Abstract
Naturally occurring coumarin compounds have received substantial attention due to their pharmaceutical effects. Esculetin is a coumarin derivative and a polyphenol compound that is used in a variety of therapeutic and pharmacological strategies. However, its effect on aldose reductase activity remains poorly understood. In this study, the potential beneficial effects of esculetin on lenticular aldose reductase were investigated in galactose-fed (GAL) rats, an animal model of sugar cataracts. Cataracts were induced in Sprague-Dawley (SD) rats via a 50% galactose diet for 2 weeks, and groups of GAL rats were orally treated with esculetin (10 or 50 mg/kg body weight). In vehicle-treated GAL rats, lens opacification was observed, and swelling and membrane rupture of the lens fiber cells were increased. Additionally, aldose reductase was highly expressed in the lens epithelium and superficial cortical fibers during cataract development in the GAL rats. Esculetin reduced rat lens aldose reductase (RLAR) activity in vitro, and esculetin treatment significantly inhibited lens opacity, as well as morphological alterations, such as swelling, vacuolation and liquefaction of lens fibers, via the inhibition of aldose reductase in the GAL rats. These results indicate that esculetin is a useful treatment for galactose-induced cataracts.
Keywords: Aldose reductase, Esculetin, Sugar cataract, Galactose, Lens fibers
INTRODUCTION
A cataract is a clouding of the eye’s lens that causes a progressive, painless loss of vision. Although this ocular disorder is the leading cause of blindness worldwide, the precise mechanism responsible for this phenomenon is unknown. The enzyme aldose reductase catalyzes the reduction of glucose to sorbitol through the polyol pathway, which is a process that is linked to cataract development in diabetes. Because sorbitol does not readily diffuse across cell membranes and because it demonstrates slow conversion to fructose, sorbitol accumulation under hyperglycemic conditions induces osmotic stress within the cell, leading to lens fiber cell swelling and eventually to membrane rupture (Kinoshita, 1974). Because aldose reductase is primarily localized to the lens epithelium, an increase in osmotic stress via sorbitol accumulation occurs in these cells first (Murata et al., 2001).
The inhibition of aldose reductase has been proposed as a therapeutic approach to ameliorate or prevent long-term diabetic complications, such as sugar cataracts. Coumarins have a ring structure that is similar to that in flavonoids, and an aldose reductase inhibitory activity of coumarins has also been reported (Wang et al., 2008). Coumarin, a natural substance found in various plants, is an organic compound in the aroma benzopyrone chemical class and is a colorless crystalline substance in a standard state. Okuda et al. have reported the inhibitory effects of coumarins, flavonoids, and flavones on rat lens aldose reductase (RLAR) (Okuda et al., 1982), and Brubaker et al. have investigated a series of synthesized coumarins using RLAR (Brubaker et al., 1986). We have previously confirmed that scopoletin, a coumarin derivative, potentially prevents cataractogenesis in an ex vivo model, in addition to sugar cataracts in galactose-fed rats, by inhibiting aldose reductase activity (Lee et al., 2010; Kim et al., 2013). The structure of esculetin is very similar to that of scopoletin. Esculetin is an abundant compound found in many medicinal plants, such as Artemisia capillaries, Artemisia scoparia, Citrus limonia and Ceratostigma willmottianum (Subramaniam and Ellis, 2011). It has been reported to have various biochemical and biological effects, including anti-proliferative and anti-oxidant activities (Egan et al., 1990; Lin et al., 2000; Wang et al., 2002; Kok et al., 2009). Esculetin has also recently been reported to have a protective effect in diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress (Prabakaran and Ashokkumar, 2013). Further, it exhibits inhibitory activity on RLAR in vitro (Jung et al., 2011). In this study, we demonstrated the effect of esculetin on cataract development in galactose-fed (GAL) rats.
MATERIALS AND METHODS
Determination of RLAR inhibitory activity in vitro
Aldose reductase activity was assayed spectrofluorophotometrically by measuring the decrease in the absorption of NADPH according to a previously described method (Kim et al., 2008). Rat lenses were removed from Sprague-Dawley rats weighting 240–260 g and preserved by freezing until use. Then, they were mixed with sodium phosphate buffer, the homogenate was centrifuged at 10,000 rpm (4°C, 20 min), and the supernatant was used as an enzyme source. Esculetin was purchased from Sigma (St. Louis, MO, USA). Briefly, the incubation mixture contained 100 mmol/l Li2SO4, 0.04 mmol/l DL-glyceraldehyde, 0.03 mmol/l NADPH, 135 mmol/l sodium/potassium-phosphate buffer (pH 7.0) and 100 μl of an enzyme preparation, RLAR homogenate with or without 50 μl esculetin or positive inhibitor, for a total volume of 1.0 ml. RLAR activity was determined by measuring the decrease in NADPH at excitation (360 nm) and emission (460 nm) wavelengths over 5 min using a spectrofluorophotometer (SynergyTM HT, Bio-Tek, VT, USA) at room temperature (RT). The concentration of esculetin leading to a 50% inhibition of aldose reductase activity (IC50) was expressed as the mean ± SEM of triplicate experiments.
