Abstract
Iridoid glycosides (mainly geniposide) and crocetin derivatives (crocins) are the two major active constituents in Gardenia jasminoides Ellis. In the present study, geniposide, crocins, crocin-1 and crocetin were separated from gardenia chromatographically. Then, mice were orally administrated with geniposide (400 mg/kg b.w.), crocins (400 mg/kg b.w.), crocin-1 (400 mg/kg b.w.) and crocetin (140 mg/kg b.w.) once daily for 7 days with CCl4. Hepatoprotective properties were evaluated by biochemical parameters: Administration of geniposide, crocins, crocin-1and crocetin significantly lowered serum alanine transaminase (ALT), aspartate transaminase (AST) and alkaline phosphatase (ALP) levels in CCl4-treated mice. The reduced glutathione (GSH) levels and antioxidant enzymes (SOD and CAT) activities were also increased by geniposide, crocins, crocin-1 and crocetin. Histopathological examination of livers showed that these components reduced deformability, irregular arrangement and rupture of hepatocyte in CCl4-treated mice. These biochemical results and liver histopathological assessment demonstrated that geniposide, crocetin derivatives and crocetin show comparative beneficial effects on CCl4-induced liver damage via induction of antioxidant defense. Therefore, contents of geniposide and crocetin derivatives should be both considered for hepatoprotective efficacy of Gardenia jasminoides Ellis.
Keywords: Gardenia jasminoides Ellis, Hepatoprotective, Geniposide, Crocin-1, Crocetin, Histopathological examination
INTRODUCTION
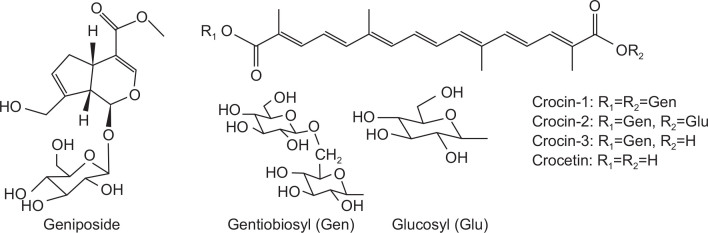
Gardenia jasminoides Ellis (Rubiaceae) is an evergreen shrub widely distributed in the tropical and subtropical regions, growing on mountain slopes or on road sides as an ornamental plant. The dried ripe fruits of this plant have been recorded as Fructus Gardeniae (Chinese herbal name is “zhizi”) in Chinese Pharmacopoeia and included in Traditional Chinese Medicine (TCM) formulations for diuretic, cholagogue, anti-inflammatory, and antipyretic effects (National Commission of Chinese Pharmacopoeia, 2010). Iridoid glycosides (mainly geniposide) and crocetin derivatives (crocins) are the two major active constituents in gardenia fruits (Fig. 1), of which components, characteristics, and activities have been investigated by many researchers (Choi et al., 2001; Wang et al., 2004). In addition, it has been reported that genipin (aglycone of geniposide) has hepatoprotective effect (Kim et al., 2010) and an in vitro study showed that crocetin has protective effect on hepatocyte (Tseng et al., 1995). However, to the best of our knowledge, there is no published literature heretofore concerning the roles of geniposide and crocetin derivatives from gardenia fruits on the CCl4-induced hepatic injury in vivo.
Fig. 1.
Structures of geniposide and crocetin derivatives.
The liver is the major site of xenobiotic metabolism and its injury can be caused by toxic chemicals, drugs, and virus in filtration from ingestion or infection (Lee et al., 2007; Mihailović et al., 2013). Conventional drugs used in pharmacotherapy, such as steroids, vaccines, and antiviral drugs, have shown limited therapeutic benefits and are usually associated with serious risks of toxicity. In the absence of a reliable liver protective drug in the modern system of medicine, natural extracts from medicinal plants are considered to be effective and safe for the treatment of liver disorders (Jaishree and Badami, 2010).
The present study, therefore, focused on the investigation of the biological activities of geniposide and crocetin derivatives from gardenia fruits on the CCl4-induced hepatic injury. Additionally, all crocins are hydrolyzed to yield the same aglycone (crocetin) in intestine before they are absorbed into the blood (Asai et al., 2005). Whether the pharmacokinetics process could influence biological effects of crocetin derivatives, however, has remained unknown. Therefore, current investigation also aimed at comparatively evaluating hepatoprotective effect of orally administered crocins and it’s aglucone crocetin.
