Ostman A, Nistér M. Identification of a SOX2-dependent subset of tumor - and sphere-forming glioblastoma cells with a distinct tyrosine kinase inhibitor sensitivity profile. Neuro Oncol. 2011;13:1178–1191. doi: 10.1093/neuonc/nor113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bourguignon LY, Wong G, Earle C, Chen L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2012;287:32800–32824. doi: 10.1074/jbc.M111.308528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leis O, Eguiara A, Lopez-Arribillaga E, Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R, Martin AG. SOX2 expression in breast tumours and activation in breast cancer stem cells. Oncogene. 2012;31:1354–1365. doi: 10.1038/onc.2011.338. [DOI] [PubMed] [Google Scholar]
- 76.Lim YC, Kang HJ, Kim YS, Choi EC. All-trans-retinoic acid inhibits growth of head and neck cancer stem cells by suppression of Wnt/β-catenin pathway. Eur J Cancer. 2012;48:3310–3318. doi: 10.1016/j.ejca.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 77.Sun Y, Han J, Lu Y, Yang X, Fan M. Biological characteristics of a cell subpopulation in tongue squamous cell carcinoma. Oral Dis. 2012;18:169–177. doi: 10.1111/j.1601-0825.2011.01860.x. [DOI] [PubMed] [Google Scholar]
- 78.Lee SH, Nam HJ, Kang HJ, Kwon HW, Lim YC. Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. Eur J Cancer. 2013;49:3210–3218. doi: 10.1016/j.ejca.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 79.Züllig L, Roessle M, Weber C, Graf N, Haerle SK, Jochum W, Stoeckli SJ, Moch H, Huber GF. High sex determining region Y-box 2 expression is a negative predictor of occult lymph node metastasis in early squamous cell carcinomas of the oral cavity. Eur J Cancer. 2013;49:1915–1922. doi: 10.1016/j.ejca.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 80.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 81.Liu T, Cheng W, Huang Y, Huang Q, Jiang L, Guo L. Human amniotic epithelial cell feeder layers maintain human iPS cell pluripotency via inhibited endogenous microRNA-145 and increased SOX2 expression. Exp Cell Res. 2012;318:424–434. doi: 10.1016/j.yexcr.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 82.Huang CF, Xu XR, Wu TF, Sun ZJ, Zhang WF. Correlation of ALDH1, CD44, OCT4 and SOX2 in tongue squamous cell carcinoma and their association with disease progression and prognosis. J Oral Pathol Med. 2014;43:492–498. doi: 10.1111/jop.12159. [DOI] [PubMed] [Google Scholar]
- 83.Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X. The multiple roles for SOX2 in stem cell maintenance and tumorigenesis. Cell Signal. 2013;25:1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Floor SL, Dumont JE, Maenhaut C, Raspe E. Hallmarks of cancer: Of all cancer cells, all the time? Trends Mol Med. 2012;18:509–515. doi: 10.1016/j.molmed.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 85.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 86.Langley RR, Fidler IJ. The seed and soil hypothesis revisited - the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Comen EA. Tracking the seed and tending the soil: Evolving concepts in metastatic breast cancer. Discov Med. 2012;14:97–104. [PubMed] [Google Scholar]
- 88.Comen E, Norton L, Massagué J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8:369–377. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]
- 89.Nakatsugawa M, Takahashi A, Hirohashi Y, Torigoe T, Inoda S, Murase M, Asanuma H, Tamura Y, Morita R, Michifuri Y, et al. SOX2 is overexpressed in stem-like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest. 2011;91:1796–1804. doi: 10.1038/labinvest.2011.140. [DOI] [PubMed] [Google Scholar]
- 90.Lee HJ, Eom DW, Kang GH, Han SH, Cheon GJ, Oh HS, Han KH, Ahn HJ, Jang HJ, Han MS. Colorectal micropapillary carcinomas are associated with poor prognosis and enriched in markers of stem cells. Mod Pathol. 2013;26:1123–1131. doi: 10.1038/modpathol.2012.163. [DOI] [PubMed] [Google Scholar]
- 91.Hu H, Chang DT, Nikiforova MN, Kuan SF, Pai RK. Clinicopathologic features of synchronous colorectal carcinoma: A distinct subset arising from multiple sessile serrated adenomas and associated with high levels of microsatellite instability and favorable prognosis. Am J Surg Pathol. 2013;37:1660–1670. doi: 10.1097/PAS.0b013e31829623b8. [DOI] [PubMed] [Google Scholar]
- 92.Han X, Fang X, Lou X, Hua D, Ding W, Foltz G, Hood L, Yuan Y, Lin B. Silencing SOX2 induced mesenchymal-epithelial transition and its expression predicts liver and lymph node metastasis of CRC patients. PLoS One. 2012;7:e41335. doi: 10.1371/journal.pone.0041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abd El-Maqsoud NM, Abd El-Rehim DM. Clinicopathologic implications of EpCAM and SOX2 expression in breast cancer. Clin Breast Cancer. 2014;14:e1–e9. doi: 10.1016/j.clbc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T, Liu Y, Li X, Xiang R, Li N. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/β-catenin signal network. Cancer Lett. 2013;336:379–389. doi: 10.1016/j.canlet.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 95.Li X, Chen S, Sun T, Xu Y, Chen Y, Liu Y, Xiang R, Li N. The transcriptional regulation of SOX2 on FOXA1 gene and its application in diagnosis of human breast and lung cancers. Clin Lab. 2014;60:909–918. doi: 10.7754/clin.lab.2013.130437. [DOI] [PubMed] [Google Scholar]
- 96.Yang F, Gao Y, Geng J, Qu D, Han Q, Qi J, Chen G. Elevated expression of SOX2 and FGFR1 in correlation with poor prognosis in patients with small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2846–2854. [PMC free article] [PubMed] [Google Scholar]
- 97.Tang XB, Shen XH, Li L, Zhang YF, Chen GQ. SOX2 overexpression correlates with poor prognosis in laryngeal squamous cell carcinoma. Auris Nasus Larynx. 2013;40:481–486. doi: 10.1016/j.anl.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 98.Schröck A, Göke F, Wagner P, Bode M, Franzen A, Braun M, Huss S, Agaimy A, Ihrler S, Menon R, et al. Sex determining region Y-box 2 (SOX2) amplification is an independent indicator of disease recurrence in sinonasal cancer. PLoS One. 2013;8:e59201. doi: 10.1371/journal.pone.0059201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.González-Márquez R, Llorente JL, Rodrigo JP, García-Pedrero JM, Álvarez-Marcos C, Suárez C, Hermsen MA. SOX2 expression in hypopharyngeal, laryngeal, and sinonasal squamous cell carcinoma. Hum Pathol. 2014;45:851–857. doi: 10.1016/j.humpath.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 100.Qiao B, He B, Cai J, Yang W. The expression profile of Oct4 and SOX2 in the carcinogenesis of oral mucosa. Int J Clin Exp Pathol. 2014;7:28–37. [PMC free article] [PubMed] [Google Scholar]
- 101.Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL, Onaitis MW. Evidence that SOX2 overexpression is oncogenic in the lung. PLoS One. 2010;5:e11022. doi: 10.1371/journal.pone.0011022. [DOI] [PMC free article] [PubMed] [Google Scholar]