Abstract
We examined whether apigenin affects the gene expression, secretion and activity of matrix metalloproteinase-3 (MMP-3) in primary cultured rabbit articular chondrocytes, as well as in vivo production of MMP-3 in the knee joint of rat to evaluate the potential chondroprotective effects of apigenin. Rabbit articular chondrocytes were cultured in a monolayer, and reverse transcription - polymerase chain reaction (RT-PCR) was used to measure interleukin-1β (IL-1β)-induced expression of MMP-3, MMP-1, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4), and ADAMTS-5. In rabbit articular chondrocytes, the effects of apigenin on IL-1β-induced secretion and proteolytic activity of MMP-3 were investigated using western blot analysis and casein zymography, respectively. The effect of apigenin on MMP-3 protein production was also examined in vivo. In rabbit articular chondrocytes, apigenin inhibited the gene expression of MMP-3, MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5. Furthermore, apigenin inhibited the secretion and proteolytic activity of MMP-3 in vitro, and inhibited production of MMP-3 protein in vivo. These results suggest that apigenin can regulate the gene expression, secretion, and activity of MMP-3, by directly acting on articular chondrocytes.
Keywords: Apigenin, Chondrocyte, Metalloproteinase, Osteoarthritis
INTRODUCTION
Osteoarthritis has been reported to be the most common degenerative articular disease, from which millions of people suffered particularly in the elderly. Among the symptoms of osteoarthritis, degeneration of articular cartilage, formation of osteophytes, synovial inflammation, and changes in subchondral bone are the major pathophysiologic features. Although the cause of osteoarthritis is not fully understood, it involves several biochemical and mechanical factors, such as disruption on the equilibrium between physiologic synthesis and degradation of articular cartilage during the progression of osteoarthritis (Mankin, 1982; Aigner and McKenna, 2002).
While the activation of degradative enzymes leads to the loss and degradation of proteoglycans and collagen in articular cartilage, the matrix metalloproteinases (MMP) play a pivotal role in the destruction of articular cartilage in osteoarthritis patients (Dean et al., 1989; Kullich et al., 2007). MMPs can be classified into collagenases (MMP-1, -8 and -13), gelatinases (MMP-2 and -9), and stromelysins (MMP-3, -7, -10 and -11) (Birkedal-Hansen et al., 1993; Burrage et al., 2006). Out of these MMPs, MMP-3 degrades proteoglycans and activates procollagenase in articular cartilage (Garnero et al., 2000; Lin et al., 2004). Since some plant-derived natural products used as arthritis remedies in folk medicine, exploration of the inhibition mechanisms of the proteolytic activity, secretion, and gene expression of MMP-3 by these plant-derived products will support the effective treatment of osteoarthritis, and may expedite a new therapeutic strategies.
As claimed by various reports, apigenin derived from Scutellariae Radix, a medicinal plant used for controlling the inflammatory diseases in folk medicine, showed the various biological activities including anti-inflammatory and anti-MMP effects (Durigova et al., 2008; Hwang et al., 2011; Palmieri et al., 2012; Du et al., 2015; Patil et al., 2015). For instance, apigenin showed the inhibitory activities on MMP-9 in rats with acute myocardial infarction (Du et al., 2015) and in endothelial cells (Palmieri et al, 2012). Also, apigenin inhibited both MMP-1 activity in human keratinocytes (Hwang et al., 2011) and hyaluronidase activity in cartilage (Durigova et al., 2008).
However, there are no research result about the effect of apigenin on the gene expression, secretion, and activity of MMP-3, an articular cartilage-degradative enzyme that decomposes proteoglycans, especially in primary cultured rabbit articular chondrocytes, or on in vivo production of MMP-3 in the rat knee. Thus, to evaluate the chondroprotective potential of apigenin, we investigated its effects on IL-1β-induced gene expression, secretion, and activity of MMP-3 in vitro, and on production of MMP-3 in vivo.
MATERIALS AND METHODS
Materials
All chemicals and reagents used in this experiment, including apigenin (purity: 98.0%), hesperidin (purity: 98.0%) and hesperetin (purity: 98.0%) were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified. Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Gibco-BRL (Grand Island, NY, USA) and recombinant human IL-1β was purchased from R&D Systems (Minneapolis, MN, USA).
Primary cultures of chondrocytes from rabbit articular cartilage
Male New Zealand White rabbits were obtained from Daehan Biolink (Seoul, Korea) at 2 weeks of age. Animals were housed 1 animal per cage, provided with distilled water and food ad libitum, and kept under a 12 h light/dark cycle (lights on from 08:00–20:00) at constant temperature (22.5°C) and humidity (55%). Animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, and care was regulated by Chungnam National University (the approval number of animal experiment: CNU-00555) (Daejeon, Korea). Rabbit articular chondrocytes were isolated from the tibial plateau and femoral condyle in cartilage of the knee joint. Cartilage was washed in phosphate-buffered saline (PBS) and minced into pieces measuring 2 mm3, approximately. Cartilage tissue was digested for 4 h with 0.2% type II collagenase at 37°C. After collection of individual cells by brief centrifugation, the cells were transferred to 100 mm culture dishes (seeding density: 105 cells/cm2) in 12 mL DMEM supplemented with 10% fetal bovine serum (FBS), in the presence of penicillin (100 units/mL) and streptomycin (100 μg/mL) (Moon et al., 2011). Cells were cultured at 37°C in a humidified, 5% CO2/95% air, water-jacketed incubator, and medium was replaced every other day.
