Abstract
Chaetominine is a quinazoline alkaloid originating from the endophytic fungus Aspergillus fumigatus CY018. In this study, we showed evidence that chaetominine has cytotoxic and apoptotic effects on human leukemia K562 cells and investigated the pathway involved in chaetominine-induced apoptosis in detail. Chaetominine inhibited K562 cell growth, with an IC50 value of 35 nM, but showed little inhibitory effect on the growth of human peripheral blood mononuclear cells. The high apoptosis rates, morphological apoptotic features, and DNA fragmentation caused by chaetominine indicated that the cytotoxicity was partially caused by its pro-apoptotic effect. Under chaetominine treatment, the Bax/Bcl-2 ratio was upregulated (from 0.3 to 8), which was followed by a decrease in mitochondrial membrane potential, release of cytochrome c from mitochondria into the cytosol, and stimulation of Apaf-1. Furthermore, activation of caspase-9 and caspase-3, which are the main executers of the apoptotic process, was observed. These results demonstrated that chaetominine induced cell apoptosis via the mitochondrial pathway. Chaetominine inhibited K562 cell growth and induced apoptotic cell death through the intrinsic pathway, which suggests that chaetominine might be a promising therapeutic for leukemia.
Keywords: Cytotoxic, Apoptosis, Mitochondrial pathway, K562 cell
INTRODUCTION
Natural products play an important role in chemotherapeutic agent discovery (Newman and Cragg, 2012), and natural products from ocean organisms have been a goldmine for anti-cancer bioactive compounds (Chau et al., 2005; Sarfaraj et al., 2012). Half of the anti-cancer drugs approved by the US FDA during 1981–2002 were bioactive compounds or metabolites from ocean organisms (Vinothkumar and Parameswaran, 2013). A great number of alkaloid secondary metabolites from plants and fungi have shown high potential in cancer studies. These alkaloids are often able to control the death, proliferation, or growth of cancer cell lines (Wink, 2007). Recent evidence shows that many of these selectively affect cancer cells by regulating mitochondria-dependent apoptosis (Kaminskyy et al., 2008; Rovini et al., 2011). Moreover, alkaloids can modify Bcl-2 family members to alter susceptibility to apoptosis (Urra et al., 2013).
Chaetominine is a secondary metabolite isolated from a submerged liquid culture of the marine endophytic fungus Aspergillus fumigatus CY018 (Lu et al., 2014). The quinazolinone alkaloid structure of chaetominine (Fig. 1) imparts attractive bioactivity (Luo et al., 2014). Chaetominine was shown to be cytotoxic against the human leukemia cell line K562 and the colon cancer cell line SW1116, with IC50 values of 21 nM and 28 nM, respectively, and was more potent than the cytotoxicity of the positive reference compound 5-fluorouracil (5-FU), with IC50 values of 33 nM and 76 nM, respectively (Jiao et al., 2006). However, the cytotoxicity of chaetominine towards normal human cells has not been determined, and the cellular mechanisms behind the mode or pathway of chaetominine-induced cell death have not been fully explored.
Fig. 1.

Chemical structure of chaetominine.
A common characteristic of cancer is uncontrolled cell growth (Brown and Attardi, 2005; Finkel et al., 2007), and induction of apoptosis is a popular strategy for cancer therapy (Brown and Attardi, 2005). Approaches aimed at controlling cell death could avoid drug resistance and enhance the efficiency of other anticancer agents (Beesoo et al., 2014). Apoptosis can be easily identified by characteristic morphological changes and the appearance of fragmented nuclear DNA (Lemasters, 2005). Mitochondrial outer membrane disruption, mainly regulated by Bcl-2 family proteins (Martinou and Youle, 2011; Czabotar et al., 2014), is known to be a central event in the intrinsic (or mitochondrial) apoptosis pathway (Danial and Korsmeyer, 2004). This mitochondrial disruption is followed by cytochrome c (cyt-c) release and formation of the apopto-some, consisting of cyt-c, activated apoptotic protease activating factor-1 (Apaf-1), and caspase-9 (Pradelli et al., 2010). Then, caspase-3 is activated prior to cell death (Lamkanfi et al., 2007).
In a previous report, the fungal metabolite chaetominine exhibited cytotoxicity against some cancer cell lines. However, studies devoted to the effects of chaetominine and the underlying mechanisms are still needed to provide insight into the performance and potential clinical applications of chaetominine. This study assessed the selective cytotoxic effects of chaetominine on human leukemia cells and normal primary cells, and elucidated the mechanism of these chaetominine-induced effects.
