Abstract
Due to frequent phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway dysregulation, AKT is typically accepted as a promising anticancer therapeutic target. mTOR, in particular, represents a suitable therapeutic target for hepatocellular carcinoma, whilst suppressor with morphogenetic effect on genitalia family member-1 (SMG-1) is believed to serve a potential tumor suppressor role in human cancer. Despite SMG-1 and mTOR belonging to the same PI3K-related kinase family, the interactions between them are not yet fully understood. In the present study, a novel pyrrolopyrimidine-derived compound, AZD5363, was observed to suppress proliferation in liver cancer Hep-G2 and Huh-7 cells by inhibiting the phosphorylation of downstream molecules in the AKT signal pathway, in a dose- and time-dependent manner. AZD5363 activated the phosphorylation of mTOR, dependent on the liver cancer cell type, as it may have differing effects in various liver cancer cell lines. Additionally, AZD5363 also activated SMG-1 within the same liver cancer cells types, which subsequently activated the phosphorylation of mTOR. In conclusion, the present study indicates that AZD5363 inhibited phosphorylation of AKT downstream molecules, and activated phosphorylation of mTOR and SMG-1, dependent on the liver cancer type.
Keywords: AZD5363, liver cancer, AKT, mTOR, SMG-1
Introduction
Liver cancer is one of the most prevalent solid tumors worldwide, with a poor prognosis and limited treatment options available (1,2); it is also the fifth most frequently diagnosed cancer and the third leading cause of cancer mortality worldwide (3). Liver cancer is well characterized as a highly refractory disease, with high levels of tumor progression and recurrence. The prognosis of advanced hepatocellular carcinoma (HCC) remains poor and no effective systemic therapy has yet been developed (4). The traditional treatment methods, including surgery, radiofrequency ablation therapy and chemotherapy, focus on reducing the bulk of the tumor mass, however, they are limited by drug resistance, various side effects and metastasis to other organs (5). Thus, there is a significant requirement to expand our understanding of the molecular mechanisms underlying liver cancer in order to develop novel therapeutic strategies. Currently, targeted therapy is considered a more effective therapeutic strategy and is receiving an increasing level of attention.
AKT has a wide range of effects on cellular signaling and has been accepted as a promising anticancer target, including for the treatment of liver cancer (6). AKT is a serine/threonine protein kinase that serves a central role in the signaling network of phosphoinositide 3-kinase (PI3K) and the mammalian target of rapamycin (mTOR). Accumulating evidence indicates that this pathway controls key cellular processes, including glucose metabolism, apoptosis, survival, cell proliferation, cell migration, transcription and angiogenesis (7,8). Under normal conditions, this signaling network may be activated by numerous receptors, including members of the epidermal growth factor receptor and vascular endothelial growth factor receptor families and their ligands. The importance of the PI3K/AKT/mTOR signaling pathway in liver cancer is underlined by the finding that mTOR inhibition can suppress HCC growth in vitro and in xenograft models (9). Additionally, either the loss of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) function or the overexpression/activation of AKT leads to HCC development in mouse models (10,11). Aberrant mTOR signaling has been detected in ~48% of HCCs (9), with a negative feedback loop, resulting in the activation of AKT following mTOR inhibition, being observed in a variety of cancer cell lines and human tumor samples of colon and breast cancer (12). Suppressor with morphogenetic effect on genitalia family member-1 (SMG-1) and mTOR each belong to the PI3K-related kinase (PIKK) family. Recently, the level of awareness regarding SMG-1 has increased due to evidence indicating that it is likely to be a potential human tumor suppressor gene product (13).
Several types of small molecular AKT inhibitors have been studied, including ATP-competitive protein kinase inhibitors [A-443654, GSK690693 and GDC0068 (14–16)], phosphatidylinositol-3,4,5-trisphosphate (PIP3) binding inhibitors [perifosine (17)] and allosteric inhibitors [MK-2206 (18)]. In order to evaluate the mechanism of AZD5363 suppression of cell proliferation and migration in liver cancer, the present study investigated the effect of AZD5363, a novel AKT inhibitor, on the phosphorylation of AKT downstream molecules, and on the SMG-1 and mTOR pathways.
Materials and methods
Inhibitor preparation
AZD5363 [(S)-4-amino-N-[1-(4-chlorophenyl)-3-hydroxypropyl]-1(7H-pyrrolo[2,3-d] pyrimidin-4-yl) piperidine-4-carboxamide (#S8019; Selleck Chemicals, Houston, TX, USA), a novel AKT inhibitor, was prepared as a 100 mM stock solution in dimethyl sulfoxide (DMSO) and stored at −80°C. The final concentration of DMSO was <0.5% in all the assays.
