Abstract
Osteoporosis is a bone pathology leading to increased fracture risk and challenging the quality of life. The aim of this study was to evaluate the effect of an anthraquinone glycoside, aloin, on osteogenic induction of MC3T3-E1 cells. Aloin increased alkaline phosphatase (ALP) activity, an early differentiation marker of osteoblasts. Aloin also increased the ALP activity in adult human adipose-derived stem cells (hADSC), indicating that the action of aloin was not cell-type specific. Alizarin red S staining revealed a significant amount of calcium deposition in cells treated with aloin. Aloin enhanced the expression of osteoblast differentiation genes, Bmp-2, Runx2 and collagen 1a, in a dose-dependent manner. Western blot analysis revealed that noggin and inhibitors of p38 MAPK and SAPK/JNK signals attenuated aloin-promoted expressions of Bmp-2 and Runx2 proteins. siRNA mediated blocking of Wnt-5a signaling pathway also annulled the influence of aloin, indicating Wnt-5a dependent activity. Inhibition of the different signal pathways abrogated the influence of aloin on ALP activity, confirming that aloin induced MC3T3-E1 cells into osteoblasts through MAPK mediated Wnt and Bmp signaling pathway.
Keywords: Aloin, MC3T3-E1 cell, MAPK, Wnt, Bmp signaling
INTRODUCTION
Bone diseases such as osteoporosis and periodontitis result from an imbalance in bone remodeling that is characterized by excessive bone resorption by osteoclasts relative to the bone formation by osteoblasts. Abnormalities in bone remodeling can also produce a variety of bone-decreasing disorders like rheumatoid arthritis, besides effecting tumor metastasis into bone and Paget’s disease (Deschaseaux et al., 2009). Two different pharmacological approaches can be used to treat such diseases; anti-resorptive agents that inhibit osteoclastic bone resorption, and anabolic agents that stimulate osteoblastic bone formation. Anti-resorptive agents such as bisphosphonates, selective estrogen receptor (ER) modulators, and calcitonin are currently available for the treatment of osteoporosis (Romas, 2005). However, these anti-resorptive agents have disadvantages such as causing unusual bone fractures and flu-like symptoms (Feldbrin et al., 2016). Currently, parathyroid hormone (PTH) therapy replacing the bisphosphonates is available (Reid, 2015). However, limited availability of natural compounds agents to enhance bone mass is still a matter of concern.
Natural compounds have historically been used as agents for the prevention and treatment of lifestyle diseases such as cancer, heart disease, diabetes, high blood pressure and urokinase plasminogen activator mediated fibrinolysis (Madhyastha et al., 2010). Natural compounds that stimulate osteoblast differentiation and bone formation could serve as useful anabolic agents. Phytochemicals, such as icariin (Chen et al., 2005), genistein (Sugimoto and Yamaguchi, 2000), epigallocatechin-3-gallate (Vali et al., 2007), resveratrol (Mizutani et al., 1998) and harmine (Yonezawa et al., 2011) can stimulate osteoblast differentiation and bone formation. Aloin, an anthraquinone glycoside, also known as barbaloin, is a bitter, yellow-brown colored compound with clinically proven pharmacological actions including anti-tumor, anti-inflammatory, anti-oxidant and anti-colitis properties (Park et al., 2011; Cui et al., 2014; Esmat et al., 2015). Since this phytochemical has not been studied previously in the context of osteogenesis, this study aims to evaluate the efficacy of aloin to stimulate osteoblast differentiation with the detailed mechanism of action, using MC3T3-E1 cells. MC3T3-E1 possesses very high osteoblast differentiation potential, expressing osteoblast phenotypic marker genes and mineralizing after the addition of suitable inducible factors (Muhammad et al., 2010; Wang et al., 2011).
Bone morphogenetic proteins (Bmp) are potent inducers of osteoblastogenesis. Bmps activate the transcription factor, Runt-related transcription factor 2 (Runx2), which translocates into the nucleus and modulates the expression of many target genes (Chen et al., 2004). Collagen 1a, osteopontin, osterix, and osteoprotegerin are essential proteins for osteoblast differentiation. Another important junction of osteoblast differentiation is MAPK family, which plays important roles in complex cellular programs like proliferation, differentiation, development, transformation, and apoptosis (Zhang and Liu, 2002).
We studied the effect of aloin on the expression of osteoblast marker genes Bmp-2, Runx2, collagen 1a, osteopontin, osterix, and osteoprotegerin. We also investigated the effect on MAPK and Wnt-dependent Bmp signaling cascades. Furthermore, we studied the effects of Bmp antagonist, noggin, p38 MAPK inhibitor, SAPK/JNK inhibitor and blockade of Wnt-5a signaling pathway using small interfering RNA (siRNA).
MATERIALS AND METHODS
Reagents
Aloin, recombinant human noggin dorsomorphin (SPR 3227), p38 MAPK inhibitor (SB 203580), and SAPK/JNK inhibitor (SP 600125) were purchased from Sigma Chemical Co (St. Louis, USA). Cell culture medium (α-MEM) was purchased from Gibco Co (Tokyo, Japan). All other reagents were obtained from Sigma Chemical Co. or Wako Pure Chemical Industries Ltd., Japan.
Cell cultures
MC3T3-E1 cells were obtained from the RIKEN Cell Bank (Tsukuba, Japan). Cells were cultured in α-MEM cell culture medium with 10% FBS and anti-bacterial cocktail (PNS) at humidified chamber (5% CO2, 37°C). At the semi-confluent stage, the cells were incubated with different concentrations of aloin for 16 h, 10 days or 2 weeks, as required.