MATERIAL AND METHODS
Plant materials
The dried gardenia (Gardenia jasminoides Ellis) fruits were harvested in Yibin City, Sichuan Province, in November 2012, and identified by Hao Zhang. The voucher specimens are deposited at the Department of Pharmacognosy, West China School of Pharmacy, Sichuan University, Sichuan Province, China.
Extraction and isolation
Separation and preparation of geniposide, crocins, crocin-1 and crocetin were conducted according to our previous study with minor modification (Chen et al., 2008a, 2008b). The dried ripe gardenia fruits (2.5 kg) were ground to coarse powder and extracted with 25 L of 40% ethanol (v/v) by cold percolation. After concentration by rotatory evaporator, the extract was dissolved in water and then subjected to a HPD-100 macroporous resin. The column was eluted with water containing increasing amounts (0, 25, 40, 60%, v/v) of ethanol. The dried residue of 25% alcohol-eluted fraction was dissolved in water and then subjected to HPD-100 macroporous resin column eluting with water, 10, 25 and 35% ethanol. The 35% alcohol-eluted fraction was evaporated to dryness and pure geniposide (1) was obtained. The 60% alcohol-eluted fraction (crocins, 2) was subjected to silica gel column chromatography, and eluted with ethylacetate, containing increasing amounts (10, 20, 30, 50%, v/v) of methanol-water (16:13 v/v). A red powder obtained from the fraction of ethylacetate containing 30% methanol-water was crocin-1 (3). Crocetin (4) was prepared by alkaline hydrolyzation with 10% potassium hydroxide solution of the total crocins (Qian et al., 2010).
HPLC analysis
The HPLC was performed as described previously (Jia et al., 2005), on a Shimadzu HPLC system equipped with two LC-10AT VP pumps, CTO-10AS VP column oven, UV-vis SPD-10A VP detector, SCL-10A VP system controller and fitted with a ODS column (4.6×150 mm, 5 μm; Agilent, USA). For determination of crocins and crocetin, the mobile phase was consisted of A (acetonitrile) and B (pure water), which was programmed as follows: from 0 to 15 min, 10 to 30% A. 15.0–20.0 min, linear increase from 30 to 35% A. 20.0–25.0 min, linear increase from 35 to 70% A. 25.0–30.0 min, 70% A. 30.0–40.0 min, linear decrease from 70 to 10% A. For determination of geniposide, isocratic elution of 15% acetonitrile was used according to the literature (National Commission of Chinese Pharmacopoeia, 2010). The flow rate was 1.0 ml/min while chromatogram were recorded at 440 nm for crocetin derivatives and 238 nm for geniposide, respectively.
Animals and treatment
All the animal experimental procedures were approved by the Animal Care and Use Committee of Sichuan University for Nationalities (Chengdu, China), and were conducted upon receipt of the approval, numbered 2014-231, from the Animal Care and Use Committee of Sichuan University. Male Kunming mice (20–22 g) were obtained from the Experimental Animal Center of Sichuan University (Chengdu, China).The animals were housed at 25 ± 2°C under a 12 h light/12 h dark cycle with access to food and water ad libitum. After acclimation for 1 week, the animals were randomly divided into seven groups comprising six mice (n=6) in each group as follows:
Group A: sterile distilled water (10 ml/kg b.w., i.g), served as a normal control.
Group B: sterile distilled water (10 ml/kg b.w., i.g) served as a CCl4 control.
Group C: 400 mg/kg (b.w.) of Geniposide.
Group D: 400 mg/kg (b.w.) of Crocins.
Group E: 400 mg/kg (b.w.) of Crocin-1.
Group F: 140 mg/kg (b.w.) of Crocetin.
Group G: 100 mg/kg (b.w.) of biphenyldicarboxylate pills (BP).
2 h after the oral administration on the sixth day, all mice except those in the normal control group were given simultaneously a CCl4-peanut oil mixture (1:1, v/v intraperitoneally, 2 ml/kg b.w.), while the normal control group received peanut oil alone. Then all the animals were fasted for 18 h and were sacrificed by cervical dislocation with the last oral administration 1 h before. Livers were dissected out immediately and blood was collected, allowed to clot, and serum was separated for assessment of enzyme activity. The weights of the liver and kidney were measured. Some specimens were properly stored at −80°C for pending tests. Part of the liver tissue was immediately transferred into 10% formalin for histopathological investigation.
Measurement of serum biochemical markers
Collected blood samples were stored at 4°C for 2 h and centrifuged at 3000 rpm for 10 min at 4°C to obtain the serum. The level of total protein (TP) and the activities of ALT, AST and ALP were determined using commercial reagent kits purchased from the Institute of Biological Engineering of Nanjing Jiancheng (Nanjing, China) according to the instruction manuals.