MATERIALS AND METHODS
Cell culture
The human leukemia cell line K562 was obtained from Shanghai Institutes for Biological Sciences. K562 cells were cultured in IMDM (GIBCO BRL, NY, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO BRL). Human peripheral blood mononuclear cells (HPBMCs) purchased from Lifeline Cell Technology (MD, USA) were grown in RPMI-1640 medium (GIBCO BRL) with 10% FBS. Cells were maintained at 37°C in a humidified incubator (SANYO, Osaka, Japan) with 5% CO2.
Reagents and antibodies
Chaetominine was extracted from a submerged liquid culture of Aspergillus fumigatus CY018 with macroporous adsorption resin. The structure was confirmed by electrospray ionization mass spectrometry (ESI-MS), and the purity of the extract (99.8%) was determined by high-performance liquid chromatography (Supplementary data). The control compound 5-FU (purity >99%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). All primary antibodies were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA).
Cytotoxicity analysis
The half-maximal inhibitory concentration (IC50) was determined by the MTT assay as described previously (Choi et al., 2015). Cells were seeded at a density of 104 cells per milliliter and then treated with chaetominine for various times, and 5-FU was used as the positive control drug. The IC50 values were calculated with a microplate reader (SpectraMax® i3, Wals, Austria) at an experimental wavelength of 570 nm and a reference wavelength of 630 nm.
Evaluation of nuclear morphology
The nuclear morphological changes in apoptotic cells were assessed using Hoechst 33258 staining (Luo et al., 2010). K562 cells were placed into a 6-well culture plate and incubated with or without chaetominine for 24 h or 48 h. The cells were collected with microtubes and washed with phosphate buffered saline (PBS). Subsequently, the cells were fixed, washed, and stained according to the manufacturer’s instructions for the Hoechst staining kit (Beyotime, JS, China). The apoptotic cells were observed and photographed under a fluorescence microscope (Olympus BX51, Tokyo, Japan).
Annexin V-FITC/PI staining
Apoptotic cell rates were determined by flow cytometry based on the fact that phosphatidylserines (PS) becomes located outside the membrane during apoptosis (Rieger et al., 2011). Following the manufacturer’s protocol for the annexin V-FITC/PI kit (Beyotime), cells were first incubated in annexin V binding buffer and annexin V-FITC for 10 min in the dark. After centrifugation, the cells were suspended in a mix containing annexin V and PI buffer. Then, the apoptotic cells were analyzed with a flow cytometer (FACSAria; Becton Dickinson, NJ, USA). The annexin V-FITC-positive and PI-negative cells were considered to be in early apoptosis, while annexin VFITC- and PI-positive cells were considered to be in late apoptosis.
DNA fragmentation detection
K562 cells were seeded in 6-well plates and incubated with the desired concentrations of chaetominine. According to the manufacturer’s instructions for the Apoptotic DNA Ladder Detection Kit (KeyGen, NJ, China), cells were pelleted in a microcentrifuge tube and washed twice with PBS before DNA preparation using the manufacturer’s enzymes. An aliquot (5–10 μL) of the sample was loaded onto a 1.5% agarose gel, and the gel was run at 2–4 V/cm for over 3 hours (Bio-Rad, Hercules, CA, USA). Ethidium bromide-stained small fragmented DNA was visualized with a trans-illuminator under UV light and photographed (Furi Co., Ltd, SH, China).
Measurement of mitochondrial membrane potential
Changes in mitochondrial membrane potential (MMP) were evaluated using the JC-1 Mitochondrial Potential Assay Kit (Beyotime). Mitochondrial depolarization occurring in the early stage of apoptosis was evidenced by a fluorescence emission shift from green (JC-1 monomer) to red (J-aggregate), and the results showed potential-dependent accumulations in mitochondria (Jang et al., 2015). K562 cells were seeded in a 6-well plate at 2×105 cells per well and then treated with chaetominine (0, 25, 50 or 100 nM) for 24 h or 48 h. Approximately 105 cells were harvested, incubated in dye-containing medium, and washed twice in staining buffer. Stained cells were resuspended in buffer and analyzed by flow cytometry (FACSAria). JC-1 monomers and J-aggregates can be detected separately in the FL1 and FL2 channels, respectively, and variations in the red/green fluorescence intensity ratio represent changes in MMP.