Cell viability and cytotoxicity assays
MC3T3-E1 cells seeded at a density of 0.3×103 cells/ml onto 96 well plate were treated with different concentrations of aloin for 10 days, and subjected to MTT and LDH assays for cell viability and cell cytotoxicity, respectively. MTT assay followed the principle that yellow tetrazolium MTT is reduced by metabolically active cells into an insoluble formazan salt. The resulting intracellular purple formazan was solubilized and quantified with a spectrophotometer at an absorbance of 570 nm (Thermo scientific, Multiskan FC, Pittsburgh PK, United States). LDH assay was performed using a CytoTox 96® Non-Radioactive cytotoxicity Assay kit (Promega, Madison, USA). After incubating cells with lactate dehydrogenase enzyme buffer for 1 h, the amount of cytosolic enzyme released into the medium upon cell lysis was measured with a spectrophotometer at an absorbance of 490 nm (Thermo scientific).
Cell proliferation assay
MC3T3-E1 cells were seeded at an initial density of 0.3×103 cells/ml in 96 well plates and treated with 0.05 μM of aloin. Cell proliferation assay was assessed by MTT assay, every alternate day, over a period of 10 days. The data represents the increase in cell number over the days.
ALP activity assay
MC3T3-E1 cells treated with aloin for 10 days were washed twice with cold PBS and lysed in cell lysis buffer. ALP activity was assayed using StemTAGTM Alkaline phosphatase activity assay kit (#CBA 301, Cell BioLabs Co. Ltd., San Diego, USA). Briefly, lysates were incubated in ALP substrate buffer (100 mM Tris-HCl pH 8.5, 2 mM MgCl2, 6.6 mM 4-nitrophenyl phosphate) for 30 min. Absorbance at 405 nm was measured as ALP activity using a microplate reader (Thermo scientific). In some studies, cells were either treated with noggin (1 μg/ ml), p38 inhibitor SB203580 (10 μM) or JNK/SAPK inhibitor SP600125 (10 μM) for 4 h or transfected with Wnt siRNA for 24 h, before treatment with aloin, followed by ALP activity assay.
ALP staining
MC3T3-E1 cells were treated with 0.05 μM aloin for 10 days in a humidified chamber (5% CO2, 37°C). ALP staining was conducted using alkaline phosphatase staining kit (#AK20, Primary Cell Co Ltd., Tokyo, Japan) according to the manufacturer’s instructions. Briefly, cells were washed twice with PBS and fixed with 10% formalin neutral buffer solution for 20 min at room temperature. After washing with deionized water, cells were stained with the chromogenic substrate and incubated at 37°C for 20 min. The stained cells were dehydrated successively at room temperature for 1 h. Images were captured using a microscope coupled to a digital camera at ×100 magnification (Model # 1X73 Olympus, Tokyo, Japan).
Mineralization assay
Mineralization of the extracellular matrix was determined by Alizarin red S staining, which detects calcium. Cells treated with aloin for 2 weeks were fixed in 0.4% paraformaldehyde and incubated in 1% Alizarin red S solution for 5 min at room temperature. Intracellular calcium deposition was monitored by light microscopy microscope (Nikon TMS 101, Tokyo, Japan).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
MC3T3-E1 cells were cultured with or without aloin for 16 h. Total RNA was isolated using ISOGEN (Nippon Gene, Toyama, Japan), and cDNA was synthesized using ReverTra Ace qPCR (Toyobo, Osaka, Japan), according to the manufacturer’s instructions. Primers used were as follows;
| Bmp-2: | Forward: AGTTCTGTCCCCAGTGACGAGTTT |
| Reverse: GTACAACATGGAGATTGCGCTGAGRT | |
| Osteopontin; | Forward: TCACCATTCGGATGAGTCTG |
| Reverse: ACTTGTGGCTCTGATGTTCC | |
| Runx2; | Forward: CCGCACGACAACCGCACCAT |
| Reverse: CGCTCCGGCCCACAAATCTC | |
| Collagen 1a; | Forward: TTCCCTGGTGCTGATGGTGTTGCT |
| Reverse: GCCTTTCCAGGTTCTCCAGCGG | |
| GAPDH; | Forward: AAATGGTGAAGGTCGGTGTG |
| Reverse: GAATTTGCCGTGAGTGGAGT |
PCR was performed with FastStart SYBR Green Master (Roche Diagnostic, Mannheim, Germany) in Gene Atlas thermocycler (Astec, Tokyo, Japan). The relative expression levels of target genes against the endogenous reference glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were calculated using Image Quant TL (GE Healthcare Life Sciences) with a digital imaging system (LAS4000, Fujifilm, Tokyo, Japan).
Western blotting
Total cell lysates were prepared using RIPA buffer (Nacalai Chemicals, Tokyo, Japan). Cytoplasmic and nuclear proteins were obtained using NE-PER reagent (Thermofisher Scientific Inc, Waltham, USA) following manufacturer’s instruction. Protein fractions were resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer containing 2-mercaptoethanol, and boiled at 95°C for 5 min. Protein samples were subjected to SDS-PAGE in 10% polyacrylamide gel and subsequently electroblotted onto polyvinylidene fluoride (PVDF) membranes (GE Healthcare, NJ, USA). After blocking non-specific binding sites for 1 h in 3% nonfat milk in TBST (TBS and 0.1% Tween 20), membranes were incubated overnight at 4°C with specific primary antibodies. Antibodies for Bmp-2, Runx2, collagen 1a, p38, p-p38, JNK/SAPK, p-JNK/SAPK, ERK1/2, p-ERK1/2 and β actin were purchased from Cell Signaling (Massachusetts, USA). Antibodies for osteoprotegerin, Sp7/osterix and osteopontin were purchased from Abcam Inc (San Francisco, USA). The membranes were washed in TBST and incubated further with horseradish peroxidase-conjugated secondary antibodies at room temperature. Protein bands were detected using an enhanced ECL kit (GE Healthcare, Tokyo, Japan) with the digital imaging system (LAS4000).
Treatment with Bmp antagonist noggin
Subconfluent cells were treated with noggin (1 μg/ml) for 4 h, followed by 16 h incubation with 0.05 μM aloin. Cell lysates were prepared using RIPA buffer. Cell lysates were used for western blot detection of Bmp-2 and Runx2. β actin was used as a housekeeping control.