Animals and experimental design
Male Sprague-Dawley rats weighing 180–200 g were randomly divided into 4 groups of 6 animals each. The normal control group (group I) received a normal diet, group II received a 50% galactose diet (GAL), and groups III and IV were treated orally with two concentrations of esculetin (10 and 50 mg/kg body weight) with GAL once per day for 2 weeks. All other groups were also treated once per day for 2 weeks. All animal procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Korea Institute of Oriental Medicine Institutional Animal Care and Use Committee.
Analysis of cataract formation
After two weeks of treatment, the rat eyes were excised under deep anesthesia via intraperitoneal injection of 10 mg/kg zolazepam (Zoletil, Virbac, Carros, France) mixed with 10 mg/kg xylazine hydrochloride (Rumpun, Bayer, Frankfurt, Germany). The lenses were removed from the eyeballs under an optical microscope, and the wet weights were calculated. The lenses were then transferred onto 24-well plates and photographed under an optical microscope with a CCD camera. The opaque areas of the lenses were analyzed using an imaging program (ImageJ, NIH, USA). The data are expressed as the percentage of the opaque area relative to the entire lens area.
Analysis of lens fiber degeneration
The isolated lenses were then fixed in 2% paraformaldehyde and 1% glutaraldehyde for 48 h, embedded in paraffin, and sectioned (6 μm). To assess lens fiber degeneration, the sections were next reacted with rhodamine-conjugated wheat germ agglutinin (Vector Laboratories, CA, USA) for 90 min. Images were captured using an Olympus BX51 microscope and DP71 digital camera (Olympus, Tokyo, Japan). The severity of lens fiber degeneration was evaluated using the following classifications: grade 0, no change in the lens fiber morphology; grade I, disorganization observed at a part of the lens fiber membrane; grade II, disorganization observed at all parts of the lens fiber membrane; grade III, morphological changes, such as swelling, of the lens fiber membrane; and grade IV, morphological changes, such as vacuolation and liquefaction, of the lens fiber membrane.
Immunohistochemical staining
For aldose reductase immunohistochemical staining, the antigen was retrieved by heating the slides in a pressure cooker in citric buffer (pH 6.0) for 10 min. Endogenous peroxidase activity was inactivated by incubation in 0.3% (v/v) hydrogen peroxidase solution in methanol for 10 min at RT. The sections were incubated at RT with goat anti-aldose reductase antibody (1:500 in 1% BSA-PBS, 2 h; Santa Cruz, CA, USA). Following three consecutive washes in PBS for 5 min each, the sections were incubated at RT for 60 min with HRP-conjugated anti-goat IgG. For signal detection, the reaction was visualized using 3,3′-diaminobenzidine tetrahydrochloride re-agent (Vector Laboratories, CA, USA) for several seconds. Photographs were taken using an Olympus BX51 microscope and DP71 digital camera (Olympus, Tokyo, Japan). For morphometric analysis, the positive signal intensities per unit area (0.32 mm2) in a total of 10 randomly selected fields were determined using Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
The values are presented as the mean ± SEM. The results were compared using a one-way analysis of variance (ANOVA) followed by Tukey’s test. A value of p<0.01 was considered significant.
RESULTS
Esculetin inhibits RLAR activity in vitro
Esculetin was tested for its inhibitory activity on RLAR activity in vitro. As shown in Table 1, esculetin dose-dependently inhibited the aldose reductase activity (IC50=18.11 ± 0.95 μM). The inhibitory activity of esculetin was greater than that of 3.3-tetramethyleneglutaric acid (TMG), a well-known aldose reductase inhibitor (IC50=28.81 ± 1.52 μM).
Table 1.
Inhibitory effect of esculetin on RLAR activity in vitro
| Sample | Concentration (μM) | Inhibition (%) | IC50 (μM) |
|---|---|---|---|
| Esculetin | 5.6 | 13.89 ± 5.01 | 18.11 ± 0.95 |
| 14.0 | 38.89 ± 6.36 | ||
| 28.1 | 78.70 ± 6.26 | ||
| TMGa | 20 | 31.42 ± 5.71 | 28.81 ± 1.52 |
| 30 | 56.42 ± 9.60 | ||
| 40 | 69.69 ± 8.15 |
Inhibitory activity is expressed as the mean ± SEM of triplicate samples. IC50 values were calculated using GraphPad Prism ® software and plotted.