Treatment of cells with apigenin and the other natural products
Chondrocytes were seeded on 6-well culture plates at a density of 105 cells/cm2. After 2 days in monolayer culture, the cells were incubated for 2 h in growth medium with 1, 10, 50, or 100 μM of apigenin, hesperidin or hesperetin, followed by incubation in the presence or absence of IL-1β (10 ng/mL) for 24 h. Apigenin, hesperidin or hesperetin was dissolved in dimethylsulfoxide, diluted in PBS, and administered in culture medium (final concentrations of dimethylsulfoxide were 0.5%). The final pH values of these solutions were between 7.0 and 7.4. Culture medium and 0.5% dimethylsulfoxide in medium did not affect the gene expression, secretion, or proteolytic activity of MMP-3 in primary cultured chondrocytes. The supernatant was collected and centrifuged, and cell and supernatant fractions were stored at −80°C until use.
Cytotoxicity assay
Chondrocytes were seeded at a density of 2×105/mL (0.1 mL/well) in a 96-well microtiter plate, and allowed to attach for 24 h to keep the log phase growth at the time of drug treatment. Apigenin was dissolved in DMSO, and administered in DMEM supplemented with 10% FBS (final concentrations of DMSO were under 0.5%). 0.5% DMSO alone did not affect the proliferation of chondrocytes. After incubation with the indicated drug concentrations for 72 h, cell proliferation was determined using the sulforhodamine B (SRB) assay (Skehan et al., 1990).
Isolation of total RNA and RT-PCR
Total RNA was isolated from chondrocytes using the Easy-BLUE Extraction Kit (INTRON Biotechnology, Inc., Kyungki-do, Korea), and reverse transcribed using AccuPower RT Premix (BIONEER Corporation, Daejeon, Korea) according to the manufacturer’s instructions. About 2 μg of total RNA was primed with 1 μg of oligo (dT) in a final reaction volume of 30 μL. 2 μL of RT reaction product was amplified in 20 μL using Thermoprime Plus DNA Polymerase (ABgene, Rochester, NY, USA). PCR was performed with the following primers: MMP-3 (5′ATG GAC CTT CTT CAG CAA 3′, 5′TCA TTA TGT CAG CCT CTC 3′), MMP-13 (5′AGG AGC ATG GCG ACT TCT AC 3′, 5′TAA AAA CAG CTC CGC ATC AA 3′), MMP-1 (5′TCA GTT CGT CCT CAC TCC AG 3′, 5′TTG GTC CAC CTG TCA TCT TC 3′), a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4) (5′CAA GGT CCC ATG TGC AAC GT 3′, 5′CAT CTG CCA CCA CCA GTG TCT 3′), and ADAMTS-5 (5′TGT CCT GCCAGC GGATGT 3′; 5′ACG GAA TTA CTG TAC GGC CTA CA 3′). GAPDH (5′ACT GGC GTC TTC ACC ACC AT 3′; 5′AAG GCC ATG CCA GTG AGC TT 3′) was used as a quantitative control. The PCR products increased as the concentration of RNA increased. The amplified fragment sizes were 350 base pairs (bp) for MMP-3, 458 bp for MMP-13, 300 bp for MMP-1, 90 bp for ADAMTS-4, 110 bp for ADAMTS-5, and 400 bp for GAPDH. After PCR, 15 μL of PCR products were subjected to 2% agarose gel electrophoresis and visualized with ethidium bromide under a transilluminator. The signal intensity of each band was analyzed by GelQuant software (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel) (Moon et al., 2011).
Western blot analysis for measuring secretion level of MMP-3 in culture supernatant
The Bradford assay was used to measure protein concentrations in culture supernatants to ensure consistent weight of protein samples subjected to electrophoresis. Culture supernatant samples containing MMP-3 protein (50 μg each) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto a polyvinylidene difluoride (PVDF) membrane. Blots were blocked using 5% skim milk in Tris-buffered saline/Tween 20 (TBS-T), and probed overnight with MMP-3 antibody in blocking buffer at 4°C. Antibody against MMP-3 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Membranes were washed with TBS-T and probed for 1 h with a secondary antibody conjugated with horseradish peroxidase (Calbiochem, La Jolla, CA, USA). After 4 washes with TBS-T, immunoreactive bands were detected using an enhanced chemiluminescence kit (Pierce ECL western blotting substrate, Thermo Scientific, Waltham, MA, USA). The signal intensity of immunoreactive bands was analyzed by GelQuant software (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel).
Casein zymography to measure the proteolytic activity of MMP-3
A modified casein-substrate zymography was carried out using culture supernatants from chondrocytes pretreated for 2 h with apigenin and stimulated for 24 h with IL-1β in DMEM containing 0.5% FBS. The Bradford assay was used to measure protein concentrations in culture media to ensure consistency across samples. Samples were electrophoresed at 4°C in a 10% SDS gel containing 0.1% casein. After electrophoresis, gels were washed with 10 mM Tris-HCl (pH 8.0) containing 2.5% Triton X-100. Next, gels were incubated at 37°C for 48 h in 50 mM Tris-HCl (pH 8.0) containing 1% Triton X-100, 0.2 M NaCl, and 5 mM CaCl2. Finally, gels were stained with 1% Coomassie Brilliant Blue, destained, and photographs were taken. The signal intensity of each band was analyzed by GelQuant software (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel) (Moon et al., 2011).