Measurement of SOD, CAT and GSH in liver homogenate
Liver homogenates (10.0%, w/v) were prepared with 50 mmol/L cold potassium phosphate buffer (pH 7.4). The resulting suspension was centrifuged at 2000 rpm for 10 min, and the supernatant was collected for further analysis. All treatments were done at 4°C. Protein and GSH concentration and the activities of SOD and CAT were assayed using commercial reagent kits purchased from the Institute of Biological Engineering of Nanjing Jiancheng (Nanjing, China) according to the instruction manuals.
Histopathological examination
Liver slices were fixed with 10% formalin in phosphate buffered saline for 24 h and embedded in paraffin. Sections of 5 μm in thickness were cut, deparaffinized, dehydrated, stained with haematoxylin-eosin (HE) and observed under microscope to evaluate histopathological lesions in the livers. Photographs of each of the slides were taken at 100× magnification.
Statistical analysis
The data were analyzed using SPSS.19 (SPSS Inc., Chicago, USA), expressed as the means ± SD and statistically analyzed by one way analysis of variance (ANOVA) test and p<0.05 was considered significant.
RESULTS
HPLC/MS analysis for the purified compounds and fractions
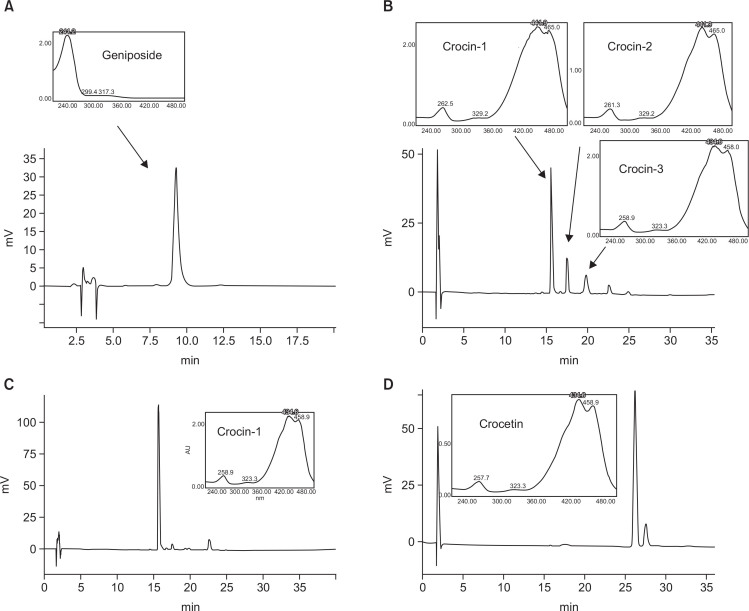
In current study, separation of 2.5 kg of gardenia fruits yielded components 1–4. Identification of the purified compounds and fraction was carried out by TLC, HPLC-UV, LC-MS and NMR, in comparison with those of reference chemicals. The main chemical in component 1 was geniposide, the major fragmentation of which was confirmed by [M+Na]+ at 411.1260 (Calcd for C17H24O11Na [M+Na]+, 411.1267). In HRESIMS spectrum of component 3, 999.3671 in [M+Na]+ indicated the presence of crocin-1 (Calcd for C44H64O24Na [M+Na]+, 999.3685). In addition, 35.1549 in [M+Na]+ of component 4 confirmed the presence of crocetin (calcd for C20H24O4Na [M+Na]+, 351.1572). These components were further identified by TLC, HPLC and NMR with reference chemicals obtained in our previous study. Additionally, the purities of components 1, 2, 3 and 4 were determined, respectively, by HPLC with reference chemicals geniposide, crocin-1, crocin-2, crocin-3 and crocetin (Table 1, Fig. 2).
Table 1.
Percentage of each ingredient
| Ingredients | Geniposide | Crocins | Crocin-1 Crocetin |
|---|---|---|---|
| Percentage of weight | 96.6% | 93.8% | 96.9% 98.1% |
Fig. 2.
The HPLC-UV chromatograms. (A) geniposide, (B) crocins, (C) crocin-1 and (D) crocetin.