Wnt-5a siRNA transfection
Cells were plated into a 6-well plate with α-MEM cell culture medium with 10% FBS and anti-bacterial cocktail (PNS) at humidified chamber (5% CO2, 37°C) as described previously. Wnt-5a siRNA and control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, Biotechnology, Inc., Texas, USA). siRNA transfection was performed according to the manufacturer’s protocol. Twenty-four hours post-transfection, cells were treated with aloin. Cell lysates were analyzed for expression of Bmp-2 and Runx2 proteins by western blotting, as described earlier.
Culture of adult human adipose-derived stem cells (hADSC)
Adult human adipose-derived stem cells were purchased from a commercial supplier (Zen-Bio, Inc., NC 27709, USA). Cells were cultured in pre-osteogenic DMEM/Ham’s F-12 medium. The initiation medium contained 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer pH 7.4, fetal bovine serum (10%), penicillin, streptomycin, and amphotericin B. Cells were incubated under standard condition (5% CO2 and 37°C) until they reached semi-confluence. Cells (8×104 cells/well) were treated with various concentrations of aloin and ALP activity was determined as described earlier.
Statistical analysis
All data are expressed as mean ± SD. Statistical analyses of the significance of differences among values were carried out by one-way ANOVA with post hoc Dunnett’s test or Students T-test with n=4 independent experiments. Values of p<0.05 were considered to indicate statistical significance.
RESULTS
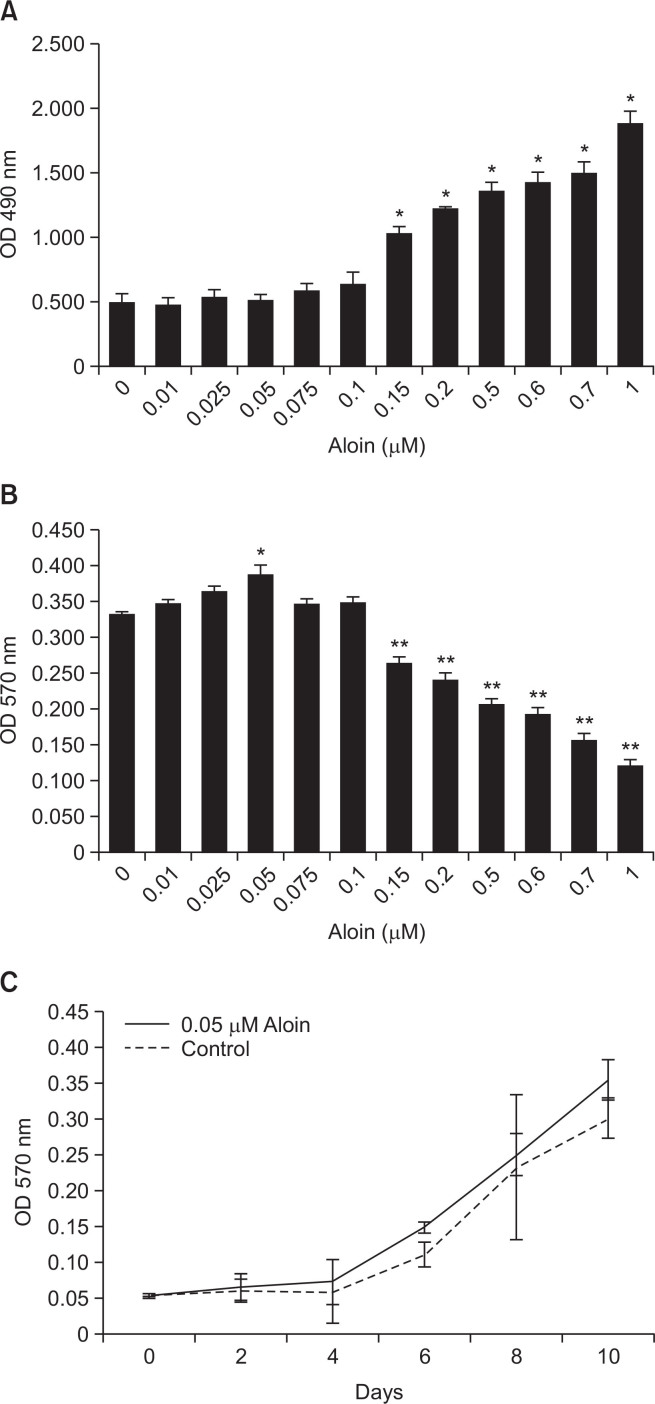
Preliminary studies were conducted to assess the effect of aloin on cell viability, proliferation, and toxicity. LDH assay showed that aloin at concentrations up to 0.1 μM was not cytotoxic to the cells (Fig. 1A). MTT assay revealed that aloin had no adverse effect on viability (Fig. 1B) or rate of proliferation of the cells (Fig. 1C), at concentrations up to 0.1 μM. Higher concentrations of aloin proved cytotoxic. Further experiments were performed using 0.05 μM aloin.
Fig. 1.
Cytotoxicity and cell viability profile of aloin: MC3T3-E1 cells were treated with different doses ofaloin for 10 days and assessed by LDH assay for cytotoxicity (A), and MTT assay for cell viability (B) and cell proliferation (C). The data represent mean ± SD of three experiments. *p<0.05 vs control cells, **p<0.05 vs 0.05 μM aloin group.