TMG (3.3-Tetramethyleneglutaric acid) was used as a positive control.
Lens opacification analysis
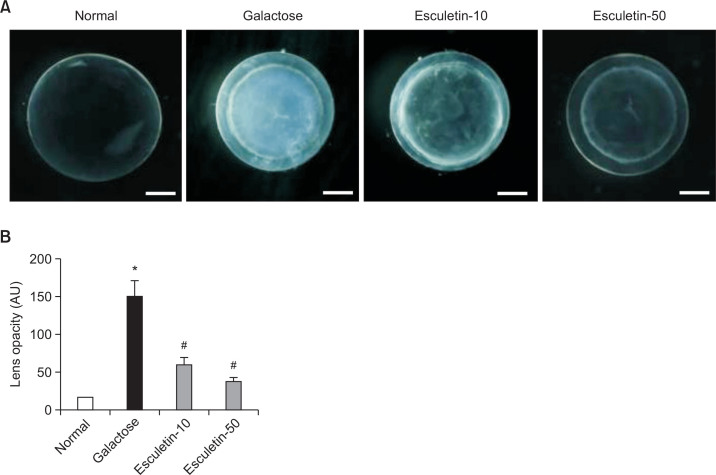
At the end of the two-week study, no lenses displayed cataract formation in the normal rats. The lenses of the vehicle-treated GAL rats became severely opaque. However, the esculetin-treated rats exhibited the dose-dependent inhibition of lens opacification (Fig. 2A). Quantitative analysis revealed that the mean opaque region of the lenses from the vehicle-treated GAL rats was significantly enhanced by eight-fold compared with the normal rats. Galactose-induced lens opacification was inhibited by esculetin administration in a concentration-dependent manner (Fig. 2B, p<0.01). This observation indicates that esculetin prevents the onset of galactose-induced cataracts.
Fig. 2.
Opacification of the lens. (A) Representative images of the lenses in each group. (B) Analysis of lens opacities. All opacities were analyzed in each lens of the normal rats (NOR) and vehicle-treated galactose-fed (GAL) rats. The GAL rats were treated with esculetin at doses of 10 mg/kg (Esculetin-10) and 50 mg/kg (Esculetin-50). All data are expressed as the mean ± SE, n=6. *p<0.01 vs. normal rats, #p<0.01 vs. GAL rats.
Lens wet weights
The amount of lens swelling was measured by determining the lens wet weights of the rats (Patterson and Bunting, 1966). As shown in Fig. 3, the lens weights of the GAL rats increased during lens opacification. However, esculetin administration significantly decreased the lens weights compared with those of the vehicle-treated GAL rats.
Fig. 3.
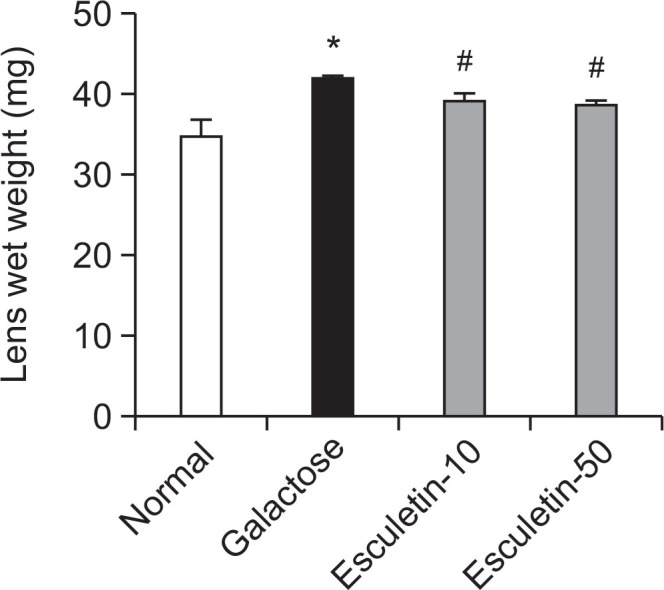
Wet weights of the lenses. Normal rats (NOR), vehicle-treated galactose-fed (GAL) rats, and GAL rats treated with esculetin at doses of 10 mg/kg (Esculetin-10) and 50 mg/kg (Esculetin-50) were examined. All data are expressed as the mean ± SE, n=8. *p<0.01 vs. normal rats, #p<0.01 vs. GAL rats.