Effect of chemicals on the animal vital signs and tissue index
After the administration of carbon tetrachloride, there was no death case in normal control group and the therapies groups, while three deaths occurred in model control group. Moreover, apathetic condition with emaciation and the rising of liver and kidney indexs (liver and kidney weight as a percentage of body weight) in mice were mostly observed in the model control group, and the therapies groups significantly improved those symptoms of liver damaged mice, among which, crocetin group showed the best effect. Liver index and kidney index in each group were listed in Table 2. It was observed that the treatment with crocins and crocetin resulted in a significant reduction in liver index (p<0.05), and interestingly, all drugs treated animals shown a remarkable reduction in kidney index (p<0.01).
Table 2.
Effect of geniposide, crocins, crocin-1 and crocetin on liver and kidney index in CCl4-induced liver damage model
| Groups | Administration | Doses (mg/kg) | n | Body wight (g) | Tissue index | |
|---|---|---|---|---|---|---|
|
| ||||||
| Liver (%) | Kidney (%) | |||||
| A | Normal control | - | 6 | 30.6 ± 1.04 | 5.31 ± 0.16 | 1.59 ± 0.10 |
| B | CCl4 model | - | 6 | 28.6 ± 0.82## | 6.17 ± 0.27## | 1.65 ± 0.06# |
| C | Geniposide | 400 | 6 | 29.5 ± 0.69 | 5.86 ± 0.33 | 1.52 ± 0.06** |
| D | Crocins | 400 | 6 | 30.2 ± 1.74 | 5.70 ± 0.11* | 1.47 ± 0.06** |
| E | Crocin-1 | 400 | 6 | 29.2 ± 1.18 | 5.95 ± 0.16 | 1.52 ± 0.06** |
| F | Crocetin | 140 | 6 | 29.8 ± 0.95* | 5.67 ± 0.18* | 1.47 ± 0.05** |
| G | Biphenyldicarboxylate | 100 | 6 | 28.7 ± 1.28 | 6.05 ± 0.24 | 1.55 ± 0.04** |
Values are mean ± SD (n=6).
p<0.01 vs. Normal,
p<0.05 vs. Normal;
p<0.01 vs. CCl4-treated group,
p<0.05 vs. CCl4-treated group.
In vivo antioxidant and hepatoprotective activity
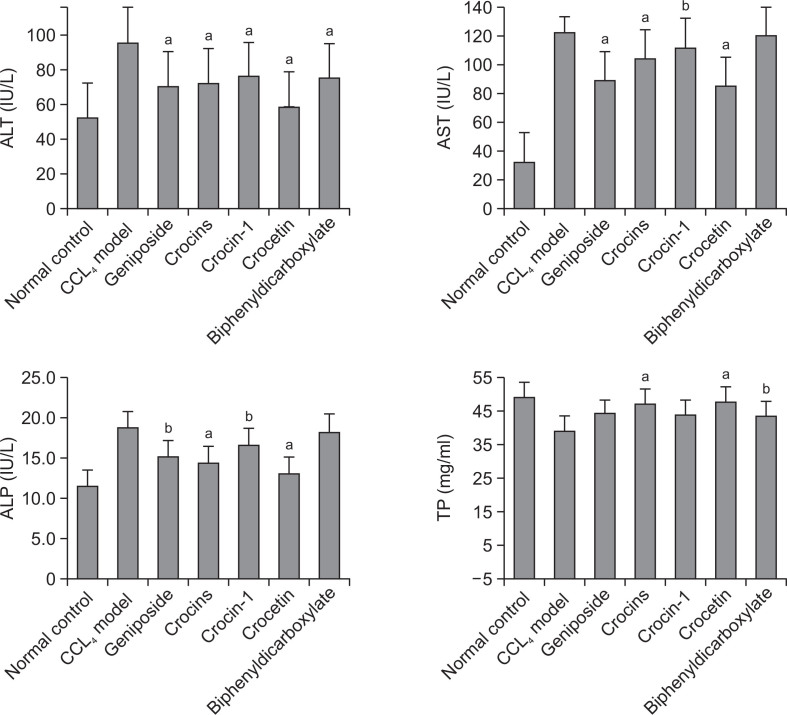
Administration of animals with carbon tetrachloride resulted in an acute hepatotoxicity which can be revealed from the levels of serum marker enzymes and the liver antioxidant levels (Fig. 3, 4). Significant increase (p<0.01) in ALT, AST and ALP levels (Fig. 3) in the serum were observed in CCl4-intoxicated group (ALT 95.38 ± 3.60 IU/L; AST 122.86 ± 6.62 IU/L; ALP 18.79 ± 1.44 IU/L) if compared with the normal control group (ALT 52.13 ± 2.36 IU/L; AST 32.48 ± 2.70 IU/L; ALP 11.55 ± 1.04 IU/L) (Fig. 3). However, the levels of these