Abstract
Naringenin (NAR) as one of the flavonoids observed in grapefruit has been reported to exhibit an anti-cancer activity. Activating transcription factor 3 (ATF3) is associated with apoptosis in human colon cancer cells. This study was performed to investigate the molecular mechanism by which NAR stimulates ATF3 expression and apoptosis in human colon cancer cells. NAR reduced the cell viability and induced an apoptosis in human colon cancer cells. ATF3 overexpression increased NAR-mediated cleaved PARP, while ATF3 knockdown attenuated the cleavage of PARP by NAR. NAR increased ATF3 expression in both protein and mRNA level, and increased the luciferase activity of ATF3 promoter in a dose-dependent manner. The responsible region for ATF3 transcriptional activation by NAR is located between −317 and −148 of ATF3 promoter. p38 inhibition blocked NAR-mediated ATF3 expression, its promoter activation and apoptosis. The results suggest that NAR induces apoptosis through p38-dependent ATF3 activation in human colon cancer cells.
Keywords: Naringenin, Activating transcription factor 3, Cancer chemoprevention, Human colon cancer
INTRODUCTION
Human colon cancer is the third most commonly diagnosed malignant disease (Siegel et al., 2014). Surgery, radiation or chemotherapy has been regarded as the most effective adjuvant therapy in many cases. However, oxaliplatin, leucovorin and irinotecan as commonly used chemotherapeutic agents are associated with severe adverse effects (Bleiberg et al., 2012). Thus, there is a need for more potent and less toxic drugs. In addition, chemoprevention using phytochemicals widely distributed in vegetables, fruits and medicinal plants has received attention as an attractive and promising strategy for human cancer prevention (Wang et al., 2012).
Many biomolecules derived from the food have various pharmacological properties and been reported to be useful in the prevention and improvement of various human diseases. Flavonoids have attracted attention as one of the potential cancer chemopreventive agents due to their anti-cancer activities (Kuo, 1997; Garcia-Lafuente et al., 2009).
Naringenin (NAR) as a common dietary flavonoid abundantly present in fruits and vegetables is formed from naringin after dietary intake in humans (Yen et al., 2015). Many studies have shown various pharmacological effects including antioxidant activity (Pietta, 2000), anti-inflammatory activity (Esmaeili and Alilou, 2014) and anti-mutagenic effects (Ganapathy et al., 2008). In addition, NAR has been reported to exhibit anti-cancer activity (Kanno et al., 2006; Lee et al., 2005a; Ekambaram et al., 2008; Yoon et al., 2013). In pro-apoptotic effect of NAR on cancer cells, NAR induced apoptosis in colon, breast and uterine cancer cell lines expressing ERα and ERβ (Totta et al., 2004; Virgili et al., 2004; Bulzomi et al., 2012). However, more detailed mechanism for NAR-mediated apoptosis still remains unanswered.
Activating transcription factor 3 (ATF3) as a member of the ATF/CREB family is activated under various physiological (Hai and Hartman, 2001) and pathological stimuli, and has been regarded to exert cell-depending effects including cell cycle arrest and apoptosis (Yin et al., 1997; Cai et al., 2000). There is growing evidence that ATF3 is one of the important molecular targets for the apoptotic effect of many anti-cancer agents in colon cancer cells (Lee et al., 2006; Yamaguchi et al., 2006; Lee et al., 2010; Lee et al., 2013), which suggests that ATF3 activation may be a promising cancer preventive and therapeutic target in human colon cancer.
In this study, we tested the effect of ATF3 on NAR-mediated apoptosis in human colon cancer and we report that NAR leads to transcriptional activation of ATF3 which may be associated with induction of apoptosis in human colon cancer cells.
MATERIALS AND METHODS
Reagents
Naringenin (NAR) was purchased from Sigma Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle medium (DMEM)/F-12 1:1 Modified medium (DMEM/F-12) was purchased from Lonza (Walkersville, MD, USA). Antibodies against ATF3, Poly ADP ribose polymerase (PARP) and β-actin were purchased from Cell Signaling (Beverly, MA, USA). PD98059 (ERK1/2 inhibitor), SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), SB216763 (GSK3β inhibitor) and BAY11-7082 (NF-κB inhibitor) were purchased from Calbiochem (San Diego, CA, USA). ATF3 siRNA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). ATF3 constructs used in this study were kindly provided from Dr. Seong Ho Lee (University of Maryland, College Park, MD, USA). All chemicals were purchased from Fisher Scientific, unless otherwise specified.