Degeneration of lens fiber cells
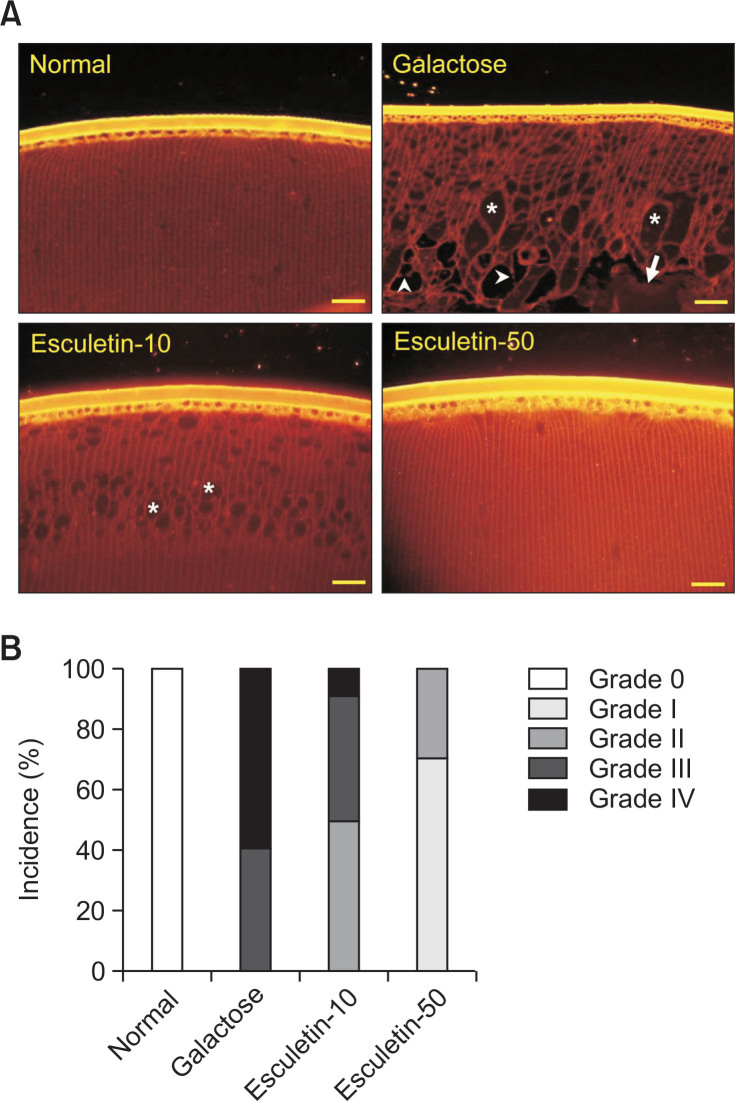
Labeling of the lens membranes demonstrated that normal lenses consistently showed complete crystal packing of fiber cells. In vehicle-treated GAL rats, swelling, multiple membrane rupture and large areas of liquefaction of lens fiber cells were detected (40% were in grade III and 60% were in grade IV, Fig. 4). However, the highest dose of esculetin inhibited these histological changes of lens fibers (70% were in grade I and 30% were in grade II, Fig. 4).
Fig. 4.
Degeneration of the lens fiber. (A) Rhodamine-conjugated wheat germ agglutinin staining. Lens sections from a normal rat (NOR) and vehicle-treated galactose-fed (GAL) rat. The GAL rat samples were treated with esculetin at doses of 10 mg/kg (Esculetin-10) and 50 mg/kg (Esculetin-50). Fiber cell liquefaction (arrow), swelling (asterisk) and membrane rupture (arrowhead) were observed in the galactosemic cataractous lenses. The Scale bar indicates 20 μm. (B) Lens fiber degeneration grading. Lens fiber degeneration was assessed on a scale of a scale of 0–IV.
