Abstract
Rosa rugosa Thunb, a deciduous shrub of the genus Rosa, has been widely used to treat stomach aches, diarrhoea, pain, and chronic inflammatory disease in eastern Asia. In recent years, our research team has extensively studied the Rosa rugosa flower extract, and specifically undertook pharmacological experiments which have optimized the extraction process. Our methods have yielded a standard extract enriched in phenolic compounds, named PRE. Herein, we expand our efforts and evaluated the anti-inflammatory activity of PRE on lipopolysaccharide (LPS)-induced inflammation in RAW 264.7 macrophages. PRE significantly inhibited production of nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor (TNF)-a, interleukin (IL)-6, and interleukin 1β (IL-1β), as well as expression of their synthesizing enzymes, inducible nitric oxide synthase (iNOS) and cyclooxygenase2 (COX-2). Furthermore, PRE inhibited activity of mitogen-activated protein kinases (MAPK) as well as nuclear factor-kappa B (NF-κB) signaling pathway. Our findings are the first to explain the anti-inflammatory mechanism by PRE in LPS-stimulated macrophages. Given these results, we propose that PRE has therapeutic potential in the prevention of inflammatory disorders.
Keywords: Rosa rugosa Thunb, Inflammation, Nuclear factor-κB, Mitogen-activated protein kinases
INTRODUCTION
Inflammation is a guarding mechanism against different harmful stimuli, such as infection, chemical exposure, tissue damage trauma or exposure to bacterial components, such as lipopolysaccharide (LPS) (Kim et al., 2013b; Nahar et al., 2014). Macrophages participate in the inflammatory process through the secretion of a series of inflammatory cytokines, including tumor necrosis factor-a (TNF-α) and interleukin-1β (Oh et al., 2013), and inflammatory mediators, such as, nitric oxide (NO) and prostaglandin E2 (PGE2), which are synthesized by inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively (An et al., 2011). An overproduction of these mediators causes harmful effects to tissues and organisms, and has been associated with the pathogenesis of various inflammation-related diseases, including rheumatoid arthritis, diabetes, inflammatory bowel disease, atherosclerosis, and cancer (Shao et al., 2013).
Accumulating evidence indicates that NF-κB is a major transcription factor that modulates the expression of COX-2, iNOS, the production of NO, PGE2, as well as the production of pro-inflammatory cytokines, such as, IL-1β, TNF-α, and IL-6 (Boje, 2004; Shao et al., 2013). Mitogen-activated protein kinases (MAPKs) are a specific class of serine/threonine kinases, which respond to extracellular signals (Park et al., 2012) and MAPKs signaling pathways play an important role in conveying information from extracellular environment into cytoplasm and finally into the nucleus (Guha and Mackman, 2001). In brief, NF-κB and MAPKs are both critical factors in the inflammatory process and important targets for the inflammatory diseases (Kim et al., 2013a).
Rosa rugosa Thunb, a deciduous shrub of the genus Rosa, is widely distributed from the temperate regions of eastern Asia including China, Japan, and Korea, to the subarctic zone of Kamchatka and Okhotsk (Gu et al., 2013). In eastern Asia, Rosa rugosa is a traditional herbal medicine for treating illnesses such as stomach aches, diarrhoea, menoxenia, diabetes mellitus, pain and chronic inflammatory disease (Gu et al., 2013). The petals of Rosa rugosa contain abundant flavonoids, terpenoids, steroids, tocopherol, and carotene (Gu et al., 2013). Scientific investigations on the medicinal properties of Rosa rugosa have indicated several potential applications, such as vasodilation, microcirculation improvement (Chen et al., 2014), inhibitory activity against HIV reverse transcriptase (Mikanagi et al., 1994; Fu et al., 2006) and anti-hyperplasia effects (Chen et al., 2014). In recent years, our laboratory has conducted in-depth research on the Rosa rugosa flower extract, and from our pharmacological experiments, we optimized the extraction process and obtained a standard extract which was named PRE. Because of the presence of various phenolic compounds in PRE, and its use in various therapeutic applications, we hypothesized that PRE exhibits anti-inflammatory effects in vitro. Among in vitro models, the LPS-treated RAW 264.7 murine macrophage cell line has been widely used for in vitro evaluation of anti-inflammatory activity (Hsu et al., 2013). The objective of this study was to evaluate the inhibitory in vitro effect of PRE in LPS-stimulated inflammation.