Cell culture reagents
Human liver cancer Hep-G2 and Huh-7 cell lines were obtained from Shanghai Saiqi Biological Engineering Co., Ltd. (Shanghai, China). The study protocol was approved by the Ethical Committee of the Shandong Provincial Hospital Affiliated to Shandong University. All cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 1% penicillin-streptomycin solution and 1% non-essential amino acids. All cells were maintained in a humidified incubator with 5% CO2 at 37°C. The structure and synthesis of the AKT inhibitor, AZD5363, has been described previously (19).
Cell counting kit-8 (CCK-8) assay
The cell growth rate was measured using CCK-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Briefly, cells seeded at 1,000–2,000/per well in 96-well plates were cultured overnight with 90 µl DMEM, containing 10% FBS, and were subsequently treated with AZD5363 at varying concentrations (5 to 30 µM) for 24, 48 and 72 h. CCK-8 One Solution Reagent (Dojindo Molecular Technologies) was added to each well according to the manufacturer's protocols. Following a total of 1.5 h in culture, the cell viability was determined by measuring the absorbance at 450 nm.
Transwell migration assay
Monolayers of serum-starved adherent cells were trypsinized (0.25% Trypsin-EDTA; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and 50,000 cell suspensions [counted using a cell counting board (Bio-Rad Laboratories, Inc., Hercules, CA, USA)] were placed in 200 µl serum-free DMEM into the upper well of Transwell filter apparatus (Corning Inc., Corning, NY, USA). The filter was suspended within a well of a 24-well plate (NEST Biotechnology Co., Ltd., Wuxi, China) and the lower reservoir was filled with 800 µl DMEM, containing 10% FBS. The cells were then incubated under normal conditions for 24 h. Migration assays were terminated by retrieving the filter and rubbing off non-migrated cells from the top surface, which was then treated with formaldehyde (Far Eastern Group, Laiyang, China), methanol (Far Eastern Group) and Giemsa (Beijing Seajet Scientific, Inc., Beijing, China) staining. The cells that were identified on the underside of the filter were fixed, stained with Giemsa and captured under a microscope (model no. BX51; Olympus Corporation, Tokyo, Japan) using a digital camera (Microshot MC55, Guangzhou Mingmei Photoelectric Technology Co. Ltd., Guangzhou, China). The cells were counted in 3 randomly selected fields for each chamber.
Western blot analysis
Protein was extracted in a 6-well plate, using a mixture of radioimmunoprecipitation assay (RIPA) and phenylmethylsulfonyl fluoride (PMSF) (RIPA:PMSF, 100:1; Beyotime Institute of Biotechnology, Haimen, China), and protein concentrations were quantified by the Pierce bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). The soluble proteins (50 µg) were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE; Bio-Rad Laboratories, Inc.), followed by immunoblotting. The proteins were separated electrophoretically in SDS-polyacrylamide gels and transferred to negative control membranes. Subsequently, the proteins were incubated at 4°C overnight with a primary antibody and incubated with HRP-conjugated secondary antibody at room temperature for a total of 2 h. Immunoreactivity was detected using the FluorChem E system (ProteinSimple, Santa Clara, CA, USA) according to the manufacturer's protocols. The majority of antibodies used were obtained from Cell Signaling Technology Inc. (Danvers, MA, USA), including rabbit monoclonal phosphor-mTOR (ser2448; D9C2; catalog no., 5536S; dilution, 1:1,000), rabbit monoclonal phosphor-Akt (Thr450; D5G4; dilution, 1:1,000; catalog no., 12178), rabbit monoclonal phosphor-glycogen synthase kinase 3β (GSK-3β; ser9; 5B3; catalog no., 9323; dilution, 1:1,000), rabbit monoclonal mTOR (7C10; catalog no., 2983S; dilution, 1:1,000), mouse polyclonal AKT (catalog. no., 9272; dilution, 1:1,000), rabbit monoclonal GSK-3β (27C10; catalog no., 9315; dilution, 1:1,000) and rabbit monoclonal SMG-1 (Q25; catalog. no., 4993s; dilution, 1:1,000). Glyceraldehyde 3-phosphate dehydrogenase (catalog no., TA-08; dilution, 1:1,000) was purchased from ZSGB-BIO (Beijing, China), as was the secondary monoclonal antibodies: Peroxidase-conjugated AffiniPure goat anti-mouse immunoglobulin (Ig)G (H+L; catalog no., ZB-2305; dilution, 1:1,5000) and peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H+L; catalog no., ZB-2301; dilution, 1:5,000).