Expression of aldose reductase protein in the lens
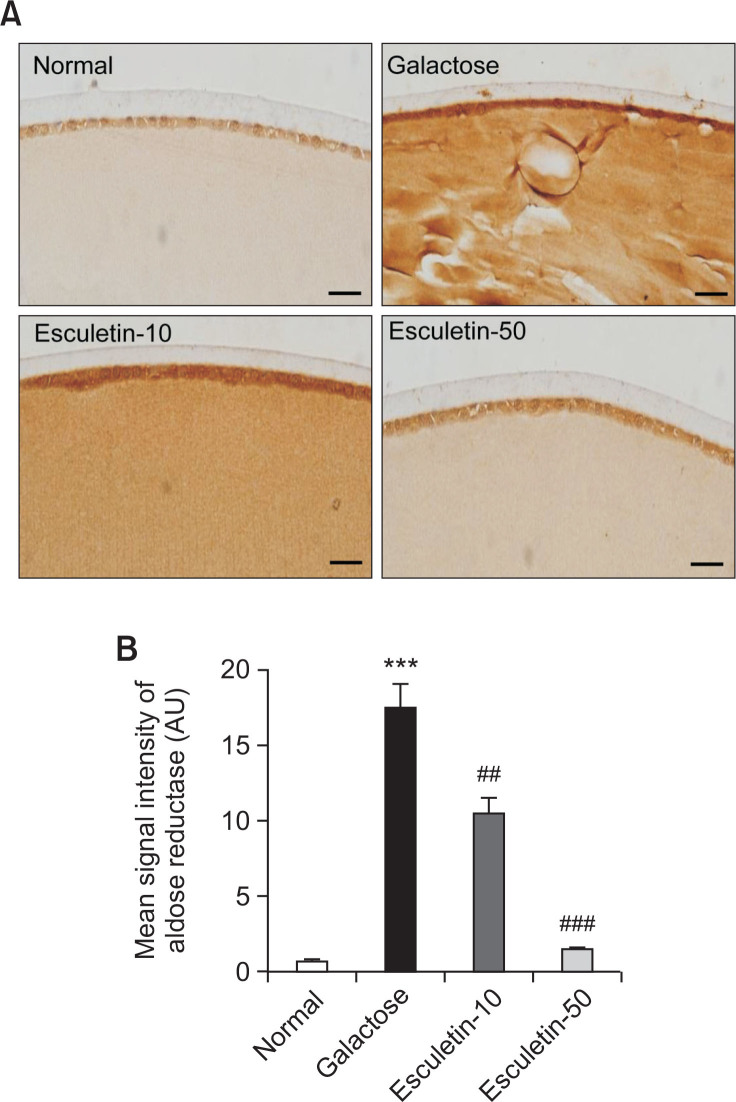
In the vehicle-treated GAL rats, an increase in immuno-reactive staining for aldose reductase was observed in the cytoplasm of the lens epithelium, extending into the deeper cortical fibers. However, esculetin administration dose-dependently reduced expression of aldose reductase protein in the lens epithelium and inhibited the extension of aldose reductase beneath the epithelial region (Fig. 5A). Quantitative analysis revealed that the intensity of aldose reductase immunolabeling was significantly increased by 24-fold in the vehicle-treated GAL rats compared with the normal rats, and it was suppressed by esculetin administration (Fig. 5B, p<0.001).
Fig. 5.
Expression of aldose reductase protein. (A) Immunohistochemical staining of aldose reductase. Representative immunostaining of aldose reductase in lenses from a normal rat (NOR) and vehicle-treated galactose-fed (GAL) rat. The GAL rats were treated with esculetin at doses of 10 mg/kg (Esculetin-10) and 50 mg/kg (Esculetin-50). Strong immunoreactivity of aldose reductase was observed in the cytoplasm of lens epithelial cells and lens cortical fibers. The Scale bar indicates 20 μm. (B) Quantitative analysis of aldose reductase signal intensity. All data are expressed as the mean ± SE, n=6. ***p<0.001 vs. normal rats. ###p<0.001, ##p<0.05 vs. GAL rats.
DISCUSSION
Cataracts represent the most common ocular disease that causes visual impairment; however, pharmacological interventions to delay or prevent the development of intraocular lens opacity remain in the experimental stage. Numerous factors are associated with the progression of this eye disease, but the exact mechanism of cataractogenesis remains unclear. Current studies are attempting to describe the mechanism of cataract formation using various animal cataract models. Galactose-induced cataract models are widely used, and the initiation factors for galactosemic cataracts in young rats can reasonably be assumed to be the same as those involved in the development of human galactosemic cataracts (Kinoshita, 1974). In this study, we evaluated the anti-cataract effects of esculetin in GAL rats using histological and morphological approaches. Treatment with esculetin had a positive effect by reducing the extent and preventing the progression of cataract formation.