In vivo experiments
Male Sprague-Dawley rats (Daehan Biolink, Seoul, Korea) weighing 200–210 g were used to investigate the effect of apigenin on production of MMP-3 in articular cartilage in vivo. Animals were housed 5 per cage, provided with distilled water and food ad libitum, and kept under a 12 h light/dark cycle (lights on from 08:00–20:00) at constant temperature (22.5°C) and humidity (55%). Animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, and care was regulated by Chungnam National University (the approval number of animal experiment: CNU-00555) (Daejeon, Korea). Rats were randomly divided into 4 groups: control, IL-1β only, 50 μM apigenin plus IL-1β, or 100 μM apigenin plus IL-1β. Rats were anesthetized with vaporized diethyl ether, and those from the 50 μM apigenin plus IL-1β and 100 μM apigenin plus IL-1β treatment groups received a 30 μL injection of 50 μM or 100 μM apigenin, respectively, into the right knee joint. After 3 h, rats from the IL-1β only group, the 50 μM apigenin plus IL-1β group, and the 100 μM apigenin plus IL-1β group received a 30 μL injection of 20 ng IL-1β in sterile PBS into the right knee joint. Rats from the control group were injected with 30 μL of sterile PBS. Rats were euthanized via CO2 asphyxiation 72 h after injections. Articular cartilage (tibial plateau and femoral condyle) was isolated from each animal, homogenized, and prepared for measurement of MMP-3 protein by western blot analysis. Tissue lysates from articular cartilage homogenates containing MMP-3 protein (50 μg each) were subjected to 10% SDS-PAGE, and transferred onto a PVDF membrane. Blots were blocked with 5% skim milk in TBS-T, and probed with MMP-3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in blocking buffer overnight at 4ºC. Membranes were washed with TBS-T, and probed for 1 h with a secondary antibody conjugated with horseradish peroxidase (Calbiochem, La Jolla, CA, USA). After 4 washes with TBS-T, immunoreactive bands were detected using an enhanced chemiluminescence kit (Pierce ECL western blotting substrate, Thermo Scientific, Waltham, MA, USA). The signal intensity of immunoreactive bands was analyzed by GelQuant software (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel).
Statistics
Means of individual group were converted to percent control and expressed as mean ± S.E.M. The difference between groups was assessed using one-way ANOVA and Holm-Sidak test as a post-hoc test. p<0.05 was considered as significantly different.
RESULTS
Effect of apigenin, hesperidin or hesperetin on MMP-3 gene expression in rabbit chondrocytes
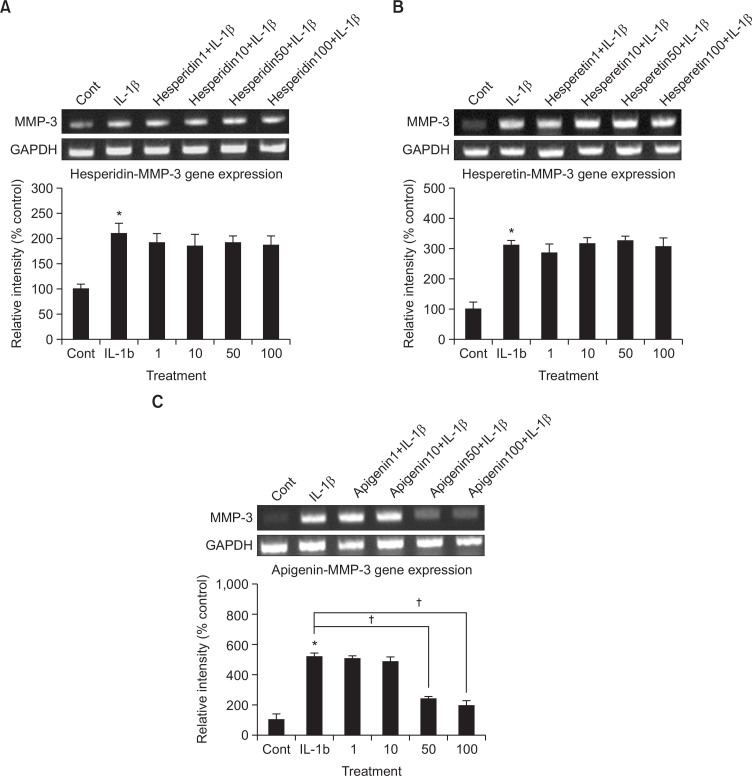
To compare the potency of activity on the gene expression of MMP-3, the key MMP involved in destruction of articular cartilage, effect on MMP-3 gene expression was investigated by pretreatment of hesperidin or hesperetin-similar flavonoidal compounds with apigeninin addition to apigenin. As shown in Fig. 1C, apigenin inhibited IL-1β-induced MMP-3 gene expression. However, hesperidin or hesperetin did not affect MMP-3 gene expression (Fig. 1A, 1B). The relative signal intensity of each band of apigenin-treated cultures were 100 ± 36%, 516 ± 23%, 506 ± 17%, 486 ± 27%, 238 ± 13% and 196 ± 27% for control, 10 ng/mL of IL-1β alone, IL-1β plus apigenin 1 μM, IL-1β plus apigenin 10 μM, IL-1β plus apigenin 50 μM, and IL-1β plus apigenin 100 μM, respectively. The relative signal intensity of each band of hesperidin-treated cultures were 100 ± 10%, 209 ± 21%, 192 ± 17%, 185 ± 23%, 192 ± 14% and 187 ± 19% for control, 10 ng/mL of IL-1β alone, IL-1β plus hesperidin 1 μM, IL-1β plus hesperidin 10 μM, IL-1β plus hesperidin 50 μM, and IL-1β plus hesperidin 100 μM, respectively. The relative signal intensity of each band of hesperetin-treated cultures were 100 ± 21%, 309 ± 17%, 287 ± 26%, 316 ± 21%, 327 ± 13% and 306 ± 29% for control, 10 ng/mL of IL-1β alone, IL-1β plus hesperetin 1 μM, IL-1β plus hesperetin 10 μM, IL-1β plus hesperetin 50 μM, and IL-1β plus hesperetin 100 μM, respectively.