Cell culture and treatment
Human colon cancer cell lines such as HCT116, SW480, Lovo and HT-29 cells were purchased from Korean Cell Line Bank (Seoul, Korea) and grown in DMEM/F-12 supplemented with 10% fatal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were maintained at 37°C under a humidified atmosphere of 5% CO2. NAR was dissolved in dimethyl sulfoxide (DMSO) and treated to cells. DMSO was used as a vehicle and the final DMSO concentration did not exceed 0.1% (v/v).
Cell viability
Cell viability was measured using MTT assay system. Briefly, cells (2×104 cells/well) were plated onto 96-well plated and grown overnight. The cells were treated with NAR for 24 h. Then, the cells were incubated with 50 μl of MTT solution (1 mg/ml) for the additional 2 h. The resulting crystals were dissolved in DMSO. The formation of formazan was measured by reading absorbance at a wavelength of 570 nm.
SDS-PAGE and western blot
After NAR treatment, cells were washed with 1×phosphate-buffered saline (PBS), and lysed in radioimmunoprecipitation assay (RIPA) buffer (Boston Bio Products, Ashland, MA, USA) supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), and centrifuged at 15,000×g for 10 min at 4°C. After determining protein concentration by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA), the proteins were separated on SDS-PAGE and transferred to PVDF membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked for non-specific binding with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBS-T) for 1 h at room temperature and then incubated with specific primary antibodies in 5% non-fat dry milk at 4°C overnight. After three washes with TBS-T, the blots were incubated with horse radish peroxidase (HRP)-conjugated immunoglobulin G (IgG) for 1 h at room temperature and chemiluminescence was detected with ECL Western blotting substrate (Amersham Biosciences, Piscataway, NJ, USA) and visualized in Polaroid film.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
After NAR treatment, total RNA was prepared using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and 1 μg of total RNA was reverse-transcribed using a Verso cDNA Kit (Thermo Scientific, Pittsburgh, PA, USA) according to the manufacturer’s protocol for cDNA synthesis. PCR was performed using PCR Master Mix Kit (Promega, Madison, WI, USA) with human primers for cyclin D1 and GAPDH as followed : human ATF3: 5′-gtttgaggattttgctaacctgac-3′, and reverse 5′-agctgcaatcttatttctttctcgt-3′; human GAPDH: forward 5′-acccagaagactgtggatgg-3′ and reverse 5′-ttctagacggcaggtcaggt-3′.
Transient transfections
Transient transfections were performed using the PolyJet DNA transfection reagent (SignaGen Laboratories, Ijamsville, MD, USA) according to the manufacturers’ instruction. HCT116 and SW480 cells were plated in 12-well plates at a concentration of 2×105 cells/well. After growth overnight, plasmid mixtures containing 1 μg of ATF3 promoter linked to luciferase and 0.1 μg of pRL-null vector were transfected for 24 h. The transfected cells were cultured in the absence or presence of NAR for 24. The cells were then harvested in 1×luciferase lysis buffer, and luciferase activity was normalized to the pRL-null luciferase activity using a dual-luciferase assay kit (Promega, Madison, WI, USA).
Transfection of small interference RNA (siRNA)
HCT116 cells were plated in 6-well plates and incubated overnight. HCT116 cells were transfected with control siRNA and ATF3 siRNA for 48 h at a concentration of 100 nM using TransIT-TKO transfection reagent (Mirus, Madison, WI) according to the manufacturer’s instruction. Then the cells were treated with 200 μM of NAR for 24 h.
Expression vector
ATF3 expression vector was provided from Addgene (Cambridge, MA, USA). Transient transfection of the vector was performed using the PolyJet DNA transfection reagent (SignaGen Laboratories, Ijamsville, MD, USA) according to the manufacturers’ instruction.
Statistical analysis
All the data are shown as mean ± SEM (standard error of mean). Statistical analysis was performed with one-way ANOVA followed by Dunnett’s test. Differences with *p<0.05 were considered statistically significant.