enzymes were significantly decreased in mice treated with geniposide (400 mg/kg) (ALT 70.32 ± 2.58 IU/L; AST 89.39 ± 2.29 IU/L, p<0.01; ALP 15.23 ± 2.88 IU/L, p<0.05), crocins (400 mg/kg) (ALT 72.60 ± 1.59 IU/L; AST 104.07 ± 10.47 IU/L, p<0.01; ALP 14.39 ± 2.18 IU/L, p<0.01), crocin-1 (400 mg/kg) (ALT 76.20 ± 2.00 IU/L; AST 111.92 ± 7.42 IU/L, p<0.05; ALP 16.74 ± 1.74 IU/L, p<0.05) and crocetin (140 mg/kg) (ALT 59.48 ± 1.81 IU/L; AST 84.59 ± 5.07 IU/L, p<0.01; ALP 13.20 ± 1.75 IU/L, p<0.01) if compared with CCl4-intoxicated group, although this decrease was minimum in the group receiving crocin-1. On the other hand, the model control group had considerably lower TP level (40.05 ± 0.76 mg/ml) than normal control group (49.33 ± 2.15 mg/ml, p<0.01). However, crocins (400 mg/kg) (47.49 ± 4.59 mg/ml, p<0.01) and crocetin (140 mg/kg) (48.12 ± 3.01 mg/ml, p<0.01) treated groups resulted in significant improvement in TP level (Fig. 3). Generally, treatments with geniposide, crocins, crocin-1 and crocetin showed significant hepatoprotective activity and, crocins and crocetin seemed more effective.
Fig. 3.
Effects of geniposide and crocetin derivatives on serum enzymes in CCl4-induced hepatotoxicity in mice (n=6). ap<0.01; bp<0.05 v.s. CCL4 control.
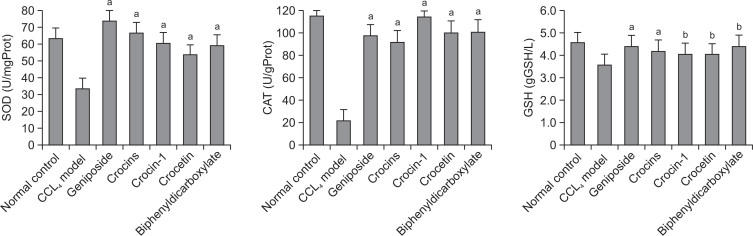
Fig. 4.
Effects of geniposide and crocetin derivatives on liver antioxidant levels in CCl4-induced hepatotoxicity in mice (n=6). ap<0.01; bp<0.05 v.s. CCL4 control.
The effects of geniposide, crocins, crocin-1 and crocetin treatments on the activities of SOD and CAT and the cellular antioxidant GSH in the liver are shown in Fig. 4. The activities of SOD and CAT and the levels of GSH in the CCl4-treated group were significantly (p<0.05) decreased (34.13 ± 2.59 U/mg protein, 21.89 ± 2.35 U/g protein and 3.54 ± 0.23 g GSH/L; p<0.01) when compared with the normal control mice. This decreasing activities were restored very significantly in the geniposide (400 mg/kg) (74.73 ± 1.77 U/mg protein, 97.74 ± 12.08 U/g protein and 4.37 ± 0.73 g GSH/L), crocins (400 mg/kg) (66.97 ± 3.36 U/mg protein, 92.27 ± 9.44 U/g protein and 4.16 ± 0.36 g GSH/L), crocin-1 (400 mg/kg) (61.30 ± 2.49 U/mg protein, 114.27 ± 4.12 U/g protein and 4.04 ± 0.31 g GSH/L) and crocetin (140 mg/kg) (54.02 ± 2.72 U/mg protein, 100.53 ± 8.89 U/g protein and 4.36 ± 0.61 g GSH/L) treated groups (p<0.01 except crocin-1 and crocetin in GSH model p<0.05).