Fig. 1.
Effect of hesperidin, hesperetin or apigenin on MMP-3 gene expression in rabbit chondrocytes. Primary cultured rabbit articular chondrocytes were pretreated with varying concentrations (1, 10, 50, and 100 μM) of hesperidin, hesperetin or apigenin for 2 h and then stimulated with IL-1β (10 ng/mL) for 24 h. MMP-3 gene expression level was measured by RT-PCR. Three independent experiments were performed and the representative data were shown (A, B and C). The signal intensity of each band was analyzed by GelQuant software (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel). Each bar represents a mean ± S.E.M. of three independent experiments in comparison with that of the control set at 100%. *Significantly different from control (p<0.05). †Significantly different from IL-1β alone (p<0.05). cont: control, concentration unit is μM.
Effect of apigenin on proliferation of rabbit chondrocytes (cytotoxicity assay)
To investigate the potential cytotoxicity of apigenin to cultured rabbit chondrocytes, effect of apigenin on proliferation of rabbit chondrocytes using SRB assay was tested. As can be seen in Fig. 2, apigenin showed no significant cytotoxicity at the concentrations of 1, 10, 50, and 100 μM. The numbers of cells in apigenin-treated cultures were 100 ± 20%, 90 ± 16%, 112 ± 9%, 86 ± 21%, and 92 ± 18% for control, 1, 10, 50, and 100 μM apigenin, respectively.
Fig. 2.
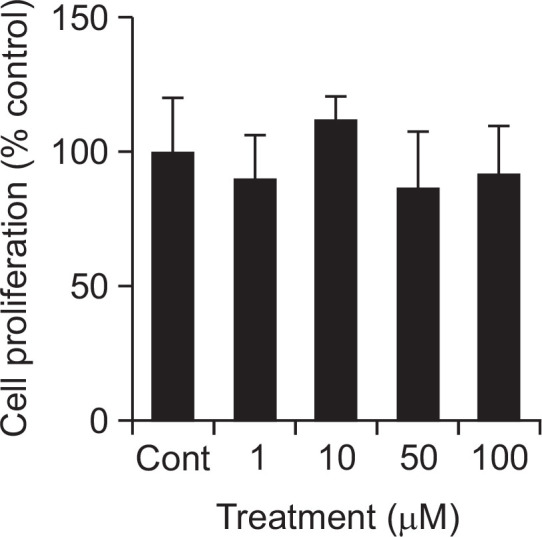
Effect of apigenin on proliferation of rabbit chondrocytes. Chondrocytes were incubated for 72 h in the presence of varying concentrations of apigenin. Cell viability was determined using SRB assay as described in Materials and Methods. Each bar represents a mean ± S.E.M. of three independent experiments in comparison with that of the control set at 100%.
Effect of apigenin on the gene expression of MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5 in rabbit chondrocytes
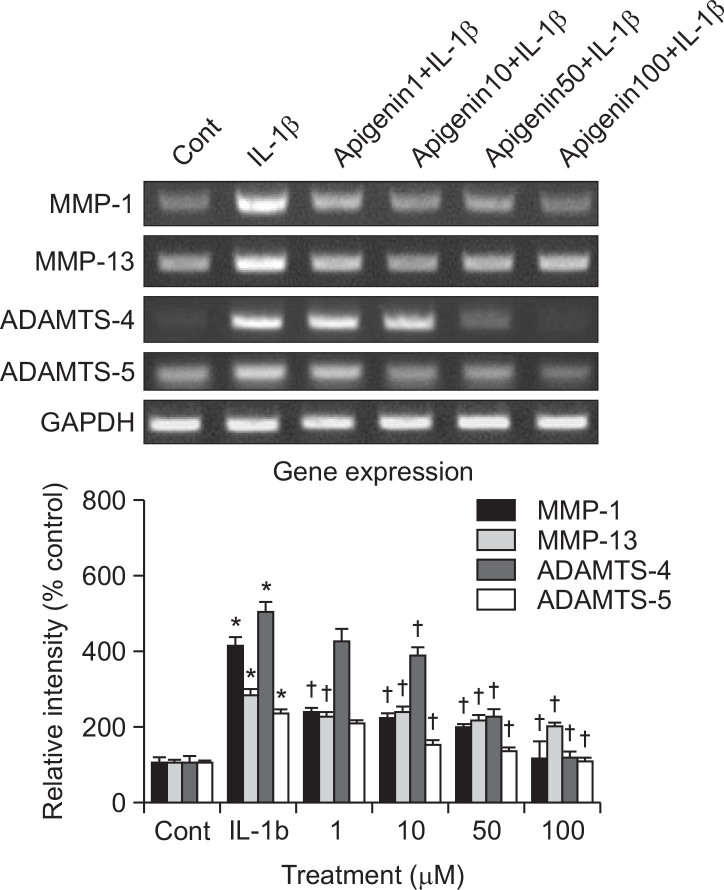
If apigenin can affect the gene expression of MMP-3, the key MMP involved in destruction of articular cartilage, it should be investigated whether apigenin affect the gene expression of MMP-1, MMP-13, ADAMTS-4 or ADAMTS-5, the other degradative enzymes related to destruction of articular cartilage, in rabbit chondrocytes. As shown in Fig. 3, apigenin showed the inhibition of IL-1β-induced gene expression of MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5. The relative signal intensity of each band of apigenin-treated cultures for measuring the effect on MMP-1 were 100 ± 17%, 418 ± 21%, 236 ± 15%, 221 ± 13%, 196 ± 12% and 113 ± 9% for control, 10 ng/mL of IL-1β alone, IL-1β plus apigenin 1 μM, IL-1β plus apigenin 10 μM, IL-1β plus apigenin 50 μM, and IL-1β plus apigenin 100 μM, respectively. The relative signal intensity of each band of