RESULTS
Effect of NAR on cell viability and apoptosis in human colon cancer cells
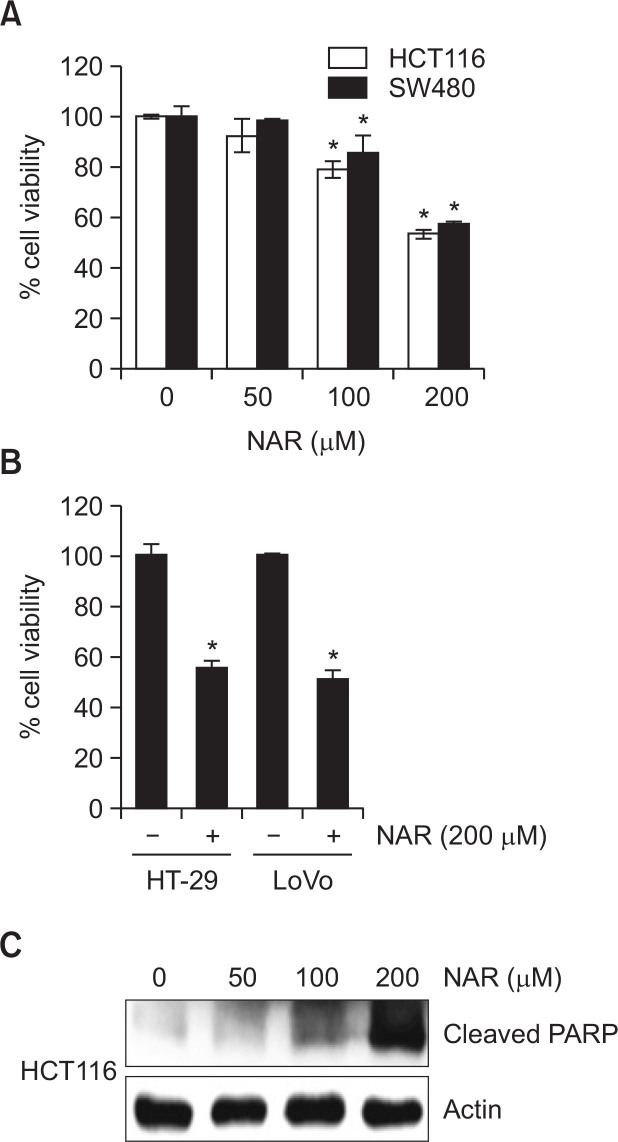
To evaluate whether NAR decreases the viability of human colon cancer cells, HCT116 and SW480 cells were treated with NAR at the different concentrations and cell viability was measured by MTT assay. As a result (Fig. 1A), NAR treatment resulted in the decrease of the cell viability by 28% and 15% at 100 μM, and 47% and 43% at 200 μM in HCT116 and SW480 cells, respectively. In addition, the viability of HT-29 and Lovo cells was decreased by 45% and 49% at 200 μM of NAR, respectively (Fig. 1B). Next, we tested cleaved PARP using Western blot to evaluate whether NAR-mediated decrease of the cell viability results from apoptosis. As shown in Fig. 1C, NAR dose-dependently induced the cleavage of PARP.
Fig. 1.
Effect of NAR on the cell viability and apoptosis. (A) HCT116 and SW480 cells were treated with NAR at the indicated concentrations for 24 h. (B) HT-29 and LoVo cells were treated with 200 μM of NAR for 24 h. Cell viability was measured using MTT assay system and expressed as % cell viability. *p<0.05 compared to cells without NAR. (C) HCT116 cells were treated with NAR at the indicated concentrations for 24 h. Cell lysates were subjected to SDS-PAGE and Western blot was performed using antibodies against Cleaved PARP and actin.
Effect of NAR on ATF3 expression in human colon cancer cells
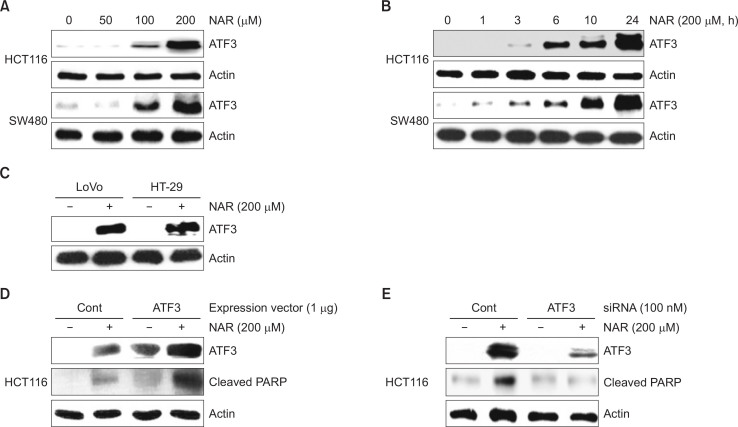
To test whether NAR affects ATF3 expression, we evaluated ATF3 expression in HCT116 and SW480 cells by Western blot. As shown in Fig. 2A, ATF3 expression was increased in NAR (100 and 200 μM)-treated HCT116 and SW480 cells. In time-course experiments (Fig. 2B), ATF3 expression started to increase at 3 h or 1 h after NAR treatment in HCT116 and SW480 cells, respectively. We also evaluated the effect of NAR on ATF3 expression in other colon cancer cells such as LoVo and HT-29. As a result (Fig. 2C), NAR-mediated ATF3 upregulation was observed in both LoVo and HT-29 cells. Next, HCT116 cells were transfected with ATF3 overexpression vector or ATF3 siRNA to investigate whether NAR-mediated ATF3 overexpression results in apoptosis. As shown in Fig. 2D, 2E, NAR-mediated cleavage of PARP was increased in ATF3 overexpression and decreased in ATF3 knockdown by ATF3 siRNA. These data indicate that ATF3 may be an important molecular target for NAR-mediated apoptosis.