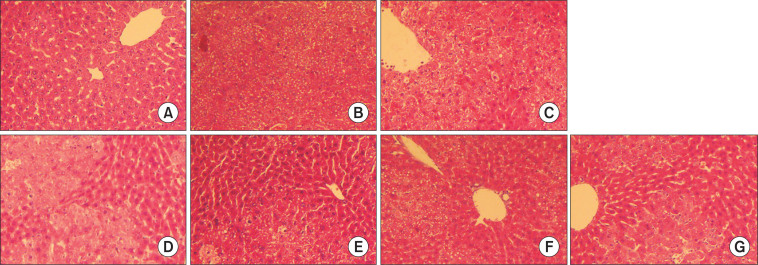
Histopathological examination
In order to assess the liver histological changes, hematoxylin and eosin staining of liver tissue sections from each group were examined. There were no pathological changes in normal control livers of healthy lobular architecture with central veins and radiating hepatic cords (Fig. 5). In the CCl4 model group, severe liver pathological changes characterized by deformability, irregular arrangement and rupture of hepatocyte. In addition, condensation of nucleus, nuclear membrane shrinkage and destruction occurred, and many vesicles appeared in the cytoplasm. These pathological changes reflected CCl4-induced acute liver injury. Compared with the CCl4-model group (Fig. 5), the pathological changes of hepatocytes induced by CCl4 was obviously improved by the treatment of compounds.
Fig. 5.
Effect of geniposide and crocetin derivatives on hepatic cells in liver tissue of CCl4-induced liver damage mice (n=6). Sections are 5 μm thick and photomicrographs are taken at 100×. (A) Normal control, (B) CCl4 control, (C) geniposide 400 mg/kg b.w.+CCl4, (D) crocins 400 mg/kg b.w.+CCl4, (E) crocin-1 400 mg/kg b.w.+CCl4, (F) crocetin 140 mg/kg b.w. + CCl4, (G) biphenyldicarboxylate 100 mg/kg b.w.+CCl4.
DISCUSSION
The whole separation of crocins and geniposide is a crucial and challenging task for pharmacological investigation of gardenia fruits. In our previous study, crocin-1 and crocetin were separated from gardenia fruits by various chromatographic means (Chen et al., 2008a, 2008b, 2010). In the present work, crocetin was prepared by alkaline hydrolyzation of the total crocins. Therefore, the yield of crocetin is significantly higher than that of the previous method. Additionally, high purities of all obtained components were achieved with the aid of the multiple chromatographic steps and the purified components were characterized and identified by HRESIMS, 1H NMR and 13C NMR (data not shown).
CCl4 is widely used to induce liver injury in laboratory rodents (Seifert et al. 1994; Manubolu et al., 2014; Cao et al., 2015). Increased levels of serum transaminases reflect hepatic injury as the enzymes are released into circulation following the exposure. CCl4 initially causes necrosis and steatosis and may lead to fibrosis, cirrhosis, and hepatocellular carcinoma when administered at higher dosages (Pierce et al., 1987). Therefore, CCl4-induced hepatic insult was selected for the hepatoprotective evaluation of components from gardenia. The increased levels of ALT, AST, ALP, and LDH are conventional indicators of liver injury (Thabrew et al., 1987). The present study revealed a significant increase in the activities of ALT, AST and ALP levels in rat serum on exposure to CCl4, indicating considerable hepatocellular injury (Bhondave et al., 2014; Raj and Gothandam, 2014). However, the serum ALT, AST and ALP activities significantly declined by treatment with geniposide (400 mg/kg), crocins (400 mg/kg), crocin-1 (400 mg/kg) and crocetin (140 mg/kg), implying that these components can rise up stabilization of plasma membrane and ameliorate biliary dysfunction effectively thereby preserving the structural integrity of cells as well as the repair of CCl4-induced liver damage, which is further confirmed by the reduced amount of histopathological injury. To compare biological effect of these components in terms of ALT, AST and ALP levels, current data indicated crocins and crocetin feature stronger hepatoprotective effect if compared with geniposide and crocin-1 (Fig. 3). In this study, total protein level in the serum was measured as well. CCl4 expectedly reduced serum total protein, while only the treatment of crocins (400 mg/ kg) and crocetin (140 mg/kg) remarkably restored the proteins content (p<0.05).