apigenin-treated cultures for measuring the effect on MMP-13 were 100 ± 9%, 283 ± 16%, 226 ± 12%, 236 ± 18%, 215 ± 16% and 198 ± 13% for control, 10 ng/mL of IL-1β alone, IL-1β plus apigenin 1 μM, IL-1β plus apigenin 10 μM, IL-1β plus apigenin 50 μM, and IL-1β plus apigenin 100 μM, respectively. The relative signal intensity of each band of apigenin-treated cultures for measuring the effect on ADAMTS-4 were 100 ± 21%, 506 ± 28%, 426 ± 36%, 390 ± 21%, 226 ± 18% and 118 ± 12% for control, 10 ng/mL of IL-1β alone, IL-1β plus apigenin 1 μM, IL-1β plus apigenin 10 μM, IL-1β plus apigenin 50 μM, and IL-1β plus apigenin 100 μM, respectively. The relative signal intensity of each band of apigenin-treated cultures for measuring the effect on ADAMTS-5 were 100 ± 7%, 235 ± 12%, 206 ± 8%, 150 ± 10%, 132 ± 12% and 106 ± 9% for control, 10 ng/mL of IL-1β alone, IL-1β plus apigenin 1 μM, IL-1β plus apigenin 10 μM, IL-1β plus apigenin 50 μM, and IL-1β plus apigenin 100 μM, respectively.
Fig. 3.
Effect of apigenin on the gene expression of MMP-1, MMP-13, ADAMTS-4, or ADAMTS-5 in rabbit chondrocytes. Primary cultured rabbit articular chondrocytes were pretreated with varying concentrations (1, 10, 50, and 100 μM) of apigenin for 2 h and then stimulated with IL-1β (10 ng/mL) for 24 h. The gene expression level of MMP-1, MMP-13, ADAMTS-4, or ADAMTS-5 was measured by RT-PCR. Three independent experiments were performed and the representative data were shown. The signal intensity of each band was analyzed by GelQuant software (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel). Each bar represents a mean ± S.E.M. of three independent experiments in comparison with that of the control set at 100%. *Significantly different from control (p<0.05). †Significantly different from IL-1β alone (p<0.05). Cont: control, concentration unit is μM.
Effect of apigenin on IL-1β-induced secretion of MMP-3 from rabbit articular chondrocytes
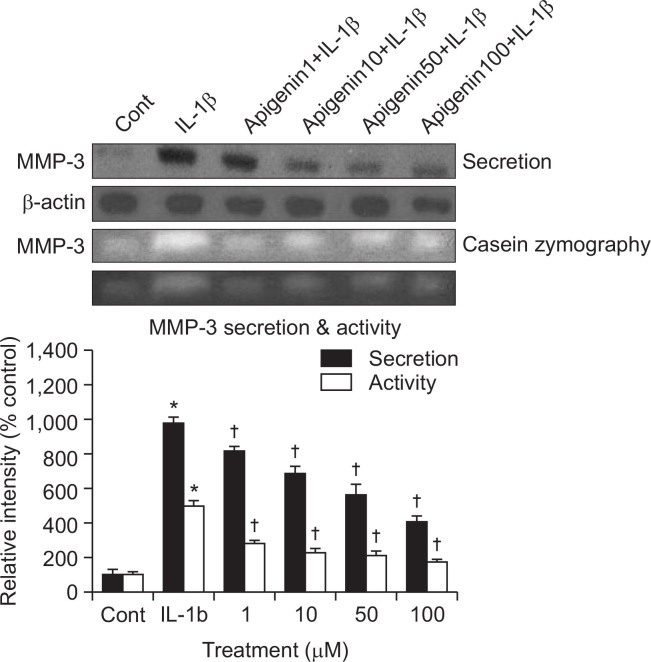
If apigenin can affect the MMP-3 gene expression at the transcriptional level, it can be examined whether apigenin affects IL-1β-induced secretion of MMP-3 proteins from rabbit articular chondrocytes. As shown in Fig. 4, stimulation with IL-1β (10 ng/mL) increased the secretion of MMP-3 from chondrocytes. However, apigenin inhibited the effects of IL-1β on MMP-3 secretion. This result means that apigenin can control the steps of protein synthesis and secretion of MMP-3, maybe due to a result of reduced MMP-3 mRNA level. The relative signal intensity of each immunoreactive band of apigenin-treated cultures were 100 ± 26%, 978 ± 38%, 816 ± 29%, 687 ± 42%, 564 ± 56% and 406 ± 31% for control, 10 ng/mL of IL-1β alone, IL-1β plus apigenin 1 μM, IL-1β plus apigenin 10 μM, IL-1β plus apigenin 50 μM, and IL-1β plus apigenin 100 μM, respectively.