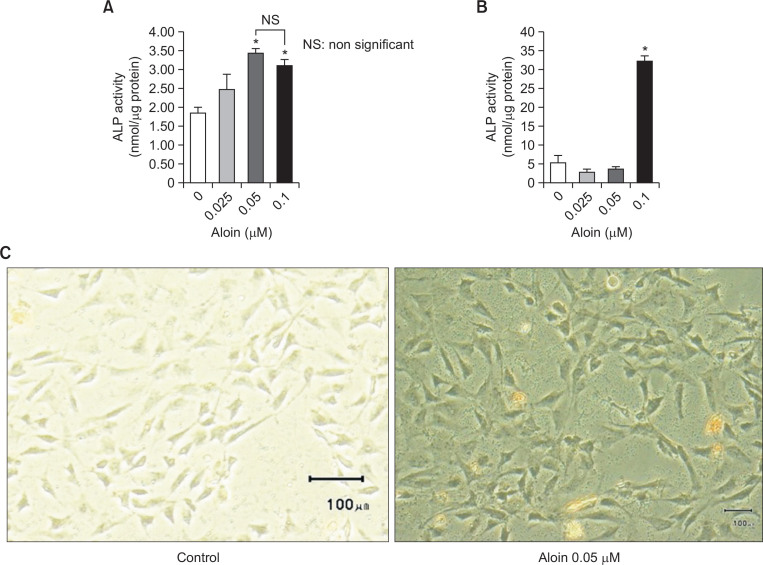
Alkaline phosphatase (ALP) activity (an early phase marker of osteoblast differentiation) and mineralization (late phase marker) were evaluated to assess the effect of aloin on osteogenic induction. Aloin significantly increased ALP activity of MC3T3-E1 cells (Fig. 2A) and hADSC (Fig. 2B) at 0.05 μM and 0.1 μM concentrations respectively. Aloin’s effect on two different distinct cell types clearly implies that effect is not cell type specific. Aloin also increased the intracellular ALP content in MC3T3-E1 cells, as evident from ALP staining (Fig. 2C).
Fig. 2.
Aloin increased the ALP activity in MC3T3-E1 cells (A) and human adult adipose derived stem cells (hADSC) (B). Cells treated with aloin were fixed and incubated in ALP substrate buffer for 30 min. Theabsorbance at 405 nm was measured as the p-nitrophenol released in nmol using a microplate reader Valueson y-axis represents the nmol ALP production (nmol/ugm protein). The data represent mean ± SD of threeexperiments. *p<0.05 vs control cells. (C) MC3T3-E1 cells treated with 0.05 μM aloin for 10 days were stained for ALP content. ALP in the form of blue droplets were monitored in microscope and photographed using Olympus microscope (Model # 1X73).
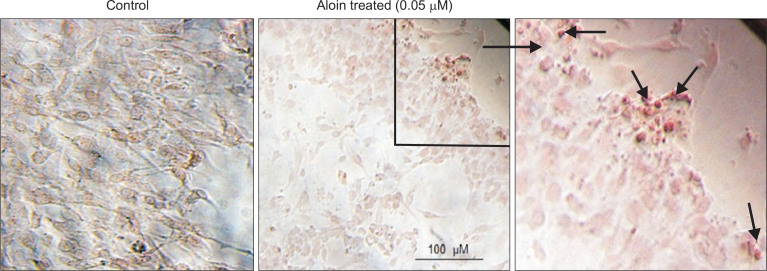
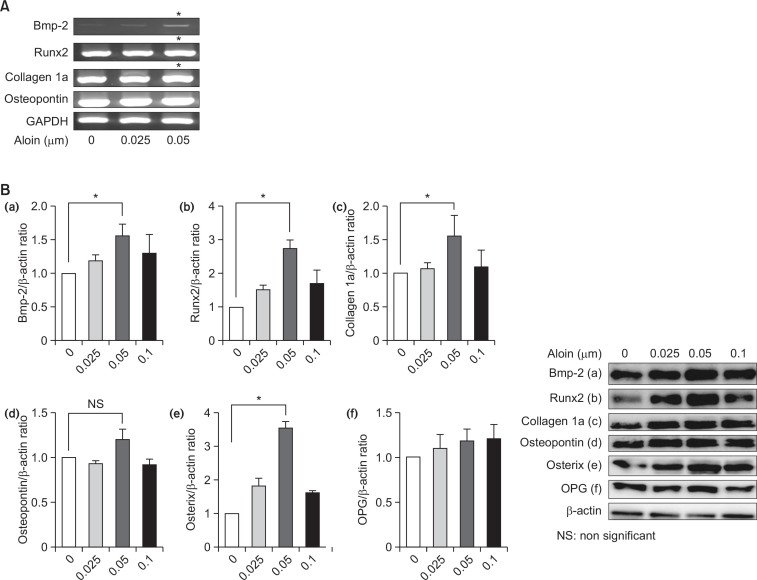
Cells treated with aloin for 2 weeks showed significant deposits of calcium, indicating stimulation of mineralization (Fig. 3). Next, effect of aloin on the expression of osteoblast marker genes, namely Bmp-2, Runx2, collagen 1a and osteopontin was evaluated. Results revealed a dose-dependent induction of Bmp-2, Runx2, and collagen 1a at the RNA level (Fig. 4A) as well as protein level (Fig. 4B). The most significant effect was observed in Bmp-2 mRNA, where 0.05 μM aloin caused a 6.2 fold increase and Runx2 protein (>2 fold increase). Aloin did not show any stimulatory effect on osteopontin, either at gene or protein level. Collectively the results indicate that aloin targets the upstream molecules like Bmp-2 and Runx2 in osteoblastogenesis pathway. The influence of aloin on expressions of Bmp-2 and Runx2 proteins was annulled in the presence of Bmp antagonist, noggin (Fig. 5), implying that Bmp activation is necessary for their expression. Results so far indicate that aloin could stimulate osteogenic differentiation of MC3T3-E1 cells through the induction of Runx2 via Bmp signaling cascade.
Fig. 3.
Aloin stimulated mineralization in the cells. Sub-confluent cells treated with/without 0.05 μM aloin; Maloin were stained with Alizarin red S, to detect calcium nodules in the cell. Calcium deposits in the form ofred droplets were monitored in microscope and photographed using Olympus microscope (Model # 1X73).
Fig. 4.