The body has a set of endogenous antioxidant enzymes as an effective defense mechanism to prevent and neutralize the free radical-induced damage (Bansal et al., 2005). In order to evaluate the antioxidant activities of geniposide, crocins and crocetin in vivo, we determined the activities of antioxidant enzymes (CAT and SOD), as well as the levels of GSH in mice liver. As enzymatic antioxidant systems, both SOD and CAT play important roles in protection against the deleterious effects of hydrogen peroxide and lipid peroxidation in diseases related to oxidative stress (Zhu et al., 2012; Abdelaziz and Ali, 2014). As a non-enzymatic antioxidant, GSH, which plays an important role in maintaining the body’s antioxidant defense mechanism, conjugates with free radicals directly to protect the integrity of cell membranes (He et al., 2012). In the present studies, we observed that the levels of CAT, SOD and GSH were significantly lower in CCl4-induced liver injury mice as compared with those of normal control group, representing severe oxidative stress status to hepatic cells. Treatment with geniposide (400 mg/kg), crocins (400 mg/kg), crocin-1 (400 mg/kg) and crocetin (140 mg/kg) resulted in restoration of antioxidant enzymes activity and GSH level in CCl4-induced liver injury mice. To compare antioxidant activities of these components, the present study implied that geniposide, crocins, crocin-1 and crocetin have comparative in vivo antioxidant effect. A series of crocetin glycosides (crocins), but not crocetin, are the main pigments of gardenia (Chen et al., 2010). Literature showed that orally administered crocetin was rapidly absorbed into the blood circulation and orally administered crocins are hydrolyzed to crocetin before or during intestinal absorption, and absorbed crocetin is partly metabolized to mono- and diglucuronide conjugates which may be biologically active (Asai et al., 2005). Thus the concentration-time profile of plasma crocetin was considerably different from that in crocins-administered mice. In current study, stronger activity of crocetin in comparison with that of crocin-1 indicated the process of this hydrolyzation probably influence hepatoprotective activity of crocins.
In the present investigation, the encouraging findings indicated that geniposide, crocetin derivatives and crocetin, separated and purified from Gardenia jasminoides Ellis, show comparatively beneficial effects on CCl4-induced liver damage via induction of antioxidant defense and these biological effects were further confirmed by histological observations. It is demonstrated that hydrolyzation might influence hepatoprotective effect of crocetin derivatives. In light of our observations, we may draw a conclusion that geniposide and crocetin derivatives are probably both responsible for hepatoprotective properties of gardenia, and therefore, contents of geniposide and crocetin derivatives should be considered for hepatoprotective evaluation of Gardenia jasminoides Ellis.
Acknowledgments
The authors acknowledge the financial support from the Construction of Seed and Seedling Breeding Base for Important TCM of National Essential Drugs Needs, the Strategic Cooperation Foundation Project of Sichuan University-Lu Zhou City (No. 2013CDLZ-S10), the Project Natural Science Foundation of China (No. 21562051, 21162046) and the project of Science and Technology Agency of Guizhou Province (J(2015)2157).
Footnotes
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- Abdelaziz DH, Ali SA. The protective effect of Phoenix dactylifera L. seeds against CCl4-induced hepatotoxicity in rats. . J Ethnopharmacol. 2014;155:736–43. doi: 10.1016/j.jep.2014.06.026. [DOI] [PubMed] [Google Scholar]
- Asai A, Nakano T, Takahashi M, Nagao A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J Agric Food Chem. 2005;53:7302–7306. doi: 10.1021/jf0509355. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Bansal M, Soni G, Bhatnagar D. Protective role of vitamin E pretreatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact. 2005;156:101–111. doi: 10.1016/j.cbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Bhondave PD, Devarshi PP, Mahadik KR, Harsulkar AM. ‘Ashvagandharishta’ prepared using yeast consortium from Woodfordia fruticosa flowers exhibit hepatoprotective effect on CCl4 induced liver damage in Wistar rats. . J Ethnopharmacol. 2014;151:183–190. doi: 10.1016/j.jep.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Cao L, Ding W, Du J, Jia R, Liu Y, Zhao C, Shen Y, Yin G. Effects of curcumin on antioxidative activities and cytokine production in Jian carp (Cyprinuscarpio var. Jian) with CCl4-induced liver damage. Fish Shellfish Immunol. 2015;43:150–157. doi: 10.1016/j.fsi.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cai L, Zhao C, Xu HC, Cao CY, Liu Y, Jia L, Yin H.-X, Chen C, Zhang H. Spectroscopic, stability and radical-scavenging properties of a novel pigment from gardenia. Food Chem. 2008a;109:269–277. doi: 10.1016/j.foodchem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang H, Tian X, Zhao C, Cai L, Liu Y, Jia L, Yin H.-X, Chen C. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides Ellis and Crocus sativus L: A relationship investigation between antioxidant activity and crocin contents. . Food Chem. 2008b;109:484–492. doi: 10.1016/j.foodchem.2007.09.080. [DOI] [Google Scholar]