Fig. 4.
Effect of apigenin on IL-1β-induced secretion of MMP-3 and caseinolytic activity of MMP-3 in rabbit articular chondrocytes. Primary cultured rabbit articular chondrocytes were pretreated with varying concentrations (1, 10, 50, and 100 μM) of apigenin for 2 h and then stimulated with IL-1β (10 ng/mL) for 24 h. Culture supernatants were collected for measurement of both the levels of produced and secreted MMP-3 by western blot analysis and the proteolytic activity of MMP-3 by casein zymography. The fourth panel was black-and-white version of casein zymography. Three independent experiments were performed and the representative data were shown. The signal intensity of each band was analyzed by GelQuant software (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel). Each bar represents a mean ± S.E.M. of three independent experiments in comparison with that of the control set at 100%. *Significantly different from control (p<0.05). †Significantly different from IL-1β alone (p<0.05). Cont: control, concentration unit is μM.
Effect of apigenin on proteolytic activity of MMP-3 in rabbit articular chondrocytes
To investigate the effect of apigenin on the enzyme activity of secreted MMP-3, which is known to degrade proteoglycans, 1 of the 2 major matrix components of cartilage, culture supernatants from rabbit articular chondrocytes were analyzed for caseinolytic activity by casein zymography, after treatment with IL-1β for 24 h. As can be seen in Fig. 4, IL-1β increased the caseinolytic activity of MMP-3 in rabbit articular chondrocytes, and this effect was inhibited by pretreatment with apigenin. The relative signal intensity of each band of apigenin-treated cultures were 100 ± 17%, 498 ± 27%, 283 ± 16%, 226 ± 27%, 213 ± 21% and 170 ± 15% for control, 10 ng/mL of IL-1β alone, IL-1β plus apigenin 1 μM, IL-1β plus apigenin 10 μM, IL-1β plus apigenin 50 μM, and IL-1β plus apigenin 100 μM, respectively.
Effect of apigenin on MMP-3 production in vivo
To investigate whether apigenin shows the potential effect in vivo, we examined the effect of intraarticular injection of apigenin into the knee joint of rats on IL-1β-stimulated production of MMP-3 from articular cartilage tissues. As shown in Fig. 5, treatment with IL-1β (20 ng/30 μL) increased MMP-3 production in articular cartilage tissues. However, apigenin inhibited IL-1β-induced MMP-3 production, in vivo. The relative signal intensity of each immunoreactive band of apigenin-treated cultures were 100 ± 9%, 190 ± 10%, 147 ± 7% and 116 ± 8% for control, 20 ng/30 μL of IL-1β alone, IL-1β plus apigenin 50 μM, and IL-1β plus apigenin 100 μM, respectively.
Fig. 5.
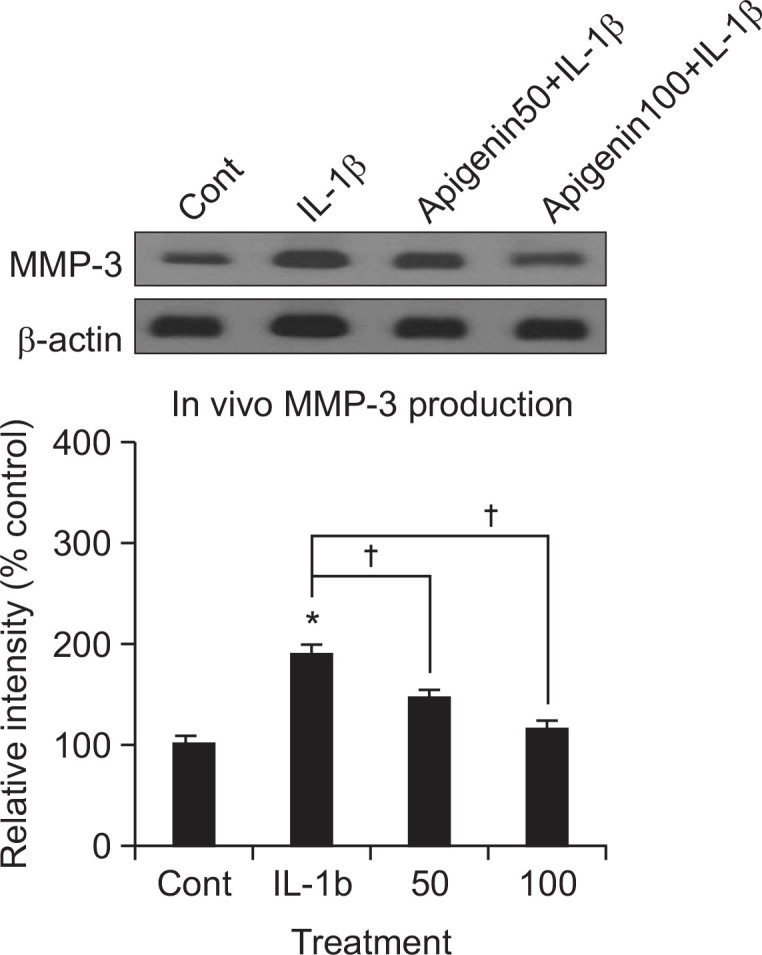
Effect of apigenin on the production of MMP-3 in vivo. The knee joint of rats were pretreated with 50 or 100 μM of apigenin for 3 h and then stimulated with IL-1β (20 ng/30 μL) for 72 h, by intraarticular injection. Tissue lysates from articular cartilage homogenates containing MMP-3 proteins were collected for measurement of the level of produced MMP-3 in vivo, by western blot analysis. The representative data were shown. Equal protein loading was evaluated by β-actin levels. The signal intensity of immunoreactive bands was analyzed by GelQuant software (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel). Each bar represents a mean ± S.E.M. of three independent experiments in comparison with that of the control set at 100%. *Significantly different from control (p<0.05). †Significantly different from IL-1β alone (p<0.05). Cont: control, concentration unit is μM.