Fig. 2.
Effect of NAR-mediated ATF3 expression on apoptosis. (A) HCT116 and SW480 cells were treated with NAR at the indicated concentrations for 24 h. (B) HCT116 and SW480 cells were treated with 200 μM of NAR for the indicated times. (C) HT-29 and LoVo cells were treated with 200 μM of NAR for 24 h. (D) HCT116 cells was transfected with empty- or ATF3 expression vector for 24 h and then treated with 200 μM of NAR for 24 h. (E) ATF3 siRNA was transfected into HCT116 for 48 h and then NAR was treated for 24 h. All cell lysates were subjected to SDS-PAGE and Western blot was performed using antibodies against ATF3, Cleaved PARP or actin.
NAR-mediated ATF3 expression is involved in transcriptional upregulation of ATF3 gene
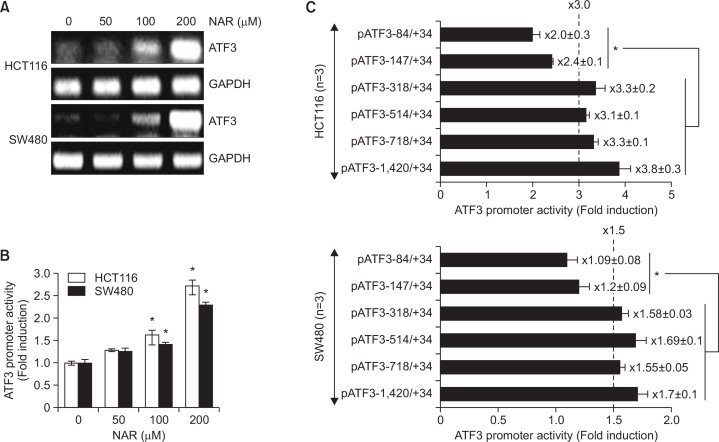
To elucidate whether NAR-mediated ATF3 increase is associated with transcriptional regulation, we measured the mRNA level of ATF3. As shown in Fig. 3A, ATF3 mRNA was increased in HCT116 and SW480 cells treated with NAR, which similar to protein expression. In addition, we measured ATF3 promoter activity using ATF3 promoter luciferase constructs (pATF3−1420/+34) to confirm the transcriptional regulation of ATF3 gene by NAR. As shown in Fig. 3B, NAR dose-dependently increased ATF3 promoter activity in HCT116 and SW480 cells.
Fig. 3.
NAR increases transcriptional activity of ATF3 gene. (A) HCT116 and SW480 cells were treated with NAR at the indicated concentrations for 24 h. Total RNA was isolated and RT-PCR was performed (B) The pATF3-1420/+34 construct (1 μg) was co-transfected with pRL-null vector (0.1 μg). The cells were treated with NAR at the indicated concentrations for 24 h and then luciferase activity was measured. *p<0.05 compared to cells without NAR. (C) HCT116 and SW480 cells were transfected with indicated ATF3 deletion promoter constructs (1 μg) with pRL-null vector (0.1 μg). The cells were treated with DMSO or 200 μM of NAR for 24 h and luciferase activity was measured. *p<0.05 compared to cells transfected with pATF3−1420/−318 (Fold induction was above 3.0 in HCT116 and 1.5 in SW480 cells).