Western blot analysis
After cells were incubated with different concentrations of chaetominine, whole cell lysates and mitochondrial protein extracts (Zhang et al., 2014) were prepared, and the protein concentration was determined with the BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Protein samples (20 μg/lane) containing 0.01% bromophenol blue were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 8–12%), and transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA). The PVDF membrane was blocked overnight with 5% non-fat dry milk in TBS-Tween and then incubated overnight with the appropriate primary antibodies (diluted with blocking buffer) at 4°C. After washing three times with TBS-T, membranes were incubated overnight with horseradish peroxidase (HRP)-labeled secondary antibodies. Proteins were detected by chemiluminescence and quantified by Quantity One software (v4.62; Bio-Rad).
Caspase activity analysis
Caspase-3 and caspase-9 activity were analyzed with a caspase assay kit (Beyotime). The kit is based on the spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the substrates Ac-DEVD-pNA and Ac-LEHD-pNA. After incubation with chaetominine, K562 cells were collected and washed with PBS. Cells were suspended in lysis buffer and incubated on ice. After centrifugation, the supernatant was incubated with the substrates. Substrate hydrolysis was monitored at 405 nm with a microplate reader (SpectraMax® i3, Austria), and the fold increase in caspase activity was determined by comparing the results of chaetomi-nine-treated samples to that of the untreated controls.
Statistical analysis
The data were expressed as mean ± SD. Group data were compared using one-way ANOVA with Newman-Keuls tests.
RESULTS
Cytotoxic effects of chaetominine on K562 cells
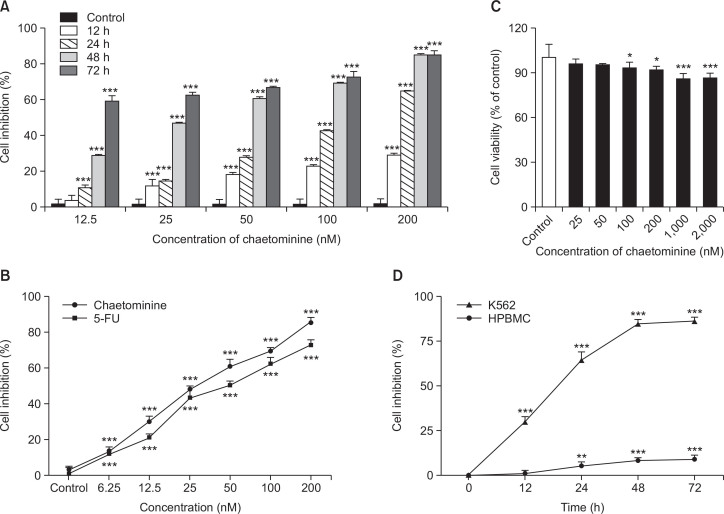
The effect of chaetominine on the growth of the human leukemia K562 cell line was tested using the MTT assay with 5-FU as a positive control. As shown in Fig. 2A, chaetominine suppressed the proliferation of K562 cells in a dose- and time-dependent manner. Chaetominine displayed greater cytotoxic activity (IC50 value, 35 ± 2.03 nM) than 5-FU (IC50 value, 55 ± 1.07 nM; Fig. 2B).
Fig. 2.
Cytotoxic effects of chaetominine and 5-FU assessed by the MTT assay. (A) Effects of chaetominine on the proliferation of K562 cells. (B) Comparison of the growth-inhibiting effects of chaetominine and 5-FU in K562 cells. Cells were exposed to different concentrations of chaetominine for 48 h before the assay. (C) Effects of chaetominine on the viability of HPBMCs. (D) Different effects of chaetominine on the inhibition of HPBMCs and K562 cells. Data are expressed as means ± SD (n=3). *p<0.05, **p<0.01, ***p<0.001, compared to the respective control group.
Cytotoxic effect of chaetominine on primary human peripheral blood cells
The cytotoxicity of chaetominine on HPBMCs was evaluated by the MTT assay. The treatment duration was 48 h, with exposure concentrations ranging from 25 to 2,000 nM. Over 86% of the HPBMCs survived at the highest concentration of chaetominine (Fig. 2C). Fig. 2D shows the toxicity of 200 nM chaetominine after different treatment durations in K562 cells and HPBMCs. In K562 cells, the percent inhibition reached a plateau after 48 h of incubation, and the maximum percent inhibition was about 90%. In comparison, under the same treatment conditions, the percent inhibition of HPBMCs was less than 10%.