Statistical analysis
Statistical analysis was performed using SPSS software, version 18.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism software, version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). The data are presented as the mean ± standard error of the mean. The differences between groups were analyzed using one-way analysis of variance, followed by Student-Newman-Keuls post hoc test for pairwise comparison. P<0.05 was considered to indicate a statistically significant difference.
Results
AZD5363 inhibits the proliferation of liver cancer cells in a dose- and time-dependent manner
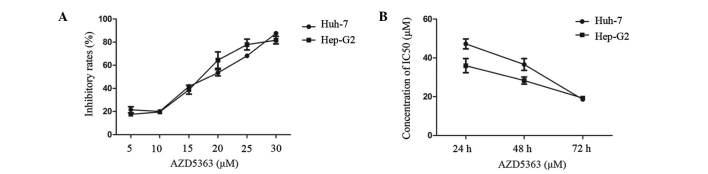
To determine the effect of AZD5363 on cell proliferation, a panel of two liver cancer cell lines was tested for anti-proliferative sensitivity using an in vitro cell growth assay. The Hep-G2 and Huh-7 cells were exposed to AZD5363 at different concentrations, ranging from 5 to 30 µM. The cells seeded in 96-well plates were cultured overnight, and were subsequently treated with AZD5363 at varying concentrations for 24, 48 and 72 h. The cell growth rate was measured using a CCK-8 assay. A dose-dependent inhibition of cell viability was observed in each cell line (Fig. 1A and B). The drug concentration required for inhibition of growth in the two liver cancer cell lines was similar, functioning in a dose- and time-dependent manner. The drug concentration of the half maximal inhibitory concentration (IC50) was lower when prolonging the cell exposure; when the exposure time reached 72 h, the IC50 of the Hep-G2 cells was 18.476 µM and the IC50 of the Huh-7 cells was 17.80 µM. In order to obtain a better inhibition curve, a higher density of Huh-7 than Hep-G2 was required. These results indicated that the AZD5363 activity was selective for the liver cancer cells.
Figure 1.
AZD5363 inhibits the proliferation of liver cancer cells. (A) The HepG2 and Huh7 cells were exposed to AZD5363 at varying concentrations (range, 5–30 µM). The cells were then cultured for 72 h, and the inhibitory rate was measured by a CCK-8 assay. Dose-dependent inhibition of each cell line was noted. (B) Concentrations of IC50 that the two cell lines were exposed to for the different time periods of AZD5363 exposure. IC50, half maximal inhibitory concentration; CCK-8, cell counting kit 8.
AZD5363 suppresses liver cancer cell migration
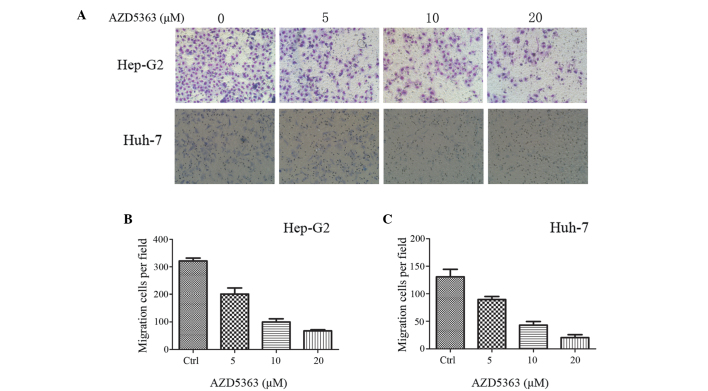
To investigate the effect of AZD5363 on liver cancer cell migration, a Transwell migration assay was performed. The treated Hep-G2 and Huh-7 cells, were inoculated in the upper chamber with serum-free medium, whilst the lower chamber held medium-containing serum. The cells were cultured for 24 h, and were subsequently treated with formaldehyde, methanol and Giemsa staining. As presented in Fig. 2, AZD5363 significantly reduced the activity of the Hep-G2 and Huh-7 cells.
Figure 2.
