Abstract
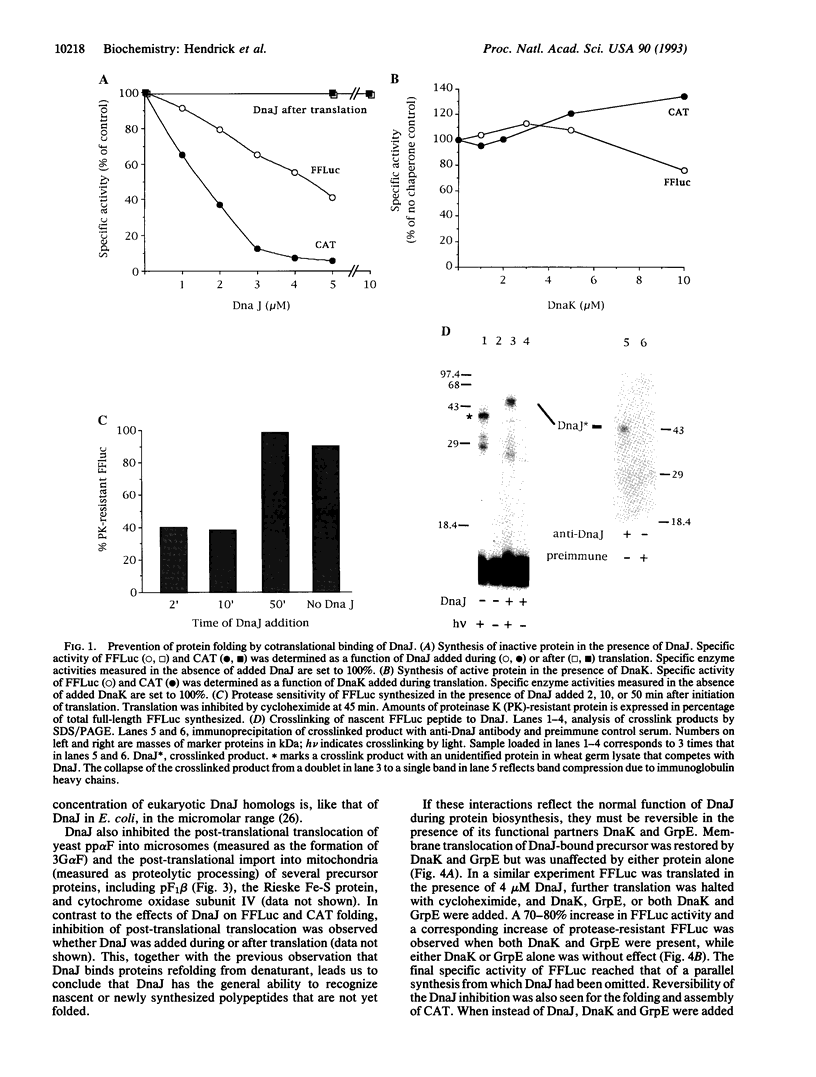
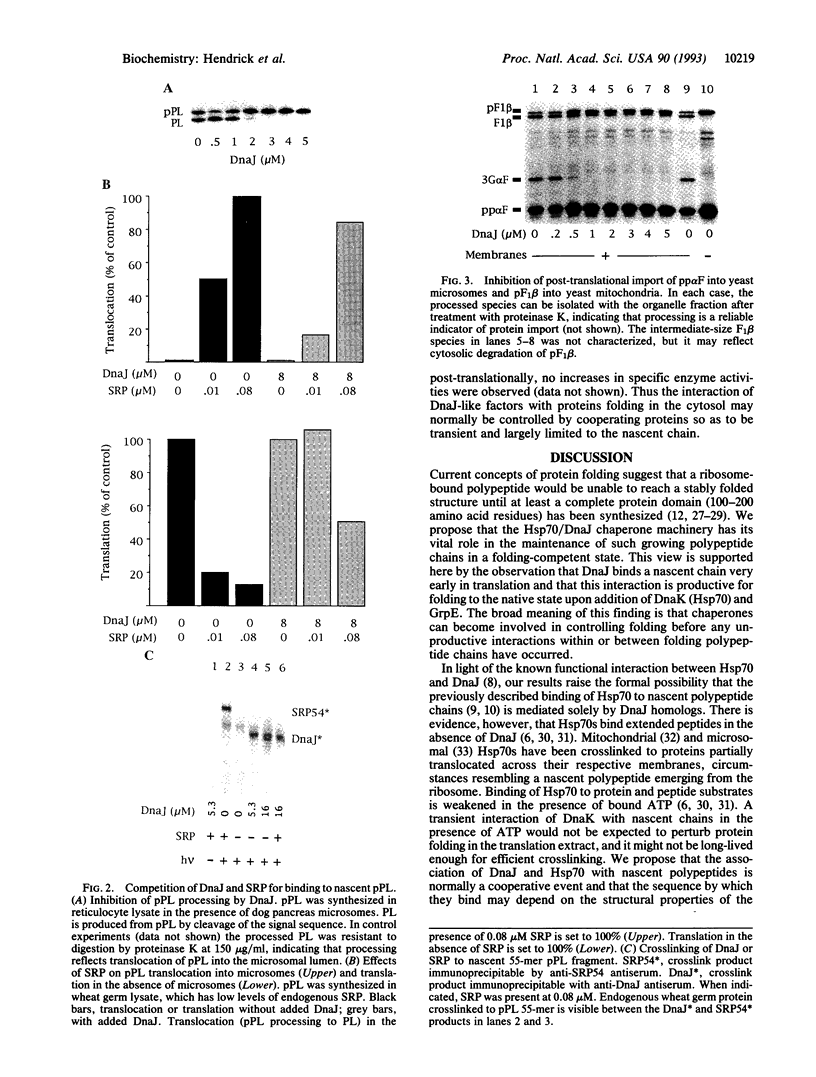
Recent evidence supports the view that cellular protein folding may be mediated by molecular chaperones. A fundamental question concerns the stage in its biogenesis at which the folding protein makes first contact with these components. We show here by crosslinking that the chaperone DnaJ binds nascent ribosome-bound polypeptide chains as short as 55 residues. Cotranslational binding of DnaJ to firefly luciferase and chloramphenicol acetyltransferase resulted in an arrest of folding as long as the functional partners of DnaJ in Escherichia coli, DnaK and GrpE, were missing. Protein uptake into microsomes and mitochondria was also interrupted by DnaJ. Both folding and post-translational translocation recommenced upon addition of DnaK and GrpE. We propose that DnaJ protects nascent polypeptide chains against aggregation and, in cooperation with Hsp70, controls their productive folding once a complete polypeptide or a polypeptide domain has been synthesized.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atencio D. P., Yaffe M. P. MAS5, a yeast homolog of DnaJ involved in mitochondrial protein import. Mol Cell Biol. 1992 Jan;12(1):283–291. doi: 10.1128/mcb.12.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Blumberg H., Silver P. A. A homologue of the bacterial heat-shock gene DnaJ that alters protein sorting in yeast. Nature. 1991 Feb 14;349(6310):627–630. doi: 10.1038/349627a0. [DOI] [PubMed] [Google Scholar]
- Caplan A. J., Cyr D. M., Douglas M. G. YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell. 1992 Dec 24;71(7):1143–1155. doi: 10.1016/s0092-8674(05)80063-7. [DOI] [PubMed] [Google Scholar]
- Caplan A. J., Douglas M. G. Characterization of YDJ1: a yeast homologue of the bacterial dnaJ protein. J Cell Biol. 1991 Aug;114(4):609–621. doi: 10.1083/jcb.114.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham M. E., Brion J. P., Anderton B. H. Human homologues of the bacterial heat-shock protein DnaJ are preferentially expressed in neurons. Biochem J. 1992 Jun 1;284(Pt 2):469–476. doi: 10.1042/bj2840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Molecular chaperones. Unfolding protein folding. Nature. 1991 Jul 4;352(6330):17–18. doi: 10.1038/352017a0. [DOI] [PubMed] [Google Scholar]