Nuclear morphology changes in K562 cells induced by chaetominine
To investigate the mode of cell death caused by chaetomi-nine, Hoechst 33258 staining was used to examine the nuclear morphological changes in K562 cells in the presence of 0, 25, 50, and 100 nM chaetominine for 24 h or 48 h. The results showed that in the absence of chaetominine, the cells proliferated significantly, with rounded normal-sized nuclei (Fig. 3A). In the chaetominine-treated groups, cell numbers decreased with increasing concentration, and the nuclei shrank and became bright blue. With increasing incubation time and/or chaetominine dose, apoptotic bodies shaped like crescents or rings emerged.
Fig. 3.
Effects of chaetominine on apoptosis of K562 cells. (A) Morphology changes by Hoechst 33258 staining. Apoptosis morphological features (white arrows) induced by chaetominine were observed by fluorescence microscopy. (a) and (e) were untreated controls incubated for 24 h and 48 h, respectively; (b), (c), (d) and (f), (g), (h) were treated with 25, 50, and 100 nM chaetominine for 24 h and 48 h, respectively. Each experiment was repeated three times. (B) Apoptosis rate determined by flow cytometry. The percentage of total apoptotic cells represent the average (mean ± SD, n=3) of the early and late apoptosis rates. The same symbols indicate no significant difference between adjacent groups (p>0.05), while different symbols above the bar graph indicate significant differences (p<0.05). (C) Chaetominine-induced DNA cleavage by DNA ladder assays. Lane 1: DNA ladder marker, lane 2: untreated control group, lane 3: treated with 25 nM chaetominine, lane 4: treated with 50 nM chaetominine, lane 5: treated with 100 nM chaetominine. The experiment was repeated three times, and a representative gel is shown.
Apoptosis of K562 cells induced by chaetominine
The rate of apoptosis induced by chaetominine was evaluated by dual staining with Annexin V-FITC and propidium iodide (PI) of K562 cells treated with chaetominine (0, 25, 50, and 100 nM) for 24 h or 48 h (Fig. 3B). Cell apoptosis rates varied significantly in a dose- and time-dependent manner. Untreated control cells were mostly viable, whereas the rate of early and late apoptosis simultaneously increased with increasing concentrations of chaetominine. Thus, chaetominine could promote apoptosis of K562 cells.
DNA fragmentation of K562 cells induced by chaetominine
To investigate the nuclear changes in apoptosis caused by chaetominine, DNA fragmentation in K562 cells was visualized using agarose gel electrophoresis (Araújo et al., 2012). Nuclear DNA was isolated from K562 cells after exposure to different concentrations of chaetominine and electrophoresed on a 1.5% agarose gel. Photographs taken under UV (312 nm) illumination (Fig. 3C) showed that the control group (lane 2) did not exhibit signs of apoptosis (DNA fragmentation), whereas the chaetominine-treated groups displayed a DNA ladder. Changes in fluorescence intensity and a ladder-like DNA fragmentation pattern, due to chaetominine-induced apoptosis, were evident in lanes 3, 4, and 5.