Unakar and Tsui described morphological changes in the lenses of GAL rats (Unakar and Tsui, 1983), such as swelling, vacuolation and liquefaction of lens fibers, beginning in the equatorial region and progressing toward the anterior and posterior cortical regions. As a result, the epithelial cells and cortical fibers of the lenses exhibited substantial damage, including cell membrane disruption. Sugar cataracts are osmotically induced by the intracellular accumulation of sorbitol produced from glucose, as catalyzed by aldose reductase. Recent studies have focused on the crucial role of the enzyme aldose reductase as the initiating factor in galactosemic cataract formation. Aldose reductase catalyzes the reduction of glucose to sorbitol in the first step of the polyol pathway, a process involved in galactosemic cataract development. Because sorbitol cannot cross cell membranes, it accumulates in lens epithelial cells and exerts osmotic pressure on cells by drawing in water. The increased sorbitol accumulation generates a hyperosmotic effect that induces fluid infusion to offset the osmotic gradient (Kinoshita, 1974; Kinoshita et al., 1981). In animal studies, sorbitol accumulation within the lens has been shown to lead to progressive liquefaction of the lens cortex and collapse of the lens capsule, eventually resulting in lens opacity (Kinoshita, 1965, 1974). These observations have led to the hyperosmotic theory of sugar cataractogenesis, which states that the intracellular increase in fluid in response to the aldose reductase-mediated accumulation of sorbitol results in lens fiber swelling associated with histological and morphological changes, which ultimately lead to lens opacity (Kador and Kinoshita, 1984; Kinoshita, 1974; Kinoshita et al., 1981). In this study, esculetin prevented the progression of lens opacity in GAL rats and also prevented galactose-induced alterations in lens morphology, such as lens fiber swelling and membrane rupture.
The inhibition of aldose reductase has been proposed to ameliorate or prevent sugar cataract formation in experimental animals (Dvornik et al., 1973; Veeresham et al., 2014). Although several synthetic inhibitors of aldose reductase (including epalrestat, sorbinil and tolrestat) have shown powerful effects, their uses are limited. In particular, these inhibitors have been withdrawn from clinical trials due to relatively poor permeation, low efficacy and safety issues (Kawanishi et al., 2003; Manzanaro et al., 2006; Peyroux and Sternberg, 2006). Among these aldose reductase inhibitors, only epalrestat is available in the Japanese market for the treatment of diabetic neuropathy. Thus, there is an urgent need for new aldose reductase inhibitors (de la Fuente and Manzanaro, 2003). Interestingly, several herbal extracts and nutritional supplements (such as naturceuticals) have shown anti-cataract activities. For example, an extensive literature survey has shown that aldose reductase inhibition with natural resources can delay or prevent cataract formation (Crabbe and Goode, 1998; de la Fuente and Manzanaro, 2003; Kim et al., 2010). Indeed, many natural compounds are known to exert potent inhibitory effects on aldose reductase in vitro (Tomlinson et al., 1994). Compounds derived from plants with aldose reductase inhibitory activity can be classified into specific chemical groups, such as alkaloids, phenolics, flavonoids and coumarins. Among these compounds, esculetin, a coumarin derivative, has also been shown to have potent aldose reductase inhibitory activity (Jung et al., 2011). In our in vitro assay, esculetin was found to exhibit strong inhibitory activity against RLAR, with a mean IC50 value of 18.11 ± 0.95 μM, whereas Jung et al. have demonstrated that esculetin isolated from Artemisia montana has an IC50 value of 172.48 ± 9.01 μM. The differences in strength of the RLAR inhibitory activity of esculetin might be attributed to differences in purity between synthetic and plant-derived compounds and differences in the experimental methods used, such as variations in the amounts of crude aldose reductase in rat lens homogenates and incubation times. Kato et al. have demonstrated that esculetin treatment (50 mg/kg body weight) completely inhibits hyperglycemia after 15 and 30 min using a sucrose loading test and that it significantly suppresses the accumulation of sorbitol in erythrocytes (Kato et al., 2008). However, the anti-cataractogenesis activities of esculetin in animal studies remain unclear. In our analysis of in vitro RLAR activity, esculetin also showed excellent inhibitory activity against aldose reductase (IC50 value of 18.11 ± 0.95 μM), and this RLAR activity corresponded with anti-cataractogenic activity in the GAL rats. Our results further showed that esculetin had a concentration-dependent effect on cataractogenesis, and a strong correlation was observed between aldose reductase activity and lens opacification. Lastly, the administration of esculetin reduced the amount of aldose reductase in the lens.
The enzymatic distribution of aldose reductase activity was demonstrated by its localized immunoreactivity. Aldose reductase is primarily localized to epithelial cells and superficial cortical fibers of the lens (Lizak et al., 1998). In the lenses of the GAL rats, increased immunoreactive positive staining of aldose reductase protein was observed in the epithelium, extending from the superficial region to the deeper cortex. However, esculetin inhibited the expression of aldose reductase protein in the lens epithelium and prevented its extension below the lens epithelium. Therefore, the decreased activity of aldose reductase observed in the esculetin-treated rats was likely due to reduced expression of aldose reductase protein.