AZD5363 inhibits liver cancer cell migration. (A) Transwell migration assays of the Hep-G2 and Huh-7 cells with varied concentrations of AZD5363. Cells on the lower surface were fixed, stained with Giemsa and counted under a microscope. A total of three fields were randomly selected from the same side (the right) or each chamber in order to count the stained cells. Magnification, ×400. AZD5363 reduced the migration of the (B) Hep-G2 and (C) Huh-7 cells in a dose-and time-dependent manner. The values reported represent the mean ± standard error of the mean. Ctrl, control.
AZD5363 inhibits phosphorylation of AKT substrates
AKT functions in cell survival signaling by phosphorylating downstream targets, with dephosphorylation of these substrates indicating the inhibition of AKT activity (19). AKT serves a key role in glucose metabolism; its substrate, GSK3β, modulates glycogen synthesis and glucose transporter function. The present study therefore investigated whether AZD5363 inhibits the phosphorylation of AKT substrates. As expected, AZD5363 inhibited the phosphorylation of GSK3β, but increased the phosphorylation of AKT in a dose- and time-dependent manner in the Hep-G2 and Huh-7 cells (Fig. 3; P<0.05).
Figure 3.
AZD5363 inhibits the phosphorylation of AKT substrates. AZD5363 inhibited the phosphorylation of GSK3β, but increased the phosphorylation of AKT in a time-dependent manner in the (A) Hep-G2 and (B) Huh-7 cells. AZD5363 also inhibited the phosphorylation of GSK3β, but increased the phosphorylation of AKT in a concentration-dependent manner in the (C) Hep-G2 and (D) Huh-7 cells. pAKT, phosphorylated AKT; GSK3β, glycogen synthase kinase 3β; pGSK3β, phosphorylated GSK3β; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
AZD5363 activates the phosphorylation of mTOR, dependent on liver cancer cell type
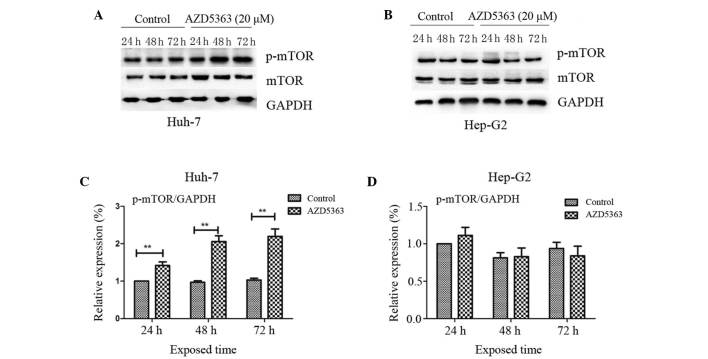
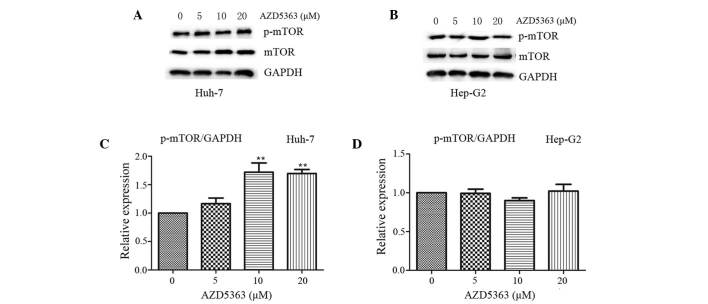
mTOR, a 289-kDa serine/threonine protein kinase, belongs to the PIKK family and is activated through the PI3K and AKT signaling pathways via phosphorylation of specific residues; once activated, mTOR mediates transcription, cytoskeleton organization, cell growth and cell survival (20,21). To investigate the effect of AZD5363 on the mTOR pathway, the phosphorylation levels of mTOR were analyzed. In contrast to the inhibited phosphorylation of AKT substrates, AZD5363 exhibited reduced activity in the mTOR pathway, as presented in panels of tumor cell lines in vitro. AZD5363 enhanced the phosphorylation of mTOR, however, this was only observed in the Huh-7 cells. This indicated that AZD5363 significantly stimulated mTOR signaling, but that this was dependent on liver cancer cell type (Fig.4 and 5; P<0.01).
Figure 4.
AZD5363 activates the phosphorylation of mTOR dependent on liver cancer cell type. AZD5363 activated the phosphorylation of mTOR in (A) the Huh-7 cells in a time-dependent manner, but not in (B) the Hep-G2 cells. Expression levels were quantified using a gel imaging scan and AZD5363 significantly stimulated mTOR signaling in (C) the Huh-7 cells, but not in (D) the Hep-G2 cells. The values are reported as the mean ± standard error of the mean. **P<0.01. mTOR, mammalian target of rapamycin; p-mTOR, phosphorylated mTOR; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Figure 5.