MMP disruption in K562 cells by chaetominine
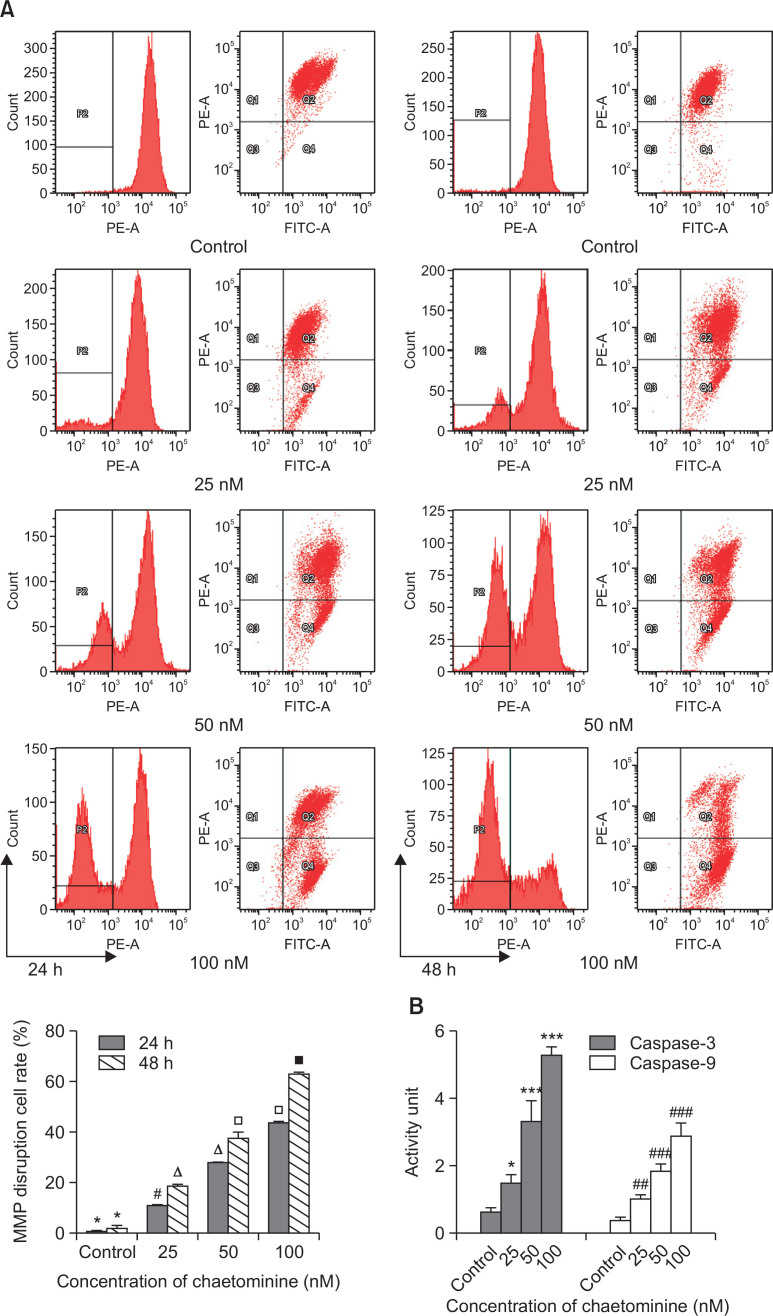
To evaluate mitochondrial membrane disruption during apoptosis, potential-dependent accumulation in mitochondria was analyzed by flow cytometry using the cationic dye JC-1. Increased red/green fluorescence intensity ratios were detected, resulting from mitochondrial depolarization in the early stage of apoptosis. Compared with the untreated groups, large numbers of chaetominine-treated cells shifted from the Q2 area to the Q4 area (Fig. 4A) due to the release of JC-1 from the membrane into the cytoplasm. The rate of MMP-disrupted cells gradually increased in a dose- and time-dependent manner (24 h: 1.4%, 11.9%, 28.3%, and 44.2%; 48 h: 3.0%, 19.4%, 37.8%, and 62.8%; at 0, 25, 50, and 100 nM, respectively). This result suggested that chaetominine-induced MMP disruption involved the intrinsic apoptosis pathway.
Fig. 4.
(A) Effects of chaetominine on mitochondrial membrane potential (MMP) of K562 cells detected by flow cytometry. Staining with JC-1 dye showed increased red fluorescence as MMP decreased. The disrupted MMP rates are the means ± SD (n=3). The same symbols indicate no significant difference between adjacent groups (p>0.05), whereas different symbols above the bar graph indicate significant differences (p<0.05). (B) Caspase-3 and caspase-9 activation by chaetominine in K562 cells. K562 cells were incubated for 48 h before detection by spectrophotometry (405 nm). Data are expressed as means ± SD (n=3). *p<0.05, ***p<0.001, #p<0.05, ##p<0.01, ###p<0.001, compared to the respective control group.
Apoptosis-related protein regulation in chaetominine-treated K562 cells
To elucidate the signaling pathway of chaetominine-induced apoptosis, the expression of several proteins was estimated by western blot analysis. Bcl-2 family proteins control cell death specifically by mediating the mitochondrial pathway (Adams and Cory, 2007). The interaction between Bax and Bcl-2 on the mitochondrial outer membrane contributes to the release of apoptogenic factors, particularly cyt-c, into the cytosol (Li et al., 1997). Then, cyt-c promotes the activation of caspase-9 and Apaf-1 prior to the downstream actions of cell death (Riedl and Salvesen, 2007). The expression levels of Bax, cytosolic cyt-c, and Apaf-1 were markedly upregulated by chaetominine, and the expression of Bcl-2 and mitochondrial cyt-c was downregulated in a dose-dependent manner (Fig. 5). After chaetominine treatment, the ratio of Bax/Bcl-2, which regulates apoptosis through the mitochondrial signaling pathway, changed from 0.3 to 8.