DISCUSSION
As an effort to control the disrupted equilibrium between synthesis and degradation of articular cartilage during the progression of osteoarthritis, development of a useful pharmacological tool can be a promising approach. Among well-known and commonly used arthritis remedies in folk medicine, apigenin is a medicinal plant-derived flavonoid that has shown anti-inflammatory and anti-matrix metalloproteinase effects (Durigova et al., 2008; Hwang et al., 2011; Palmieri et al., 2012; Du et al., 2015; Patil et al., 2015). However, no information has been reported on the effects of apigenin on the gene expression, secretion, and enzyme activity of MMP-3, which is an articular cartilage-degradative enzyme that decomposes proteoglycans. Therefore, we examined the effects of apigenin on MMP-3 gene expression, secretion, and proteolytic activity in primary cultured rabbit articular chondrocytes, as well as on MMP-3 production in the rat knee.
Even as defined as a non-inflammatory disease, development and progression of osteoarthritis have been caused by low-grade inflammation in intraarticular sites, as well as to various inflammatory cytokines in articular tissues and fluids that are produced by chondrocytes and/or interact with chondrocytes (Bonnet and Walsh, 2005; Kobayashi et al., 2005; Loeser, 2006; Goldring et al., 2008). IL-1β is an inflammatory cytokine that is produced by cells in articular tissues, including chondrocytes, and can increase expression of MMPs, thus stimulating the progression of osteoarthritis. IL-1β contributes to the initiation and progression of destruction of articular cartilage by suppressing synthesis of collagen and stimulating MMP expression (Aida et al., 2005; Kobayashi et al., 2005; Pantsulaia et al., 2010). Especially, it has been studied that MMP-3 plays a pivotal pathophysiological role in osteoarthritis by degrading components of the extracellular matrix, such as proteoglycans. Compared with the control group, patients suffering from osteoarthritis in knee joints have shown more increased MMP-3 levels than MMP-1 levels (Garnero et al., 2000; Lijnen, 2002).
As shown in Results, we have shown that apigenin inhibited IL-1β-induced MMP-3 gene expression. However, another flavonoidal compound similar to apigenin, such as hesperidin or hesperetin, had no effect on MMP-3 gene expression (Fig. 1A–1C). This suggests that apigenin suppresses the gene expression of MMP-3 at the transcriptional level.
In addition to MMP-3, it has also been reported that MMP-1 and MMP-13 contribute to the destruction of cartilage in osteoarthritis. MMP-1 is a commonly detected metalloproteinase in synovial fluid from patients suffering from osteoarthritis (Freemont et al., 1997; Goupille et al., 1998; Kanyama et al., 2000; Yoshihara et al., 2000; Neuhold et al., 2001; Jo et al., 2003; Little et al., 2009). Another degradative enzymes were considered in this paper. ADAMTS-4 is a major aggrecanase in cartilage of mouse and ADAMTS-5 has been known to be important in cartilage matrix destruction during osteoarthritis (Stanton et al., 2005; Echtermeyer et al., 2009).
For that reason, we examined the effect of apigenin on expression of MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5. As shown in Fig. 3, apigenin inhibited IL-1β-induced gene expression of MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5, in rabbit chondrocytes. In other words, the chondroprotective effects of apigenin are supported by its regulation of the gene expression of diverse proteases involved in the destruction of articular cartilage in osteoarthritis.
In order to test if apigenin influences the MMP-3 gene expression at the transcriptional level, we investigated whether apigenin affects IL-1β-induced secretion of MMP-3 proteins from rabbit articular chondrocytes. As shown in Fig. 4, stimulation with IL-1β increased the secretion of MMP-3 from chondrocytes, and this effect was suppressed by apigenin. This result tells that apigenin can regulate the step of protein synthesis and secretion of MMP-3.