AZD5363 activates the phosphorylation of mTOR dependent of liver cancer cell type. AZD5363 activated the phosphorylation of mTOR in (A) the Huh-7 cells in a dose-dependent manner, but this was not observed in (B) the Hep-G2 cells. The expression levels in the (C) Huh-7 and (D) Hep-G2 cells were quantified using a gel imaging scan. The values are reported as the mean ± standard error of the mean. **P<0.01. mTOR, mammalian target of rapamycin; pmTOR, phosphorylated mTOR; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
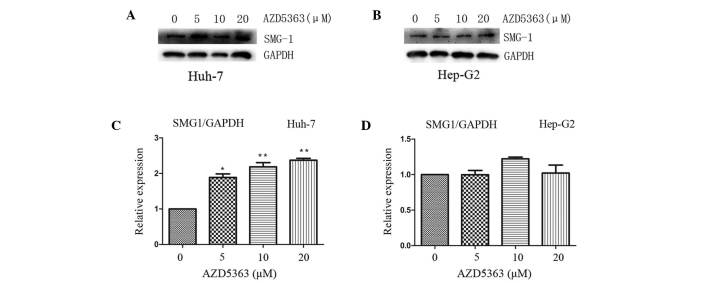
AZD5363 activates SMG-1, dependent on liver cancer cell type
SMG-1 and mTOR each belong to the PIKK family. Yamashita et al (22) reported that the nonsense-mediated mRNA decay (NMD) pathway was suppressed following inhibition of SMG-1. A follow-up study confirmed that the phosphorylation of up-frameshift protein 1 (Upf1), via SMG-1, is an important step required to trigger the NMD reaction (23). Therefore, SMG-1 may combine with Upf1 to promote its phosphorylation, subsequently forming the SMG-1-Upf1-eRF1-eRF3 surveillance complex. The phosphorylation of Upfl recruits SMG-5/SMG-7 composites and leads to the degradation of nonsense mRNA (24,25). To investigate the effect of AZD5363 on SMG-1 in the present study, the levels of SMG-1 were analyzed. It was observed that AZD5363 promoted the expression of SMG-1 in the Huh-7 cells, but not in the Hep-G2 cells (Fig. 6 and 7; P<0.01). However, the mechanism by which AZD5363 induced SMG-1 was different from that of the AKT signaling; furthermore, it was observed that the effect became more marked with an increasing dose and time of exposure, which indicated that AZD5363 activated SMG-1 in a dose-and time-dependent manner (Figs. 6 and 7).
Figure 6.
AZD5363 activates SMG-1 dependent of liver cancer cell type. AZD5363 activated the expression of SMG-1 in (A) the Huh-7 cells in a time-dependent manner, but this was not observed in (B) the Hep-G2 cells. The expression levels in the (C) Huh-7 and (D) Hep-G2 cells were quantified using a gel imaging scan. The values are reported as the mean ± standard error of the mean. *P<0.005 and **P<0.001. SMG-1, suppressor with morphogenetic effect on genitalia family member-1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Figure 7.
AZD5363 activates SMG-1 dependent on liver cancer cell type. AZD5363 activated the expression of SMG-1 in (A) the Huh-7 cells in a dose-dependent manner, but this was not observed in (B) the Hep-G2 cells. The expression levels in the (C) Huh-7 and (D) Hep-G2 cells were quantified using a gel imaging scan. The values are reported as the mean ± standard error of the mean. *P<0.01 and **P<0.005. SMG-1, suppressor with morphogenetic effect on genitalia family member-1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Emerging evidence supports a crucial role for the PI3K-AKT-mTOR pathway in tumorigenesis and drug resistance. Regarding the treatment of solid tumors, including liver cancer, a number of novel small molecule inhibitors that target PI3K, AKT and mTOR are currently at varying phases of drug development. However, the detailed effects that AZD5363 may exert on this signaling pathway remain an unresolved issue. In the present study, the novel AKT kinase inhibitor, AZD5363, exhibited strong specificity for the AKT kinase in Hep-G2 and Huh-7 cells. AZD5363 suppressed proliferation through the inhibition of AKT kinase activity in vitro.