Next, we performed the promoter assay using different size of ATF3 promoter constructs to elucidate the promoter region responsible for NAR-mediated transcriptional upregulation of ATF3 gene. HCT116 and SW480 cells were transfected with six different size of ATF3 promoter constructs (pATF3−1420/+34, pATF3−718/+34, pATF3−514/+34, pATF3−318/+34, pATF3−147/+34 and pATF3−84/+34) and then treated with NAR or DMSO. As shown in Fig. 3C, NAR-mediated increase of ATF3 transactivation was above 3- or 1.5-fold in HCT116 and SW480 cells transfected with pATF3−318/+34, pATF3−514/+34, pATF3−718/+34 and pATF3−1420/+34, respectively. However, NAR slightly increased ATF3 promoter activity in pATF3−84/+34 and −147/+34 transfected HCT116 and SW480 cells. These data indicate that −317/−148 region of ATF3 promoter may be responsible for NAR-mediated ATF3 promoter activation.
NAR-mediated ATF3 expression is dependent on p38 activation, but not ERK1/2, JNK, GSK3β and NF-κB
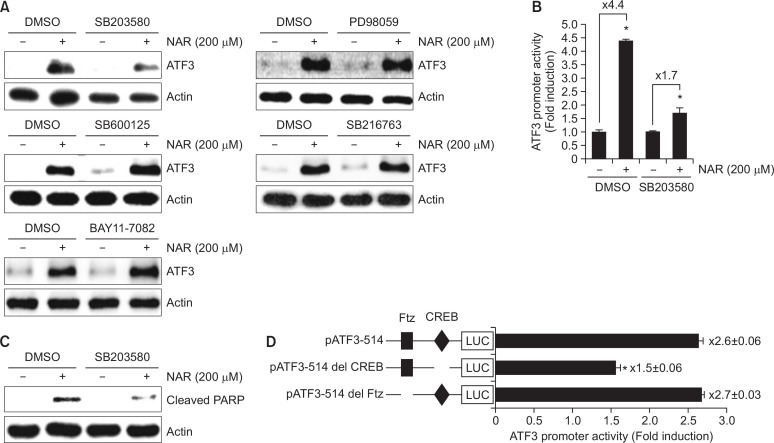
To elucidate upstream kinases involved in NAR-mediated ATF3 expression, HCT116 cells were pretreated with PD98059 (ERK1/2 inhibitor), SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), BAY 11-7082 (NF-κB inhibitor) or SB216763 (GSK3β inhibitor) for 2 h and then co-treated with NAR for the additional 6 h. As shown in Fig. 4A, p38 inhibition by SB203580 attenuated NAR-mediated ATF3 expression. However, inhibitions of ERK1/2, JNK, NF-κB and GSK3β did not affect ATF3 expression by NAR compared to the cell without the treatment of inhibitors. To confirm that the effect of p38 in NAR-mediated ATF3 expression, ATF3 promoter activity was tested in HCT116 cells transfected with ATF3 promoter constructs (pATF3-1420/+34). As shown in Fig. 4B, p38 inhibition by SB203580 attenuated NAR-mediated ATF3 promoter activation. Next, we evaluated the effect of p38 inhibition on NAR-mediated cleavage of PARP and observed that p38 inhibition by SB203580 blocked PARP cleavage by NAR (Fig. 4C). These data indicate that p38 might act as a regulator for NAR-mediated ATF3 expression and apoptosis. To identify the potential regulatory cis-acting elements associated with p38-dependent ATF3 activation by NAR, each site-deleted ATF3 promoter constructs (Del-CREB and Del-Ftz) were transfected into HCT116 cells and treated with 200 μM of NAR for 24 h. As shown in Fig. 4D, NAR-induced ATF3 promoter activity was significantly decreased when the CREB site was deleted. However, the deletion of Ftz sites did not affect ATF3 promoter activity by NAR. These data indicated that CREB is an important region in NAR-induced ATF3 expression.
Fig. 4.