Fig. 5.
Expression of apoptosis-related proteins in chaetominine-treated K562 cells. Protein levels of Bax and Bcl-2 (A), cytochrome c (B), and Apaf-1 (C) were determined by western blot analysis in K562 cells after treatment with 0, 25, 50, and 100 nM chaetominine. The bar graphs show densitometric analysis of protein expression relative to the internal reference protein β-actin. All the data are expressed as means ± SD (n=3), different symbols indicate significance difference (p<0.05) compared to the adjacent group.
Caspase activation in chaetominine-treated K562 cells
The activities of the key regulators caspase-3 and -9 in the chaetominine-induced apoptosis pathway were detected by spectrophotometry (Liu et al., 2012). Following induction of apoptosis by chaetominine, caspase-3 and -9 were cleaved to their respective active forms. Compared to the controls (Fig. 4B), cells treated with 25, 50, and 100 nM chaetominine showed 2.5-, 5.5-, and 8.8-fold increases in caspase-3 activity and 2.1-, 4.3-, and 5.6-fold increases in -9 activity, respectively. Chaetominine activated caspase-3 more significantly than caspase-9.
DISCUSSION
For decades, invaluable potential therapeutic agents have been obtained from nature. In our efforts to identify novel candidates for cancer therapy from endophytic fungal secondary metabolites, we isolated chaetominine, a quinazolinone alkaloid from Aspergillus fumigatus CY018, which inhibited cancer cell proliferation in vitro. Various substituted quinazoline or quinazolinone derivatives have been shown to have anti-infectious, anti-inflammatory, and anti-cancer properties (Belofsky et al., 2000; Nakao et al., 2003; Babu et al., 2014). However, the potent effects of chaetominine and the underlying mechanisms have not been well characterized. Our preliminary study showed that the cytotoxic effects of chaetominine were correlated with induction of apoptosis via the mitochondrial pathway in a leukemia cell line.
In this study, we first assessed the cytotoxic effects of chaetominine on a leukemia cell line versus a normal human primary cell line. Chaetominine treatment of K562 cells inhibited proliferation in a dose- and time-dependent manner, with a lower IC50 value (35 nM) than that of 5-FU (55 nM). In contrast, chaetominine showed little cytotoxicity towards normal blood cells at concentrations below 200 nM, which suggested that cancer cells were more sensitive to chaetominine. Therefore, the underlying mechanism should be further explored to understand the effect of chaetominine on cancer cell death.
Morphological changes in chaetominine-treated K562 cells, such as chromatin condensation, were then observed, which confirmed that the cytotoxic effect was associated with its apoptotic effect. The ladder-like DNA fragmentation pattern, containing small DNA fragments, observed in K562 cells treated with chaetominine is another hallmark of apoptosis, which further confirmed that chaetominine-induced apoptotic activity. Flow cytometry analysis of chaetominine-treated K562 cells demonstrated that the apoptotic rate was time- and dose-dependent. These results consistently indicated that chaetominine induced cell death by interfering with apoptosis. Considering the difference between the rates of inhibition in the cell proliferation and apoptosis assays, the high/low inhibition demonstrated that cell death was partially regulated by chaetominine-induced apoptosis. Chaetominine may also affect other cellular functions, including the cell cycle and cell autophagy (Helleday et al., 2008).
Cell apoptosis can be initiated by the intrinsic or extrinsic pathway (Pradelli et al., 2010). Mitochondria are studied as a judgment site of the intrinsic pathway (Kroemer et al., 2007). Mitochondrial outer membrane permeabilization, generally considered one of the earliest cellular events of apoptosis (Pradelli et al., 2010), is regulated by caspase activation in the intrinsic pathway (Martinou and Youle, 2011). Previous studies have shown that the anticancer effect of chaetominine on K562 cells was related to its pro-apoptotic activity. The MMP in K562 cells decreased sharply after chaetominine treatment, which suggested induction of the mitochondrial pathway leading to apoptosis. In addition, two types of caspases (caspase-3 and -9) were simultaneously activated by chaetominine in K562 cells, suggesting the caspase-dependent mitochondrial pathway. Caspase-9 is generally required for apoptosome formation with cyt-c/dATP/Apaf-1, which then transmits the apoptotic signal (Riedl and Salvesen, 2007). In K562 cells, cytochrome c was released from the mitochondria into the cytosol and downstream Apaf-1 was activated in response to chaetominine treatment.