- Chen Y, Zhang H, Li YX, Cai L, Huang J, Zhao C, Jia L, Buchanan R, Yang T, Jiang LJ. Crocin and geniposide profiles and radical scavenging activity of gardenia fruits (Gardenia jasminoides Ellis.) from different cultivars and during fruit maturation. Fitoterapia. 2010;81:269–273. doi: 10.1016/j.fitote.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Choi H.-J, Park YS, Kim MG, Kim TK, Yoon NS, Lim YJ. Isolation and characterization of the major colorant in Gardenia fruit. Dyes Pigm. 2001;49:15–20. doi: 10.1016/S0143-7208(01)00007-9. [DOI] [Google Scholar]
- He J, Huang B, Ban X, Tian J, Zhu L, Wang Y. In vitro and in vivo antioxidant activity of the ethanolic extract from Meconopsis quintuplinervia. . J Ethnopharmacol. 2012;141:104–110. doi: 10.1016/j.jep.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Jaishree V, Badami S. Antioxidant and hepatoprotective effect of swertiamarin from Enicostemmaaxillare against D-galactosamine induced acute liver damage in rats. . J Ethnopharmacol. 2010;130:103–106. doi: 10.1016/j.jep.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Jia L, Zhang H, Chen C, Liu Y, Chen Y. Simultaneous determination of geniposide and crocin-1 in Fructus Gardeniae by HPLC. Huaxi Yaoxue Zazhi. 2005;20:223–225. [Google Scholar]
- Kim SJ, Kim JK, Lee DU, Kwak JH, Lee SM. Genipin protects lipopolysaccharide-induced apoptotic liver damage in D-galactosamine-sensitized mice. Eur J Pharmacol. 2010;635:188–193. doi: 10.1016/j.ejphar.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Lee CP, Shih PH, Hsu CL, Yen GC. Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. . Food Chem Toxicol. 2007;45:888–895. doi: 10.1016/j.fct.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Manubolu M, Goodla L, Ravilla S, Thanasekaran J, Dutta P, Malmlöf K, Obulum VR. Protective effect of Actiniopteris radiata (Sw.) Link. against CCl4 induced oxidative stress in albino rats. . J Ethnopharmacol. 2014;153:744–752. doi: 10.1016/j.jep.2014.03.040. [DOI] [PubMed] [Google Scholar]
- Mihailović V, Mihailović M, Uskoković A, Arambašić J, Mišić D, Stanković V, Katanić J, Mladenović M, Solujić S, Matić S. Hepatoprotective effects of Gentiana asclepiadea L. extracts against carbon tetrachloride induced liver injury in rats. . Food Chem Toxicol. 2013;52:83–90. doi: 10.1016/j.fct.2012.10.034. [DOI] [PubMed] [Google Scholar]
- National Commission of Chinese Pharmacopoeia . Pharmacopoeia of the People’s Republic of China. Vol. 1. China Medical Science and Technology Press; Beijing: 2010. p. 231. [Google Scholar]
- Pierce RA, Glaug MR, Greco RS, Mackenzie JW, Boyd CD, Deak SB. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987;262:1652–1658. [PubMed] [Google Scholar]
- Qian H, Zhao B, Xu D, Huang X. Preparation of crocetin from Gardenia yellow pigment. Chinese Wild Plant Resources. 2010;29:26–28. [Google Scholar]
- Raj S, Gothandam KM. Hepatoprotective effect of polyphenols rich methanolic extract of Amorphophallus commutatus var. wayanadensis against CCl4 induced hepatic injury in swiss albino mice. . Food Chem Toxicol. 2014;67:105–112. doi: 10.1016/j.fct.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Seifert WF, Bosma A, Brouwer A, Hendriks HF, Roholl PJ, van Leeuwen RE, van Thiel-de Ruiter GC, Seifert-Bock I, Knook DL. Vitamin A deficiency potentiates carbon tetrachloride-induced liver fibrosis in rats. Hepatology. 1994;19:193–201. doi: 10.1002/hep.1840190129. [DOI] [PubMed] [Google Scholar]
- Thabrew MI, Joice PD, Rajatissa W. Comparative study of efficacy of Paettaindica and Osbeckiaoctandra in the treatment of liver dysfunction. . Planta Med. 1987;53:239–241. doi: 10.1055/s-2006-962691. [DOI] [PubMed] [Google Scholar]
- Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995;97:61–67. doi: 10.1016/0304-3835(95)03964-X. [DOI] [PubMed] [Google Scholar]
- Wang SC, Tseng TY, Huang CM, Tsai TH. Gardenia herbal active constituents: applicable separation procedures. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:193–202. doi: 10.1016/S1570-0232(04)00680-4. [DOI] [PubMed] [Google Scholar]
- Zhu R, Wang Y, Zhang L, Guo Q. Oxidative stress and liver disease. Hepatol Res. 2012;42:741–749. doi: 10.1111/j.1872-034X.2012.00996.x. [DOI] [PubMed] [Google Scholar]