Dependency of p38 in NAR-mediated ATF3 activation (A) HCT116 cells were pretreated with SB203580 (40 μM, p38 inhibitor), PD98059 (40 μM, ERK1/2 inhibitor), SP600125 (40 μM, JNK inhibitor), SB216763 (20 μM, GSK3β inhibitor) or BAY11-7082 (20 μM, NF-κB inhibitor) for 2 h and then co-treated with 200 μM of NAR for 6 h. Cell lysates were subjected to SDS-PAGE and Western blot was performed using antibodies against ATF3 or actin. (B) HCT116 cells were transfected with pATF3-1420/+34 construct (1 μg) and pRL-null vector (0.1 μg). The cells were pretreated with 40 μM of SB203580 for 2 h and then co-treated with 200 μM of NAR for 24 h. Then, luciferase activity was measured. *p<0.05 compared to cells without NAR. (C) HCT116 cells were pretreated with SB203580 (40 μM, p38 inhibitor) for 2 h and then co-treated with 200 μM of NAR for 24 h. Cell lysates were subjected to SDS-PAGE and Western blot was performed using antibodies against cleaved PARP or actin. (D) HCT116 cells were transfected with the indicated ATF3 constructs (1 μg) and pRL-null vector (0.1 μg). The cells were treated with 200 μM of NAR for 24 h. Then, luciferase activity was measured. *p<0.05 compared to cells transfected with ATF3 promoter construct without the deletion.
DISCUSSION
The risk of human cancer can be reduced by the consumption of fruits and vegetables (Pan and Ho, 2008) and approximately 70% of anti-cancer drugs have been developed from natural products (Newman et al., 2002; Yeh et al., 2012). One of the mechanisms for the natural anti-cancer products is through the apoptosis (Pan et al., 2008). According recent reports, NAR expresses ERα and ERβ, which induces apoptosis in colon, breast and uterine cancer cell lines (Totta et al., 2004; Virgili et al., 2004; Bulzomi et al., 2012). However, no other molecular targets involved in NAR-mediated apoptosis have been described. Thus, the elucidation of the additional potential molecular target of NAR for proapoptotic effect may be necessary.
Phytochemicals exert their proapoptotic effect through the regulation of various molecular targets associated with apoptosis. There is a growing body of evidences to suggest that phytochemicals with anti-cancer activity induce apoptosis through upregulating ATF3 expression (Baek et al., 2004; Lee et al., 2005b; Lee et al., 2006; Lee et al., 2013; Kim et al., 2015), which indicates that ATF3 may be one of the important molecular targets for chemoprevention of human colon cancer. In our study, NAR activated ATF3 expression in human colon cancer cell lines such as HCT116, SW480, LoVo and HT-29. In addition, ATF3 overexpression increased NAR-mediated cleavage of PARP and ATF3 knockdown attenuated PARP cleavage by NAR. These findings suggest that ATF3 may be one of the molecular targets for NAR-mediated apoptosis in human colon cancer cells. There are observations indicating that ATF3 inhibits cancer cell proliferation through activating p53 (Yan et al., 2005; Wang et al., 2010) and downregulating cyclin D1 by transcriptional suppression (Lu et al., 2006b). We have reported that NAR downregulates cyclin D1 protein level but not mRNA level resulting in the growth arrest of human colon cancer cells (Song et al., 2015), which indicates that NAR-mediated downregulation of cyclin D1 may be independent on ATF3 activation. Although ATF3 has been regarded as a common molecular target for the proapoptotic effect, specific mechanisms for the apoptotic effect of ATF3 remain nuclear. Thus, the further mechanistic study how NAR-mediated ATF3 activation induces apoptosis will be demanded.
ATF3 expression is mainly regulated through its transcription (Hai et al., 1999). Our data shows that NAR activated the expression of ATF3 mRNA and ATF3 promoter activity, which indicates NAR-mediated ATF3 expression may be dependent on transcription. In addition, we determined that NAR-responsible sites for ATF3 transcriptional activity might be between the −317/−148 region in ATF3 promoter.
There is growing evidence that ATF3 expression is regulated by a variety of the upstream kinases (Cai et al., 2000; Baek et al., 2004; Totta et al., 2004). So, we examined whether NAR-mediated ATF3 activation is associated with the activation of ERK1/2, p38, JNK, NF-κB or GSK3β, and found that NAR-mediated ATF3 expression and promoter activity was suppressed in p38 inhibition, but not in the inhibition of other kinases such as ERK1/2, JNK, NF-κB or GSK3β, which indicates that p38 may be an important upstream kinase associated with NAR-mediated ATF3 activation. In the previous study, we have reported that NAR induces p38 activation, which induces cyclin D1 proteasomal degradation in human colon cancer cells (Song et al., 2015). To date, there has been agreement that p38 activation is involved in the induction of ATF3 via various signals such as transcription factors and ROS (Lu et al., 2006a). Among transcription factors, CREB has been reported to plays a role in ATF3 induction (Lu et al., 2006a). In present study, we found that CREB deletion attenuated NAR-mediated ATF3 promoter activation, indicating that CREB may be an important regulator for p38 dependent-ATF3 activation by NAR. In addition, Ahamad et al. has reported that NAR-mediated apoptotic and anti-proliferative activity may result from ROS generation and cell cycle arrest (Ahamad et al., 2014) and ROS have been known to activate p38. Therefore, NAR-induced p38 activation may result from ROS and ROS production may contribute in part to NAR-mediated ATF3 activation.