Recently, numerous studies have demonstrated that AKT inhibitors may activate varying feedback loops, subsequently affecting the efficacy of AKT inhibitors, including the MAPK and HER3 pathways (26,27). A number of feedback loops and layers of cross-talk have been reported to connect the mTOR complex 1 (mTORC1) and PI3K/AKT pathways. The proximal mTORC1 activator, Ras homolog enriched in brain, is involved in the direct inhibition of C-Raf activity and B-Raf/C-Raf heterodimerization, highlighting the complexity of the connections among the mTORC1 and PI3K/AKT pathways (28). In the present study, however, the mechanism by which AZD5363 induced mTOR signaling was different from that of the AKT depleted situation. It was observed that while each cell line was sensitive to AZD5363, they exhibited different reactions to the mTOR pathway. AZD5363 activated phosphorylation of mTOR only in the Huh-7 cells. Nonetheless, the explicit mechanism between mTOR and PI3K/AKT pathway activation by AZD5363 remains elusive.
A number of studies focused on the development of inhibitors of the mTOR signaling pathway are currently in progress (29). In contrast to inhibitors of the mTOR kinase (19,30), a recent study has reported that AZD5363 exerts a reduced range of activity in panels of tumor cell lines in vitro, for which a drug concentration required to reduce growth rates to 50% of the maximum rate (GI50) value of 3 µM was used as a cutoff; only 41 of 182 (23%) of the cell lines were sensitive to AZD5363 (31). Furthermore, the same study also observed that these tumor types, which have a high frequency of phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α (PIK3CA) mutation and PTEN loss, appear to have more contact with AKT signaling and are sensitive to monotherapy inhibition by AZD5363. In the present study, the Hep-G2 and Huh-7 cells also exhibited differing reactions to mTOR kinase. Therefore, to determine whether the liver cancer cells have these characteristics (including HER2 amplification, RAS mutations, PIK3CA mutation and PTEN loss) is necessary. It follows that certain tumor types may be enriched for responders to an AKT inhibitor, such as AZD5363, whereas in cell types that are insensitive to a specific AKT inhibitor, targeting the AKT pathway alone is not sufficient enough to eradicate cancer.
SMG-1 is a member of the PIKK family of mammalian genes that includes mTOR, ataxia telangiectasia mutated (ATM), ATM and Rad3-related, the DNA-dependent protein kinase catalytic subunit and transformation/transcription domain-associated protein (32). The NMD pathway has now been identified and widely exists within eukaryotic organisms as a highly-conserved RNA monitoring mechanism (33). Although its role as an NMD effector is well recognized, SMG-1 also serves well-characterized roles in other biological aspects, including the maintenance of genomic integrity, the response to hypoxia and protection against tumor necrosis factor-α-induced apoptosis, as well as possessing essential roles in the DNA damage response, embryogenesis, the regulation of diverse genes, the regulation of lifespan and oxidative stress resistance, and the activation of p53 in response to DNA double-strand breaks (34–37). Recently, an increased level of awareness was focused on the downregulation of SMG-1 in response to promoter hypermethylation in human papillomavirus-positive head and neck squamous cell carcinoma (38). Roberts et al (39) reported that SMG-1 heterozygous mice exhibited a predisposition to various types of cancer, including hematopoietic malignancies and lung cancer, and the development of chronic inflammation. González-Estévez et al (13) reported that SMG-1 and mTORC1 act antagonistically to regulate response to injury and growth in planarians; the study also indicated that SMG-1 is likely to be a potential human tumor suppressor gene product. Based on the studies discussed, the present study analyzed the expression of SMG-1, with a significant difference identified between the liver cancer cells. In the Huh-7 cells, when exposed to AZD5363, SMG-1 was activated in a time- and dose-dependent manner, but this was not observed in the Hep-G2 cells. With continued investigation, SMG-1 is expected to become one of the breakthrough targets in cancer treatment, possibly providing novel ideas and methods for the diagnosis and treatment of tumor-associated diseases.