Effect of aloin on osteogenic marker genes and proteins. (A) Total RNA isolated from MC3T3-E1cells treated with different doses of aloin was subjected to reverse transcription polymerase chain reactionusing specific primers. The band intensities were normalized to the housekeeping gene, GAPDH. The datarepresent mean ± SD of triplicate determinations. *p<0.05 vs. control cells. (B) Relative protein expressions of (a) BMP-2, (b) RunX 2, (c) Collagen 1a, (d) Osteopontin, (e) Osterix and (f) Osteoprotegerin (OPG) wereassessed by WB analysis. β-actin was used as housekeeping control. Data represents the results of threeindependent experiments. Level of significance was calculated at *p<0.05 vs. control cells.
Fig. 5.
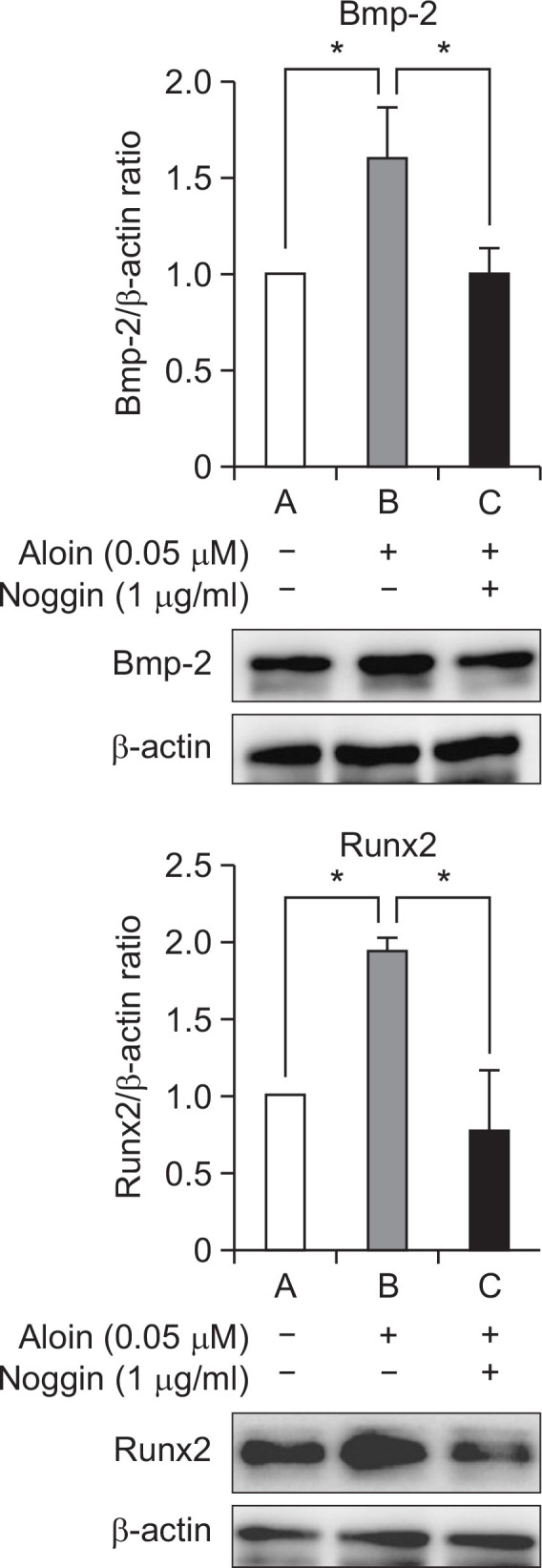
The influence of aloin was annulled in the presence of noggin. Cells were treated with aloin (0.05 μM) in the presence of Bmp antagonist noggin (1 μg/mL). Protein expressions of Bmp-2 and RunX 2 were assessed by WB analysis. The data represent mean ± SD of triplicate determinations. *p<0.05 vs. control cells.
Involvement of signaling pathways
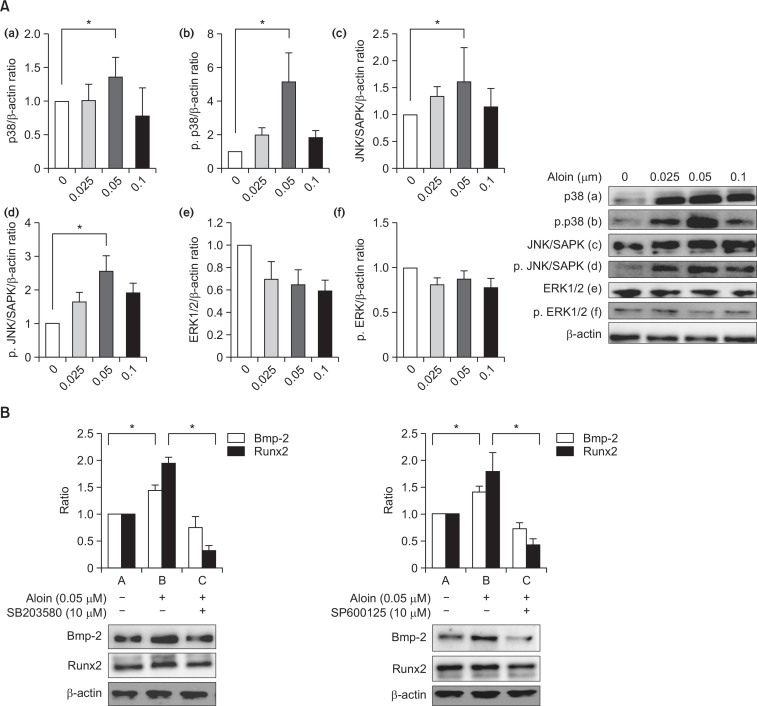
Members of the MAPK signaling pathways (p38, JNK/SAPK and ERK1/2) were studied to elucidate the possible participation of MAPK signaling pathways in the effects of aloin. Treatment with aloin caused a significant increase in the activation of p38 and JNK/SAPK proteins; on the other hand, ERK1/2 was downregulated (Fig. 6A). To test the role of MAPK activation in the regulation of Bmp-2 and Runx2, cells were treated with aloin in the presence of p38 inhibitor (SB203580) and SAPK/JNK inhibitor (SP600125). Co-treatment with the inhibitors attenuated the effect of aloin on Bmp-2 and Runx2 proteins (Fig. 6B), confirming the involvement of MAPK members, p38, and JNK/SAPK.