Taken together, our findings demonstrate that NAR increases the transcriptional activation and increase of ATF3 protein level through p38 activation, and NAR-mediated ATF3 expression contributes least in part to apoptosis in human colon cancer cells. Furthermore, the current study provides information on the apoptotic effect and the potential molecular mechanism of NAR.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2053448).
REFERENCES
- Ahamad MS, Siddiqui S, Jafri A, Ahmad S, Afzal M, Arshad M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS One. 2014;9:e110003. doi: 10.1371/journal.pone.0110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004;25:2425–2432. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]
- Bleiberg H, Vandebroek A, Deleu I, Vergauwe P, Rezaei Kalantari H, D’Haens G, Paesmans M, Peeters M, Efira A, Humblet Y. A phase II randomized study of combined infusional leucovorin sodium and 5- FU versus the leucovorin calcium followed by 5-FU both in combination with irinotecan or oxaliplatin in patients with metastatic colorectal cancer. Acta Gastroenterol Belg. 2012;75:14–21. [PubMed] [Google Scholar]
- Bulzomi P, Bolli A, Galluzzo P, Acconcia F, Ascenzi P, Marino M. The naringenin-induced proapoptotic effect in breast cancer cell lines holds out against a high bisphenol a background. IUBMB Life. 2012;64:690–696. doi: 10.1002/iub.1049. [DOI] [PubMed] [Google Scholar]
- Cai Y, Zhang C, Nawa T, Aso T, Tanaka M, Oshiro S, Ichijo H, Kitajima S. Homocysteine-responsive ATF3 gene expression in human vascular endothelial cells: activation of c-Jun NH(2)-terminal kinase and promoter response element. Blood. 2000;96:2140–2148. [PubMed] [Google Scholar]
- Ekambaram G, Rajendran P, Magesh V, Sakthisekaran D. Naringenin reduces tumor size and weight lost in N-methyl-N′-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Nutr Res. 2008;28:106–112. doi: 10.1016/j.nutres.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Esmaeili MA, Alilou M. Naringenin attenuates CCl induced hepatic inflammation by the activation of Nrf2 mediated pathway in rats. Clin Exp Pharmacol Physiol. 2014;41:416–422. doi: 10.1111/1440-1681.12230. [DOI] [PubMed] [Google Scholar]
- Ganapathy E, Peramaiyan R, Rajasekaran D, Venkataraman M, Dhanapal S. Modulatory effect of naringenin on N-methyl-N′-nitro-N-nitrosoguanidine- and saturated sodium chloride-induced gastric carcinogenesis in male Wistar rats. Clin Exp Pharmacol Physiol. 2008;35:1190–1196. doi: 10.1111/j.1440-1681.2008.04987.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Lafuente A, Guillamon E, Villares A, Rostagno MA, Martinez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/S0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- Kanno S, Tomizawa A, Ohtake T, Koiwai K, Ujibe M, Ishikawa M. Naringenin-induced apoptosis via activation of NF-kappaB and necrosis involving the loss of ATP in human promyelo-leukemia HL-60 cells. Toxicol Lett. 2006;166:131–139. doi: 10.1016/j.toxlet.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Lee J, Park Y, Lee SH. ATF3 Mediates Anti-Cancer Activity of Trans-10, cis-12-Conjugated Linoleic Acid in Human Colon Cancer Cells. Biomol Ther. 2015;23:134–140. doi: 10.4062/biomolther.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SM. Dietary flavonoid and cancer prevention: evidence and potential mechanism. Crit Rev Oncog. 1997;8:47–69. doi: 10.1615/CritRevOncog.v8.i1.30. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park CH, Jung KC, Rhee HS, Yang CH. Negative regulation of beta-catenin/Tcf signaling by naringenin in AGS gastric cancer cell. Biochem Biophys Res Commun. 2005a;335:771–776. doi: 10.1016/j.bbrc.2005.07.146. [DOI] [PubMed] [Google Scholar]
- Lee SH, Bahn JH, Whitlock NC, Baek SJ. Activating transcription factor 2 (ATF2) controls tolfenamic acid-induced ATF3 expression via MAP kinase pathways. Oncogene. 2010;29:5182–5192. doi: 10.1038/onc.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim JS, Yamaguchi K, Eling TE, Baek SJ. Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun. 2005b;328:63–69. doi: 10.1016/j.bbrc.2004.12.138. [DOI] [PubMed] [Google Scholar]