The Bcl-2 protein family controls apoptosis sensitivity as a regulator of MMP. Once the integrity of the mitochondrial membrane is destroyed, mitochondrial permeability transition pores will open to allow efflux of apoptotic factors (Dalla Via et al., 2014). The ratio of Bax/Bcl-2 was upregulated following treatment, indicating that chaetominine induced apoptosis by increasing Bax expression and reducing Bcl-2 expression. Consistent with previous data, the increased ratio of the pro-apoptotic and anti-apoptotic proteins of the Bcl-2 family altered mitochondrial membrane integrity, resulting in cyt-c removal and Apaf-1 activation. Eventually, caspase-3 was activated to trigger the signaling cascade as an effector of the apoptosis pathway.
Most successful current cancer therapeutics rely heavily on triggering tumor cell death. In this study, chaetominine exhibited greater cytotoxicity than the positive control 5-FU in K562 cells. In addition, it selectively killed tumor cells and not normal cells. It is clear that chaetominine could induce apoptotic cell death, which was evidenced by the high apoptosis rate, DNA fragmentation, and caspase activation. The results also showed that chaetominine acts via the intrinsic apoptosis pathway (Fig. 6), as evidenced by the increased Bax/Bcl-2 ratio and consequent cyt-c release and Apaf-1 activation. Based on the current data, which signaling pathway caused the increase in the Bax/Bcl-2 ratio could not be determined. Whether the inhibitory effect of chaetominine on cancer cell growth is related to cell cycle arrest or reversed drug resistance requires further study (Helleday et al., 2008). The present findings provide the necessary incentive for further in vitro and in vivo studies of chaetominine, with an aim to develop a potent leukemia therapy.
Fig. 6.
The apoptosis signaling pathway mediated by chaetominine.
SUPPLEMENTARY DATA
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (2013AA09-2901), and partially financed by the Fundamental Research Funds for the Central Universities (WF1113010), the National Special Fund for State Key Laboratory of Bioreactor Engineering (2060204).
Footnotes
CONFLICT OF INTEREST STATEMENT
All the authors declare that there are no conflicts of interest.
REFERENCES
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo AJ, de Souza AA, da Silva Júnior EN, Marinho-Filho JD, de Moura MA, Rocha DD, Vasconcellos MC, Costa CO, Pessoa C, de Moraes MO, Ferreira VF, de Abreu FC, Pinto AV, Montenegro RC, Costa-Lotufo LV, Goulart MO. Growth inhibitory effects of 3′-nitro-3-phenylamino nor-beta-lapachone against HL-60: a redox-dependent mechanism. Toxicol. In Vitro. 2012;26:585–594. doi: 10.1016/j.tiv.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Babu YR, Bhagavanraju M, Reddy GD, Peters GJ, Prasad VV. Design and synthesis of quinazolinone tagged acridones as cytotoxic agents and their effects on EGFR tyrosine kinase. Arch. Pharm. (Weinheim) 2014;347:624–634. doi: 10.1002/ardp.201400065. [DOI] [PubMed] [Google Scholar]
- Beesoo R, Neergheen-Bhujun V, Bhagooli R, Bahorun T. Apoptosis inducing lead compounds isolated from marine organisms of potential relevance in cancer treatment. Mutat Res. 2014;768:84–97. doi: 10.1016/j.mrfmmm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Belofsky GN, Anguera M, Jensen PR, Fenical W, Köck M. Oxepinamides A–C and fumiquinazolines H–I: bioactive metabolites from a marine isolate of a fungus of the genus Acremonium. Chemistry. 2000;6:1355–1360. doi: 10.1002/(SICI)1521-3765(20000417)6:8<1355::AID-CHEM1355>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- Chau M, Phan K, Nguyen D. Marine natural products and their potential application in the future. AJSTD. 2005;22:297–311. [Google Scholar]