Fig. 6.
(A) Effects of aloin on MAPK signaling pathway. MC3T3-E1 cells were treated with or without aloinand analyzed for (a) p38, (b) phospho-p38, (c) JNK/SAPK, (d) phospho- JNK/SAPK, (e) ERK1/2 and (f) phospho-ERK1/2 by western blotting. β-actin was used as housekeeping protein. The data represent mean ± SD of triplicate determinations. *p<0.05 vs. control cells. (B) Effect of inhibitors of p38 (SB203580) and SAPK/JNK (SP600125) on the activity of aloin: Cells were treated with aloin (0.05 μM) in thepresence of SB203580 or SP600125. Protein expressions of Bmp-2 and RunX 2 were assessed by WB analysis. β-actin was used as housekeeping protein. Data represents the results of three independent experiments and significant level (*) was calculated at p<0.05 vs. control cells.
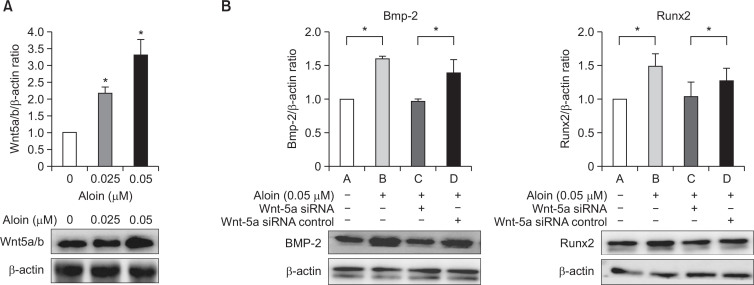
Besides MAPK, Wnt family also plays important roles in many aspects of osteogenesis. In the present investigation, we selected Wnt 5a/b to evaluate the Wnt signaling pathway. Aloin (0.05 μM) increased Wnt 5a/b protein activity significantly (Fig. 7A). To study the involvement of Wnt on the activity of aloin, we employed the siRNA-mediated silencing technique to knock down Wnt5a. Silencing of Wnt-5a inhibited both Bmp-2 and Runx2 protein expressions (Fig. 7B), highlighting that the effect of aloin on Bmp-2 and Runx2 was indeed, Wnt-dependent.
Fig. 7.
(A) Effect of aloin on Wnt signaling pathway. MC3T3-E1 cells were treated with or without aloin andanalysed for Wnt 5a/b expression by western blotting. The data represent mean ± SD of triplicatedeterminations. *p<0.05 vs. control cells. (B) Effect of Wnt-5a silencing on activity of aloin. Cells weretransfected with Wnt-5a siRNA or control siRNA before treatment with aloin (0.05 μM). Total proteinwas analysed by western blotting for Bmp-2 and RunX 2 proteins. The data represent mean ± SD of triplicatedeterminations. *p<0.05 vs. control cells.
ALP production is the hallmark of differentiation of MC3T3-E1 cells into osteoblasts, through a complex process of signaling circuits (Chen et al., 2004). Inhibition of Bmp-2, p38, JNK/ SAPK and Wnt pathways annulled the effect of aloin on ALP activity (Fig. 8).
Fig. 8.
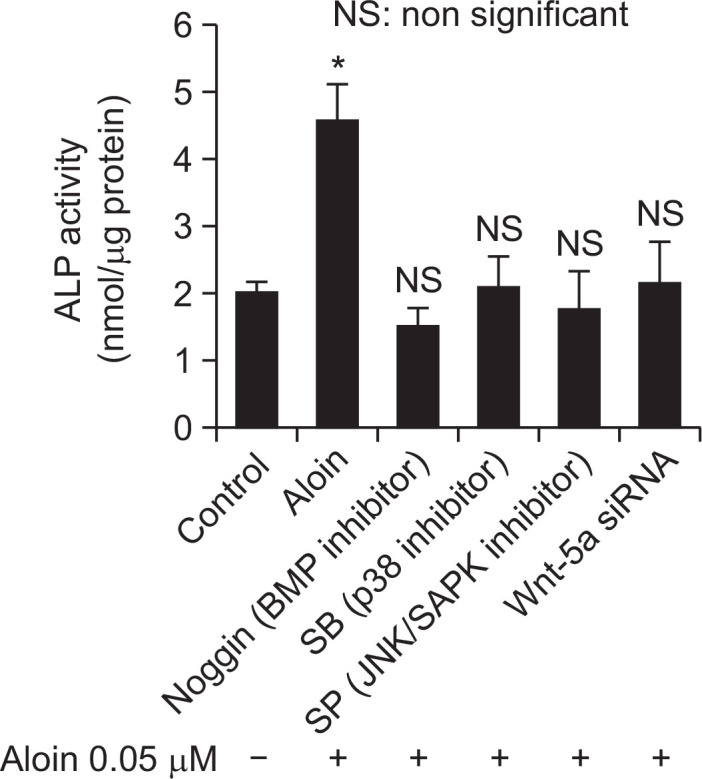
The rate of ALP production in the presence of signal pathway inhibitors. Cells were treated with aloin (0.05 μM) in the presence of Bmp-2 antagonist noggin (1 μg/mL), p38 inhibitor SB203580 (10 μM), JNK/SAPK inhibitor SP600125 (10 μM) or Wnt-5a siRNA, and cultured for a period of 10 days. ALP activity was assessed and expressed as nmol ALP produced/mg protein. The data represent mean ± SD of triplicate determinations. *p<0.05 vs. control cells.
DISCUSSION
Many research groups are currently attempting to identify molecules that stimulate osteoblast differentiation, for the development of drugs to treat osteoporosis. Plant-derived natural compounds including flavonoids, polyphenols, lignans, coumarins, terpenoids, carotenoids, and alkaloids can stimulate in vitro osteoblast cellular differentiation and in vivo bone mass formation (Woo et al., 2010). Phytochemicals can differentiate mesenchymal stem cells and MC3T3-E1 cells, to osteoblasts, through several crucial molecular and cellular processes in bone formation, and can be used as agents to treat various bone diseases (Woo et al., 2010).