- Lee SH, Min KW, Zhang X, Baek SJ. 3,3′-diindolyl-methane induces activating transcription factor 3 (ATF3) via ATF4 in human colorectal cancer cells. J Nutr Biochem. 2013;24:664–671. doi: 10.1016/j.jnutbio.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Yamaguchi K, Kim JS, Eling TE, Safe S, Park Y, Baek SJ. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis. 2006;27:972–981. doi: 10.1093/carcin/bgi268. [DOI] [PubMed] [Google Scholar]
- Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. Biochem J. 2006a;401:559–567. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Wolfgang CD, Hai T. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J Biol Chem. 2006b;281:10473–10481. doi: 10.1074/jbc.M509278200. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM, Holbeck S, Sausville EA. Natural products and derivatives as leads to cell cycle pathway targets in cancer chemotherapy. Curr. Cancer Drug Targets. 2002;2:279–308. doi: 10.2174/1568009023333791. [DOI] [PubMed] [Google Scholar]
- Pan MH, Ghai G, Ho CT. Food bioactives, apoptosis, and cancer. Mol Nutr Food Res. 2008;52:43–52. doi: 10.1002/mnfr.200700380. [DOI] [PubMed] [Google Scholar]
- Pan MH, Ho CT. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev. 2008;37:2558–2574. doi: 10.1039/b801558a. [DOI] [PubMed] [Google Scholar]
- Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Song HM, Park GH, Eo HJ, Lee JW, Kim MK, Lee JR, Lee MH, Koo JS, Jeong JB. Anti-Proliferative Effect of Naringenin through p38-Dependent Downregulation of Cyclin D1 in Human Colorectal Cancer Cells. Biomol Ther. 2015;23:339–344. doi: 10.4062/biomolther.2015.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totta P, Acconcia F, Leone S, Cardillo I, Marino M. Mechanisms of naringenin-induced apoptotic cascade in cancer cells: involvement of estrogen receptor alpha and beta signalling. IUBMB Life. 2004;56:491–499. doi: 10.1080/15216540400010792. [DOI] [PubMed] [Google Scholar]
- Virgili F, Acconcia F, Ambra R, Rinna A, Totta P, Marino M. Nutritional flavonoids modulate estrogen receptor alpha signaling. IUBMB Life. 2004;56:145–151. doi: 10.1080/15216540410001685083. [DOI] [PubMed] [Google Scholar]
- Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, Kong AN. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12:1281–1305. doi: 10.2174/187152012803833026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Mo P, Ren S, Yan C. Activating transcription factor 3 activates p53 by preventing E6-associated protein from binding to E6. J Biol Chem. 2010;285:13201–13210. doi: 10.1074/jbc.M109.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Lee SH, Kim JS, Wimalasena J, Kitajima S, Baek SJ. Activating transcription factor 3 and early growth response 1 are the novel targets of LY294002 in a phosphatidylinositol 3-kinase-independent pathway. Cancer Res. 2006;66:2376–2384. doi: 10.1158/0008-5472.CAN-05-1987. [DOI] [PubMed] [Google Scholar]
- Yan C, Lu D, Hai T, Boyd DD. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. EMBO J. 2005;24:2425–2435. doi: 10.1038/sj.emboj.7600712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CC, Yang JI, Lee JC, Tseng CN, Chan YC, Hseu YC, Tang JY, Chuang LY, Huang HW, Chang FR, Chang HW. Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement Altern Med. 2012;12:142. doi: 10.1186/1472-6882-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HR, Liu CJ, Yeh CC. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem Biol Interact. 2015;235:1–9. doi: 10.1016/j.cbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, Hai T, Whelan J. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- Yoon H, Kim TW, Shin SY, Park MJ, Yong Y, Kim DW, Islam T, Lee YH, Jung KY, Lim Y. Design, synthesis and inhibitory activities of naringenin derivatives on human colon cancer cells. Bioorg Med Chem Lett. 2013;23:232–238. doi: 10.1016/j.bmcl.2012.10.130. [DOI] [PubMed] [Google Scholar]