In conclusion, our results show that the beneficial protective effect of esculetin against sugar cataract development occurs through the inhibition of aldose reductase. Moreover, a degree of protection against cataracts can likely be obtained through dietary intake of esculetin. Our results indicate that esculetin inhibits galactose-induced cataractogenesis by suppressing aldose reductase expression. Multiple mechanisms, such as non-enzymatic glycation and oxidative stress, are also involved in cartaractogenesis as a consequence of hypergalactosemia or hyperglycemia. Therefore, further research is needed to investigate other possible mechanisms of action for esculetin.
Fig. 1.
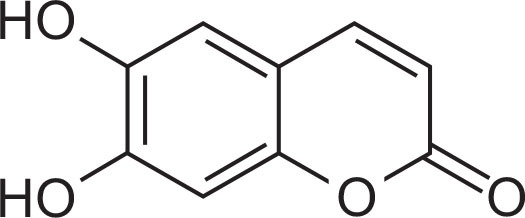
Chemical structure of esculetin.
Acknowledgments
This research was supported by a grant [K14040] from the Korea Institute of Oriental Medicine (KIOM).
REFERENCES
- Brubaker AN, DeRuiter J, Whitmer WL. Synthesis and rat lens aldose reductase inhibitory activity of some benzopyran-2-ones. J Med Chem. 1986;29:1094–1099. doi: 10.1021/jm00156a031. [DOI] [PubMed] [Google Scholar]
- Crabbe MJ, Goode D. Aldose reductase: a window to the treatment of diabetic complications? Prog Retin Eye Res. 1998;17:313–383. doi: 10.1016/S1350-9462(97)00013-X. [DOI] [PubMed] [Google Scholar]
- de la Fuente JA, Manzanaro S. Aldose reductase inhibitors from natural sources. Nat Prod Rep. 2003;20:243–251. doi: 10.1039/b204709h. [DOI] [PubMed] [Google Scholar]
- Dvornik E, Simard-Duquesne N, Krami M, Sestanj K, Gabbay KH, Kinoshita JH, Varma SD, Merola LO. Polyol accumulation in galactosemic and diabetic rats: control by an aldose reductase inhibitor. Science. 1973;182:1146–1148. doi: 10.1126/science.182.4117.1146. [DOI] [PubMed] [Google Scholar]
- Egan D, O’Kennedy R, Moran E, Cox D, Prosser E, Thornes RD. The pharmacology, metabolism, analysis, and applications of coumarin and coumarin-related compounds. Drug Metab Rev. 1990;22:503–529. doi: 10.3109/03602539008991449. [DOI] [PubMed] [Google Scholar]
- Jung HA, Islam MD, Kwon YS, Jin SE, Son YK, Park JJ, Sohn HS, Choi JS. Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem Toxicol. 2011;49:376–384. doi: 10.1016/j.fct.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Kador PF, Kinoshita JH. Diabetic and galactosaemic cataracts. Ciba Found Symp. 1984;106:110–131. doi: 10.1002/9780470720875.ch7. [DOI] [PubMed] [Google Scholar]
- Kato A, Minoshima Y, Yamamoto J, Adachi I, Watson AA, Nash RJ. Protective effects of dietary chamomile tea on diabetic complications. J Agric Food Chem. 2008;56:8206–8211. doi: 10.1021/jf8014365. [DOI] [PubMed] [Google Scholar]
- Kawanishi K, Ueda H, Moriyasu M. Aldose reductase inhibitors from the nature. Curr Med Chem. 2003;10:1353–1374. doi: 10.2174/0929867033457304. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim CS, Lee YM, Sohn E, Jo K, Shin SD, Kim JS. Scopoletin inhibits rat aldose reductase activity and cataractogenesis in galactose-fed rats. Evid Based Complement Alternat Med. 2013;2013:787138. doi: 10.1155/2013/787138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NH, Kim YS, Lee YM, Jang DS, Kim JS. Inhibition of aldose reductase and xylose-induced lens opacity by puerariafuran from the roots of Pueraria lobata. Biol Pharm Bull. 2010;33:1605–1609. doi: 10.1248/bpb.33.1605. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim NH, Jung DH, Jang DS, Lee YM, Kim JM, Kim JS. Genistein inhibits aldose reductase activity and high glucose-induced TGF-beta2 expression in human lens epithelial cells. Eur J Pharmacol. 2008;594:18–25. doi: 10.1016/j.ejphar.2008.07.033. [DOI] [PubMed] [Google Scholar]
- Kinoshita JH. Cataracts in galactosemia. The Jonas S. Friedenwald Memorial Lecture. Invest Ophthalmol. 1965;4:786–799. [PubMed] [Google Scholar]