In this study, we attempted to clarify the effect of aloin, an anthrocyclic glycoside, on in vitro osteogenic induction and the associated mechanisms, employing MC3T3-E1 cells. Undifferentiated cells such as MC3T3-E1 and C3H10T1/2 are model cell lines utilized for in vitro studies on osteoblast differentiation. 3T3 fibroblasts, which are already committed to a specific differentiation phenomenon, can be induced to express osteoblast markers, but these cells have to be reprogrammed by adding epigenetic modifiers (Muhammad et al., 2010). MC3T3-E1 cells can also differentiate into chondrocytes, adipocytes and myoblasts by physiological inducers through Bmp, Wnt signaling circuits (Kobayashi et al., 2008).
Aloin stimulated the process of osteoblast induction through an increase in ALP production at the initial stage, and mineralization at the later stage. It is reported that the methoxyl substituent in anthraquinone derivatives is important to elicit osteogenic activity (Lee et al., 2008). Several natural compounds are reported to enhance the ALP activity and calcium deposition during initial osteogenesis process (Chen et al., 2005; Lee et al., 2008). Since aloin has methoxyl group, we believe that structure-activity relationship of aloin could be important for inducing initial osteogenic activity.
In this study, aloin induced Bmp-2 gene at the initial stage (Fig. 4A), stimulated ALP accumulation (Fig. 2A) at an early stage, and intracellular calcium deposition at a later stage (Fig. 3). Taken together, these findings collectively indicate that aloin induced molecular initiation of osteoblastogenesis in MC3T3-E1 cells.
MAPK family regulates multiple cellular activities related to osteoblast initiation process, and can be activated in response to a wide range of external stimuli including natural compounds (Trzeciakiewicz et al., 2009). Various reports highlight that the MAPK pathway can phosphorylate Runx2 and osterix, implying that MAPK is an obligatory transducer for bone healing (Xiao et al., 2000; Celil and Campbell, 2005). In addition, MAPK family proteins, p38 and JNK, are reported to regulate osteoblast differentiation via activation of transcriptional factors such as activator protein 1 (AP-1) (Lee et al., 2008). MAPK activation can induce Runx2 dependent osteocalcin and osteopontin genes (Zhang and Liu, 2002). Stimulation of cells with aloin resulted in the activation of p38 and JNK/ SAPK MAPK pathways and also in an increased expression of Runx2 and osterix proteins. Inhibition of MAPK using specific inhibitors annulled the effect of aloin on Runx2 and Bmp-2 proteins, indicating that osteogenesis parameters are initiated through MAPK members. Runx2 is a key transcription factor associated with differentiation of bone forming cells (Holleville et al., 2007). It can differentiate mesenchymal stem cells to osteochondroblast progenitor through Bmp signaling pathways, and also differentiate pre-osteoblast to mature osteoblast through MAPK signaling pathways (Nakashima et al., 2002; Ge et al., 2007). Bmp pathway is crucial for progression and maturation of osteogenesis (Nohe et al., 2002; Chen et al., 2004; Seib et al., 2009). Bmp-2 is also crucial for proliferation and differentiation of osteogenesis through pre-osteoblast cells, which could depend on the transcription factor osterix acting downstream of Runx2 (Lum and Beachy, 2004). Inactivation of Bmp-2 using specific inhibitor, noggin, attenuated the increase in Runx2 protein caused by aloin.
In addition to MAPK and Bmp pathways, aloin also induced Wnt signaling. Wnt signaling is required for commitment of mesenchymal stem cells to the osteoblast lineage (You et al., 2004; Baron and Kneissel, 2013; Kumawat et al., 2014). Wnt 5a/b has a significant role in bone formation (Liu et al., 2008; Bennett et al., 2005; Bodine et al., 2005). Silencing of Wnt signaling via siRNA technique nullified the effect of aloin on Bmp-2 and Runx2 proteins.
In conclusion, our study reveals that aloin can stimulate osteogenic initiation of MC3T3-E1 cells via the induction of transcription factors (Runx2, osterix) and osteogenic factors (Bmp-2). These inductive effects are in turn mediated by the regulation of MAPK (p38, JNK/SAPK) and Wnt pathways.
The present investigation revealed that aloin differentiates MC3T3-E1 cells and adipose-derived stem cells to osteoblasts. First, the cells become osteochondroblast progenitor cells as evidenced by overexpression of transcription factor Runx2, through Bmp signaling pathway. Later, transcription factors Runx2 and osterix induce the osteochondroblast progenitors to mature osteoblasts. These processes are mediated by Wnt and MAPK signaling pathways. Taken together, these results indicate that aloin differentiates MC3T3-E1 cells into osteoblasts through MAPK- and Wnt-dependent Bmp signaling pathways.
Acknowledgments
This research was supported by the University of Miyazaki, Japan, and Prince of Songkla University, Thailand.