mTOR is a key component of the PIKK/AKT/mTOR signaling pathway and functions as a kinase-activating molecule downstream of PIKK/AKT (12). Despite SMG-1 and mTOR each belonging to the PIKK family, the interactions between them are not yet fully understood. In the present study, it was observed that AZD5363 activated the phosphorylation of mTOR in the Huh-7 cells, but this was not observed in the Hep-G2 cells; this indicates that AZD5363 activated SMG-1 only in the Huh-7 cells. Therefore, it can be inferred that mTOR expression is positively correlated with that of SMG-1, possibly suggesting that SMG-1 may interact with mTOR signaling in a direct or indirect manner. González-Estévez et al (13) also described near opposite roles for mTOR and SMG-1 in planarian regeneration. Altman et al (40) reported that tumor suppressor molecules may target and inhibit the mTOR pathway, resulting in regulatory effects on mRNA translation. It has also been noted that in planarian worms, SMG-1 may function as a regulator of injury and growth responses, primarily through cross-talk interactions with mTOR (13). González-Estévez et al (13) observed that SMG-1 was essential for the tight control of stem cell proliferation and differentiation caused by injury or nutrient status in planarian flatworms (Schmidtea mediterranea). The knockdown of SMG-1 in planarian flatworms, similar to the knockdown of several known human suppressors, such as PTEN or p53, leads to lethal outgrowths (13,41,42). Such findings suggest that SMG-1 may serve a potential role as a tumor suppressor in human cancer.
In conclusion, extensive genetic and biochemical research may aid the clarification of the association between mTOR and SMG-1. Nevertheless, the present study has uncovered novel roles of AZD5363 that may be targeted in the treatment of liver cancer, with the investigation of the possible evolutionary conservation of these roles also likely to be advantageous.
Acknowledgements
This study was supported by the National Natural Science Foundation of China, 2014 (grant no. 81373172).
References
- 1.Bruix J, Sherman M. American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 6.Mitsui H, Takuwa N, Maruyama T, Maekawa H, Hirayama M, Sawatari T, Hashimoto N, Takuwa Y, Kimura S. The MEK1-ERK map kinase pathway and the PI 3-kinase-Akt pathway independently mediate anti-apoptotic signals in HepG2 liver cancer cells. Int J Cancer. 2001;92:55–62. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1143>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983. doi: 10.1053/j.gastro.2008.08.008. 1983.e1-1983.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvisi DF, Wang C, Ho C, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–1083. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stiles B, Wang Y, Stahl A, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected] Proc Natl Acad Sci USA. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Estévez C, Felix DA, Smith MD, Paps J, Morley SJ, James V, Sharp TV, Aboobaker AA. SMG-1 and mTORC1 act antagonistically to regulate response to injury and growth in planarians. PLoS Genet. 2012;8:e1002619. doi: 10.1371/journal.pgen.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han EK, Leverson JD, McGonigal T, Shah OJ, Woods KW, Hunter T, Giranda VL, Luo Y. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26:5655–5661. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 15.Levy DS, Kahana JA, Kumar R. AKT inhibitor, GSK690693, induces growth inhibition and apoptosis in acute lymphoblastic leukemia cell lines. Blood. 2009;113:1723–1729. doi: 10.1182/blood-2008-02-137737. [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Sampath D, Nannini MA, Lee BB, Degtyarev M, Oeh J, Savage H, Guan Z, Hong R, Kassees R, et al. Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res. 2013;19:1760–1772. doi: 10.1158/1078-0432.CCR-12-3072. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Oh DY, Nakamura K, Thiele CJ. Perifosine-induced inhibition of Akt attenuates brain-derived neurotrophic factor/TrkB-induced chemoresistance in neuroblastoma in vivo. Cancer. 2011;117:5412–5422. doi: 10.1002/cncr.26133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu R, Liu D, Trink E, Bojdani E, Ning G, Xing M. The Akt-specific inhibitor MK2206 selectively inhibits thyroid cancer cells harboring mutations that can activate the PI3K/Akt pathway. J Clin Endocrinol Metab. 2011;96:E577–E585. doi: 10.1210/jc.2010-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, Li J, Gao B, Ji Q, Maynard J, et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11:873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 20.Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: Hitting the bull's-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374–391. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong ZZ, Maiese K. Mammalian target of rapamycin signaling in diabetic cardiovascular disease. Cardiovasc Diabetol. 