As examining the effect of apigenin on the proteolytic activity of secreted MMP-3, culture supernatants from rabbit articular chondrocytes stimulated with IL-1β for 24 h were analyzed for caseinolytic activity by casein zymography. Fig. 4 shows that the proteolytic activity of MMP-3 was increased when rabbit articular chondrocytes were stimulated with IL-1β, and this effect was suppressed by pretreatment with apigenin. From this result, it is probable and possible that apigenin also affect the proteolytic activity of overproduced and oversecreted MMP-3 in osteoarthritic articular cartilage tissues.
Moreover, we explored the effect of intraarticular injection of apigenin into the knee joint of rats on IL-1β-stimulated production of MMP-3 in articular cartilage tissue. As can be seen in Fig. 5, apigenin inhibited IL-1β-stimulated production of MMP-3 in articular cartilage tissue. This result shows that, in addition to its in vitro effects, apigenin exerts chondroprotective effects in vivo when administered via intraarticular injection.
In summary, our experiments show that the chondroprotective effects of apigenin are produced by its regulation of the gene expression, secretion, and enzyme activity of MMP-3 in articular chondrocytes. In further studies, developing apigenin as a novel agent for the control of cartilage damage in osteoarthritis via intraarticular administration will be considered.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Aida Y, Maeno M, Suzuki N, Shiratsuchi H, Motohashi M, Matsumura H. The effect of IL-1β on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci. 2005;77:3210–3221. doi: 10.1016/j.lfs.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Aigner T, McKenna L. Molecular pathology and pathobiology of osteoarthritic cartilage. Cell Mol Life Sci. 2002;59:5–18. doi: 10.1007/s00018-002-8400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005;44:7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989;84:678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Hao J, Liu F, Lu J, Yang X. Apigenin attenuates acute myocardial infarction of rats via the inhibitions of matrix metalloprotease-9 and inflammatory reactions. Int J Clin Exp Med. 2015;8:8854–8859. [PMC free article] [PubMed] [Google Scholar]
- Durigova M, Roughley PJ, Mort JS. Mechanism of proteoglycan aggregate degradation in cartilage stimulated with oncostatin M. Osteoarthr Cartil. 2008;16:98–104. doi: 10.1016/j.joca.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C, Theilmeier G, Pap T. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56:542–549. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnero P, Rousseau JC, Delmas PD. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 2000;43:953–968. doi: 10.1002/1529-0131(200005)43:5<953::AID-ANR1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008;67:iii75–iii82. doi: 10.1136/ard.2008.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration. Spine. 1998;23:1612–1626. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- Hwang YP, Oh KN, Yun HJ, Jeong HG. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J Dermatol Sci. 2011;61:23–31. doi: 10.1016/j.jdermsci.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Jo H, Park JS, Kim EM, Jung MY, Lee SH, Seong SC, Park SC, Kim HJ, Lee MC. The in vitro effects of dehydroepiandrosterone on human osteoarthritic chondrocytes. Osteoarthr Cartil. 2003;11:585–594. doi: 10.1016/S1063-4584(03)00094-3. [DOI] [PubMed] [Google Scholar]
- Kanyama M, Kuboki T, Kojima S, Fujisawa T, Hattori T, Takigawa M, Yamashita A. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids of patients with temporomandibular joint osteoarthritis. J. Orofac. Pain. 2000;14:20–30. [PubMed] [Google Scholar]
- Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- Kullich W, Fagerer N, Schwann H. Effect of the NSAID nimesulide on the radical scavenger glutathione S-transferase in patients with osteoarthritis of the knee. Curr Med Res Opin. 2007;23:1981–1986. doi: 10.1185/030079907X223486. [DOI] [PubMed] [Google Scholar]
- Lijnen HR. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry Mosc. 2002;67:92–98. doi: 10.1023/A:1013908332232. [DOI] [PubMed] [Google Scholar]
- Lin PM, Chen CT, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthr Cartil. 2004;12:485–496. doi: 10.1016/j.joca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators and aging collide. Arthritis Rheum. 2006;54:1357–1360. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin HJ. The response for articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. [PubMed] [Google Scholar]
- Moon PD, Jeong HS, Chun CS, Kim HM. Baekjeolyusin-tang and its active component berberine block the release of collagen and proteoglycan from IL-1β-stimulated rabbit cartilage and down-regulate matrix metalloproteinases in rabbit chondrocytes. Phytother Res. 2011;25:844–850. doi: 10.1002/ptr.3353. [DOI] [PubMed] [Google Scholar]
- Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri D, Perego P, Palombo D. Apigenin inhibits the TNF-α-induced expression of eNOS and MMP-9 via modulating Akt signalling through oestrogen receptor engagement. Mol Cell Biochem. 2012;371:129–136. doi: 10.1007/s11010-012-1429-1. [DOI] [PubMed] [Google Scholar]
- Pantsulaia I, Kalichman L, Kobyliansky E. Association between radiographic hand osteoarthritis and RANKL, OPG and inflammatory markers. Osteoarthr Cartil. 2010;18:1448–1453. doi: 10.1016/j.joca.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Patil RH, Babu RL, Naveen Kumar M, Kiran Kumar KM, Hegde SM, Ramesh GT, Chidananda Sharma S. Apigenin inhibits PMA-induced expression of pro-inflammatory cytokines and AP-1 factors in A549 cells. Mol Cell Biochem. 2015;403:95–106. doi: 10.1007/s11010-015-2340-3. [DOI] [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]