- Kinoshita JH. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974;13:713–724. [PubMed] [Google Scholar]
- Kinoshita JH, Kador P, Catiles M. Aldose reductase in diabetic cataracts. JAMA. 1981;246:257–261. doi: 10.1001/jama.1981.03320030049032. [DOI] [PubMed] [Google Scholar]
- Kok SH, Yeh CC, Chen ML, Kuo MY. Esculetin enhances TRAIL-induced apoptosis through DR5 upregulation in human oral cancer SAS cells. Oral Oncol. 2009;45:1067–1072. doi: 10.1016/j.oraloncology.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim NH, Nam JW, Lee YM, Jang DS, Kim YS, Nam SH, Seo EK, Yang MS, Kim JS. Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis ex vivo. Arch Pharm Res. 2010;33:1317–1323. doi: 10.1007/s12272-010-0904-z. [DOI] [PubMed] [Google Scholar]
- Lin WL, Wang CJ, Tsai YY, Liu CL, Hwang JM, Tseng TH. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch Toxicol. 2000;74:467–472. doi: 10.1007/s002040000148. [DOI] [PubMed] [Google Scholar]
- Lizak MJ, Secchi EF, Lee JW, Sato S, Kubo E, Akagi Y, Kador PF. 3-FG as substrate for investigating flux through the polyol pathway in dog lens by 19F-NMR spectroscopy. Invest Ophthalmol Vis Sci. 1998;39:2688–2695. [PubMed] [Google Scholar]
- Manzanaro S, Salva J, de la Fuente JA. Phenolic marine natural products as aldose reductase inhibitors. J Nat Prod. 2006;69:1485–1487. doi: 10.1021/np0503698. [DOI] [PubMed] [Google Scholar]
- Murata M, Ohta N, Sakurai S, Alam S, Tsai J, Kador PF, Sato S. The role of aldose reductase in sugar cataract formation: aldose reductase plays a key role in lens epithelial cell death (apoptosis) Chem. Biol. Interact. 2001;130–132:617–625. doi: 10.1016/S0009-2797(00)00289-1. [DOI] [PubMed] [Google Scholar]
- Okuda J, Miwa I, Inagaki K, Horie T, Nakayama M. Inhibition of aldose reductases from rat and bovine lenses by flavonoids. Biochem Pharmacol. 1982;31:3807–3822. doi: 10.1016/0006-2952(82)90297-0. [DOI] [PubMed] [Google Scholar]
- Patterson JW, Bunting KW. Sugar cataracts, polyol levels and lens swelling. Doc Ophthalmol. 1966;20:64–72. doi: 10.1007/BF00165406. [DOI] [PubMed] [Google Scholar]
- Peyroux J, Sternberg M. Advanced glycation endproducts (AGEs): Pharmacological inhibition in diabetes. Pathol. Biol. (Paris) 2006;54:405–419. doi: 10.1016/j.patbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Prabakaran D, Ashokkumar N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie. 2013;95:366–373. doi: 10.1016/j.biochi.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Subramaniam SR, Ellis EM. Esculetin-induced protection of human hepatoma HepG2 cells against hydrogen peroxide is associated with the Nrf2-dependent induction of the NAD(P)H: Quinone oxidoreductase 1 gene. Toxicol Appl Pharmacol. 2011;250:130–136. doi: 10.1016/j.taap.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Stevens EJ, Diemel LT. Aldose reductase inhibitors and their potential for the treatment of diabetic complications. Trends Pharmacol Sci. 1994;15:293–297. doi: 10.1016/0165-6147(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Unakar NJ, Tsui JY. Inhibition of galactose-induced alterations in ocular lens with sorbinil. Exp Eye Res. 1983;36:685–694. doi: 10.1016/0014-4835(83)90106-9. [DOI] [PubMed] [Google Scholar]
- Veeresham C, Rama Rao A, Asres K. Aldose reductase inhibitors of plant origin. Phytother Res. 2014;28:317–333. doi: 10.1002/ptr.5000. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Hsieh YJ, Chu CY, Lin YL, Tseng TH. Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett. 2002;183:163–168. doi: 10.1016/S0304-3835(02)00031-9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ling B, Zhang R, Liu Y. Docking and molecular dynamics study on the inhibitory activity of coumarins on aldose reductase. J Phys Chem B. 2008;112:10033–10040. doi: 10.1021/jp8033227. [DOI] [PubMed] [Google Scholar]