2012;11:45. doi: 10.1186/1475-2840-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita A, Ohnishi T, Kashima I, Taya Y, Ohno S. Human SMG-1, a novel phosphatidylinositol 3-kinase-related protein kinase, associates with components of the mRNA surveillance complex and is involved in the regulation of nonsense-mediated mRNA decay. Genes Dev. 2001;15:2215–2228. doi: 10.1101/gad.913001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimson A, O'Connor S, Newman CL, Anderson P. SMG-1 is a phosphatidylinositol kinase-related protein kinase required for nonsense-mediated mRNA decay in Caenorhabditis elegans. Mol Cell Biol. 2004;24:7483–7490. doi: 10.1128/MCB.24.17.7483-7490.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang J, Maquat LE. Nonsense-mediated mRNA decay (NMD) in animal embryogenesis: To die or not to die, that is the question. Curr Opin Genet Dev. 2011;21:422–430. doi: 10.1016/j.gde.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita A. Role of SMG-1-mediated Upf1 phosphorylation in mammalian nonsense-mediated mRNA decay. Genes Cells. 2013;18:161–175. doi: 10.1111/gtc.12033. [DOI] [PubMed] [Google Scholar]
- 26.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karbowniczek M, Robertson GP, Henske EP. Rheb inhibits C-raf activity and B-raf/C-raf heterodimerization. J Biol Chem. 2006;281:25447–25456. doi: 10.1074/jbc.M605273200. [DOI] [PubMed] [Google Scholar]
- 29.Barrett D, Brown VI, Grupp SA, Teachey DT. Targeting the PI3K/AKT/mTOR signaling axis in children with hematologic malignancies. Paediatr Drugs. 2012;14:299–316. doi: 10.1007/BF03262236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 31.Yu K, Shi C, Toral-Barza L, Lucas J, Shor B, Kim JE, Zhang WG, Mahoney R, Gaydos C, Tardio L, et al. Beyond rapalog therapy: Preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res. 2010;70:621–631. doi: 10.1158/0008-5472.CAN-09-2340. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd JP, Davies B. SMG1 is an ancient nonsense-mediated mRNA decay effector. Plant J. 2013;76:800–810. doi: 10.1111/tpj.12329. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira V, Romanow WJ, Geisen C, Otterness DM, Mercurio F, Wang HG, Dalton WS, Abraham RT. A protective role for the human SMG-1 kinase against tumor necrosis factor-alpha-induced apoptosis. J Biol Chem. 2008;283:13174–13184. doi: 10.1074/jbc.M708008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson P, Yepiskoposyan H, Metze S, Orozco Zamudio R, Kleinschmidt N, Mühlemann O. Nonsense-mediated mRNA decay in human cells: Mechanistic insights, functions beyond quality control and the double-life of NMD factors. Cell Mol Life Sci. 2010;67:677–700. doi: 10.1007/s00018-009-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masse I, Molin L, Mouchiroud L, Vanhems P, Palladino F, Billaud M, Solari F. A novel role for the SMG-1 kinase in lifespan and oxidative stress resistance in Caenorhabditis elegans. PLoS One. 2008;3:e3354. doi: 10.1371/journal.pone.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McIlwain DR, Pan Q, Reilly PT, Elia AJ, McCracken S, Wakeham AC, Itie-Youten A, Blencowe BJ, Mak TW. Smg1 is required for embryogenesis and regulates diverse genes via alternative splicing coupled to nonsense-mediated mRNA decay. Proc Natl Acad Sci USA. 2010;107:12186–12191. doi: 10.1073/pnas.1007336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gewandter JS, Bambara RA, O'Reilly MA. The RNA surveillance protein SMG1 activates p53 in response to DNA double-strand breaks but not exogenously oxidized mRNA. Cell Cycle. 2011;10:2561–2567. doi: 10.4161/cc.10.15.16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gubanova E, Brown B, Ivanov SV, Helleday T, Mills GB, Yarbrough WG, Issaeva N. Downregulation of SMG-1 in HPV-positive head and neck squamous cell carcinoma due to promoter hypermethylation correlates with improved survival. Clin Cancer Res. 2012;18:1257–1267. doi: 10.1158/1078-0432.CCR-11-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts TL, Ho U, Luff J, Lee CS, Apte SH, MacDonald KP, Raggat LJ, Pettit AR, Morrow CA, Waters MJ, et al. Smg1 haploinsufficiency predisposes to tumor formation and inflammation. Proc Natl Acad Sci USA. 2013;110:E285–E294. doi: 10.1073/pnas.1215696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget. 2011;2:510–517. doi: 10.18632/oncotarget.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oviedo NJ, Pearson BJ, Levin M, Sánchez Alvarado A. Planarian PTEN homologs regulate stem cells and regeneration through TOR signaling. Dis Model Mech. 2008;1:131–143. doi: 10.1242/dmm.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson BJ, Sánchez Alvarado A. A planarian p53 homolog regulates proliferation and self-renewal in adult stem cell lineages. Development. 2010;137:213–221. doi: 10.1242/dev.044297. [DOI] [PMC free article] [PubMed] [Google Scholar]