REFERENCES
- Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatment. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine PV, Billiard J, Moran RA, Ponce-de-Leon H, McLarney S, Mangine A, Scrimo MJ, Bhat RA, Stauffer B, Green J, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem. 2005;96:1212–1230. doi: 10.1002/jcb.20599. [DOI] [PubMed] [Google Scholar]
- Celil AB, Campbell PG. BMP-2 and insulin-like growth factor-Immediate Osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. J Biol Chem. 2005;280:31353–31359. doi: 10.1074/jbc.M503845200. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Chen KM, Ge BF, Ma HP, Liu XY, Ba MH, Wang Y. Icariin, a flavonoid from the herb Epimedium enhances the osteogenic differentiation of rat primary bone marrow stromal cells. Pharmazie. 2005;60:939–942. [PubMed] [Google Scholar]
- Cui Y, Ye Q, Wang H, Li Y, Xia X, Yao W, Qian H. Aloin protects against chronic alcoholic liver injury via attenuating lipid accumulation, oxidative stress and inflammation in mice. Arch Pharm Res. 2014;37:1624–1633. doi: 10.1007/s12272-014-0370-0. [DOI] [PubMed] [Google Scholar]
- Deschaseaux F, Sensébé L, Heymann D. Mechanisms of bone repair and regeneration. Trends Mol Med. 2009;15:417–429. doi: 10.1016/j.molmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Esmat AY, Said MM, Khalil SA. Aloin: a natural anti-tumor anthraquinone glycoside with iron chelating and non-atherogenic activities. Pharm Biol. 2015;53:138–146. doi: 10.3109/13880209.2014.912239. [DOI] [PubMed] [Google Scholar]
- Feldbrin Z, Luckish A, Shargorodsky M. Effects of long-term risedronate treatment on serum ferritin levels in postmenopausal women with osteoporosis: the impact of cardiovascular risk factor load. Menopause. 2016;23:55–59. doi: 10.1097/GME.0000000000000480. [DOI] [PubMed] [Google Scholar]
- Ge C, Xiao G, Jiang D, Franceschi RT. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol. 2007;176:709–718. doi: 10.1083/jcb.200610046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleville N, Matéos S, Bontoux M, Bollevot K, Monsoro-Burg AH. Dlx5 drives Runx2 expression and osteogenic differentiation in developing cranial suture mesenchyme. Dev Biol. 2007;304:860–874. doi: 10.1016/j.ydbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Maeba K, Takahashi N. Roles of Wnt signaling in bone formation and resorption. Jpn Dent Sci Rev. 2008;44:76–82. doi: 10.1016/j.jdsr.2007.11.002. [DOI] [Google Scholar]
- Kumawat K, Menzen MH, Slegtenhorst RM, Halayko AJ, Schmidt M, Gosens R. TGF-β-activated kinase 1 (TAK1) signaling regulates TGF-β-induced WNT-5A expression in airway smooth muscle cells via Sp1 and β-catenin. PLoS One. 2014;9:e94801. doi: 10.1371/journal.pone.0094801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SU, Shin HK, Min YK, Kim SH. Emodin accelerates osteoblast differentiation through phosphatidylinositol 3-kinase activation and bone morphogenetic protein-2 gene expression. Int Immunopharmacol. 2008;8:741–747. doi: 10.1016/j.intimp.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Liu F, Kohlmeier S, Wang CY. Wnt signaling and skeletal development. Cell Signal. 2008;20:999–1009. doi: 10.1016/j.cellsig.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L, Beachy PA. The hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. Curcumin facilitates fibrinolysis and cellular migration during wound healing by modulating urokinase plasminogen activator expression. Pathophysiol Haemost Thromb. 2010;37:59–66. doi: 10.1159/000321375. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Ikeda K, Kawai Y, Yamori Y. Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 1998;253:859–863. doi: 10.1006/bbrc.1998.9870. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Heris YI, Knaus P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277:5330–5338. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- Park MY, Kwon HJ, Sung MK. Dietary aloin, aloesin, or aloe-gel exerts anti-inflammatory activity in a rat colitis model. Life Sci. 2011;88:486–492. doi: 10.1016/j.lfs.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11:418–428. doi: 10.1038/nrendo.2015.71. [DOI] [PubMed] [Google Scholar]
- Romas E. Bone loss in inflammatory arthritis: mechanisms and therapeutic approaches with bisphosphonates. Best Pract Res Clin Rheumatol. 2005;19:1065–1079. doi: 10.1016/j.berh.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Seib FP, Franke M, Jing D, Werner C, Bornhäuser M. Endogenous bone morphogenetic proteins in human bone marrow-derived multipotent mesenchymal stromal cells. Eur J Cell Biol. 2009;88:257–271. doi: 10.1016/j.ejcb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Sugimoto E, Yamaguchi M. Anabolic effect of genistein in osteoblastic MC3T3-E1 cells. Int J Mol Med. 2000;5:515–520. doi: 10.3892/ijmm.5.5.515. [DOI] [PubMed] [Google Scholar]
- Trzeciakiewicz A, Habauzit V, Horcajada MN. When nutrition interacts with osteoblast function: molecular mechanisms of polyphenols. Nutr Res Rev. 2009;22:68–81. doi: 10.1017/S095442240926402X. [DOI] [PubMed] [Google Scholar]
- Vali B, Rao LG, El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem. 2007;18:341–347. doi: 10.1016/j.jnutbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sugano E, Isago H, Hiroi T, Tamai M, Tomita H. Differentiation of neuronal cells NIH/3T3 fibroblast under defined conditions. Dev Growth Differ. 2011;53:357–365. doi: 10.1111/j.1440-169X.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- Woo JT, Yonezawa T, Nagai K. Phytochemical that stimulate osteoblastic differentiation and bone formation. J Oral Biosci. 2010;52:15–21. doi: 10.1016/S1349-0079(10)80003-9. [DOI] [Google Scholar]
- Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- Yazid MD, Ariffin SH, Senafi S, Razak MA, Wahab RM. Determination of the differentiation capacities of murine’s primary mononucleated cells and MC3T3-E1. Cancer Cell Int. 2010;10:42. doi: 10.1186/1475-2867-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa T, Lee JW, Hibino A, Asai M, Hojo H, Cha BY, Teruya T, Nagai K, Chung UI, Yagasaki K, Woo JT. Harmine promotes osteoblast differentiation through bone morphogenetic protein signaling. Biochem Biophys Res Commun. 2011;409:260–265. doi: 10.1016/j.bbrc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- You L, He B, Uematsu K, Xu Z, Mazieres J, Lee A, McCormick F, Jablons DM. Inhibition of Wnt-1 signaling induces apoptosis in β-catenin-deficient mesothelioma cells. Cancer Res. 2004;64:3474–3478. doi: 10.1158/0008-5472.CAN-04-0115. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]