- Cyr D. M., Lu X., Douglas M. G. Regulation of Hsp70 function by a eukaryotic DnaJ homolog. J Biol Chem. 1992 Oct 15;267(29):20927–20931. [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Feldheim D., Rothblatt J., Schekman R. Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol Cell Biol. 1992 Jul;12(7):3288–3296. doi: 10.1128/mcb.12.7.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G., Schmid F. X. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry. 1990 Mar 6;29(9):2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Pohl J., Flocco M. T., Rothman J. E. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991 Oct 24;353(6346):726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- Frydman J., Nimmesgern E., Erdjument-Bromage H., Wall J. S., Tempst P., Hartl F. U. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J. 1992 Dec;11(13):4767–4778. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. The emergence of the chaperone machines. Trends Biochem Sci. 1992 Aug;17(8):295–299. doi: 10.1016/0968-0004(92)90439-g. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Görlich D., Kurzchalia T. V., Wiedmann M., Rapoport T. A. Probing the molecular environment of translocating polypeptide chains by cross-linking. Methods Cell Biol. 1991;34:241–262. doi: 10.1016/s0091-679x(08)61684-2. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Protein folding: local structures, domains, subunits, and assemblies. Biochemistry. 1991 Apr 2;30(13):3147–3161. doi: 10.1021/bi00227a001. [DOI] [PubMed] [Google Scholar]
- Krieg U. C., Walter P., Johnson A. E. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T. V., Wiedmann M., Girshovich A. S., Bochkareva E. S., Bielka H., Rapoport T. A. The signal sequence of nascent preprolactin interacts with the 54K polypeptide of the signal recognition particle. Nature. 1986 Apr 17;320(6063):634–636. doi: 10.1038/320634a0. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Nelson R. J., Ziegelhoffer T., Nicolet C., Werner-Washburne M., Craig E. A. The translation machinery and 70 kd heat shock protein cooperate in protein synthesis. Cell. 1992 Oct 2;71(1):97–105. doi: 10.1016/0092-8674(92)90269-i. [DOI] [PubMed] [Google Scholar]
- Palleros D. R., Welch W. J., Fink A. L. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Krieg U. C., Hwang S. T., Vestweber D., Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990 Dec;9(13):4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Walter P. The affinity of signal recognition particle for presecretory proteins is dependent on nascent chain length. EMBO J. 1988 Jun;7(6):1769–1775. doi: 10.1002/j.1460-2075.1988.tb03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Lingappa V. R. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Bielka H., Rapoport T. A. Direct probing of the interaction between the signal sequence of nascent preprolactin and the signal recognition particle by specific cross-linking. J Cell Biol. 1987 Feb;104(2):201–208. doi: 10.1083/jcb.104.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]