- Choi DW, Lim MS, Lee JW, Chun W, Lee SH, Nam YH, Park JM, Choi DH, Kang CD, Lee SJ, Park SC. The Cytotoxicity of Kahweol in HT-29 Human Colorectal Cancer Cells Is Mediated by Apoptosis and Suppression of Heat Shock Protein 70 Expression. Biomol. Ther. (Seoul) 2015;23:128–133. doi: 10.4062/biomolther.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Dalla Via L, García-Argáez AN, Martínez-Vázquez M, Grancara S, Martinis P, Toninello A. Mitochondrial permeability transition as target of anticancer drugs. Curr Pharm Des. 2014;20:223–244. doi: 10.2174/13816128113199990033. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- Jang YJ, Won JH, Back MJ, Fu Z, Jang JM, Ha HC, Hong S, Chang M, Kim DK. Paraquat Induces Apoptosis through a Mitochondria-Dependent Pathway in RAW264.7 Cells. Biomol. Ther. (Seoul) 2015;23:407–413. doi: 10.4062/biomolther.2015.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao RH, Xu S, Liu JY, Ge HM, Ding H, Xu C, Zhu HL, Tan RX. Chaetominine, a cytotoxic alkaloid produced by endophytic chaetomium sp. IFB-E015. Org Lett. 2006;8:5709–5712. doi: 10.1021/ol062257t. [DOI] [PubMed] [Google Scholar]
- Kaminskyy V, Kulachkovskyy O, Stoika R. A decisive role of mitochondria in defining rate and intensity of apoptosis induction by different alkaloids. Toxicol Lett. 2008;177:168–181. doi: 10.1016/j.toxlet.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ. Dying a thousand deaths: redundant pathways from different organelles to apoptosis and necrosis. Gastroenterology. 2005;129:351–360. doi: 10.1053/j.gastro.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic pro-tease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhao Y, Li J, Xu S, Liu C, Zhu Y, Liang S. The venom of the spider Macrothele raveni induces apoptosis in the myelogenous. Leuk Res. 2012;36:1063–1066. doi: 10.1016/j.leukres.2012.02.025. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhu Y, Jiao R, Tan R, Yao L, Hu W. inventors; Univ. East. China Science & Tech. and Univ. Nanjing, assignee. [Method of preparing fumigaclavine C by symbiotic aspergillus fumigatus of sea crab and culture medium of fumigaclavine C]. Chinese patent CN 103849663. 2014 Jun 11. Chinese.
- Luo M, Liu X, Zu Y, Fu Y, Zhang S, Yao L, Efferth T. Cajanol, a novel anticancer agent from Pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem Biol Interact. 2010;188:151–160. doi: 10.1016/j.cbi.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Luo S-P, Peng Q-L, Xu C-P, Wang A-E, Huang P-Q. Bio-inspired step-economical, redox-economical and protecting-group-free enantioselective total syntheses of (−)-chaetominine and analogues. Chin J Chem. 2014;32:757–770. doi: 10.1002/cjoc.201400413. [DOI] [Google Scholar]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao Y, Kuo J, Yoshida WY, Kelly M, Scheuer PJ. More kapakahines from the marine sponge Cribrochalina olemda. Org Lett. 2003;5:1387–1390. doi: 10.1021/ol026830u. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradelli LA, Bénéteau M, Ricci JE. Mitochondrial control of caspase-dependent and -independent cell death. Cell Mol Life Sci. 2010;67:1589–1597. doi: 10.1007/s00018-010-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp. 2011;50:e2597. doi: 10.3791/2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovini A, Savry A, Braguer D, Carré M. Microtubule-targeted agents: When mitochondria become essential to chemotherapy. Biochim. Biophys. Acta. 2011;1807:679–688. doi: 10.1016/j.bbabio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Sarfaraj HM, Sheeba F, Saba A, Mohd SK. Marine natural products: a lead for anti-cancer. Indian J Geomarine Sci. 2012;41:27–39. [Google Scholar]
- Urra FA, Cordova-Delgado M, Pessoa-Mahana H, Ramírez-Rodríguez O, Weiss-Lopez B, Ferreira J, Araya-Maturana R. Mitochondria: a promising target for anticancer alkaloids. Curr Top Med Chem. 2013;13:2171–2183. doi: 10.2174/15680266113139990150. [DOI] [PubMed] [Google Scholar]
- Vinothkumar S, Parameswaran PS. Recent advances in marine drug research. Biotechnol Adv. 2013;31:1826–1845. doi: 10.1016/j.biotechadv.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Wink M. Molecular modes of action of cytotoxic alkaloids: from DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance. Alkaloids Chem Biol. 2007;64:1–47. doi: 10.1016/S1099-4831(07)64001-2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang H, Xu J, Zhu J, Ding K. Inhibition of cathepsin S induces autophagy and apoptosis in human glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K and JNK signaling pathways. Toxicol Lett. 2014;228:248–259. doi: 10.1016/j.toxlet.2014.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.