MATERIALS AND METHODS
Sample preparation
The flowers of Rosa rugosa were collected from Hotan County, Xinjiang Uygur Autonomous Region, China, and identified by Guanmian Shen, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences. The flowers of Rosa rugosa were extracted with 50% (V/V) ethanol under re-flux three times. After recovery of the solvent under reduced pressure, the residue was dissolved in water and then subjected to a NKA-9 macroporous resin glass chromatography column, and the diameter height ration (inner diameter of the column bed height ratio) was 1:7. Elution solvent was 50% (V/V) ethanol and collected 5 resin bed volume eluent. Next, the eluent was concentrated under reduced pressure and the obtained fraction was named PRE.
Reagents
Lipopolysaccharide (LPS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), dimethylsufoxide (DMSO) were purchased from Sigma Chemical Co. (St. Louis, Mo, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were produced by Gibco BRL (Grand Island, NY, USA). Enhanced Bradford protein assay kit was obtained from Beijing Biomed Co. (Beijing, China). Phenylmethylsulfonyl fluoride (PMSF) and the components of the whole cell lysis buffer for Western blot analysis were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The Griess reagent kit was from Beyotime Chemical Co. (Jiang su, china). Mouse PGE2 ELISA kit was from Blue Gene Biological Technology Co. (Shanghai, China) and TNF-α, IL-1β and IL-6 ELISA kits were from Multi Sciences Biological Technology Co. (Hangzhou, China). Antibodies for iNOS, COX-2, β-actin, phosphor-NF-κB p65, NF-κB p65, phosphor-IκBa, IκBa, phospho-ERK1/2, ERK1/2, phospho-p38, p38, phospho-JNK/SAPK and JNK/SAPK were obtained from Cell Signaling Technology (Danvers, MA, USA). Acetonitrile (HPLC grade) was purchased from Fisher Scientific (Fair Lawn, NJ, USA) and the other solvents such as formic acid (analytical grade) from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). All other chemicals used in the experiments were commercial products of reagent grade.
Cell culture
RAW 264.7 mouse macrophage cells were obtained from Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, China). The cells were cultured in DMEM medium supplemented with 10% heat-inactivated FBS, 100U/ mL penicillin and 100 μg/mL streptomycin. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2. RAW 264.7 cells were plated in a 96 well for MTT; and in 6-well and 12-well plates for the Western blot and ELISA. To stimulate the cells, the medium was changed with DMEM medium and LPS (1 μg/mL) was added in the presence or absence of PRE (10, 25, 50, and 100 μg/mL) for the indicated periods.
Cell viability assay
Cytotoxicity was analyzed using the MTT assay according to the method described by Mosmann (Mosmann, 1983). RAW 264.7 cells were plated into 96-well microtiter plates at a density of 1×104 cells per well and incubated in a 37°C, 5% CO2 incubator overnight. Next, the culture medium was replaced with 200 μL serial dilutions of PRE followed by a 24 h incubation at 37°C with 5% CO2. After 24 h incubation, 50 μL of MTT solution (2 mg/mL) was added to each well, followed by incubation for 4 h. MTT reagent was removed and cells were lysed with 150 μL/well DMSO and the cellular viability was measured at 570 nm using an ELISA plate-reader (Spec-traMaxM2, Molecular Devices Corp, Sunnyvale, CA).
Effect on NO production
RAW 264.7 cells (1×105 cells/mL) were pre-incubated for 1 h with various concentrations of PRE and stimulated with LPS (1 μg/mL) at 37°C for 24 h in medium. The level of NO production was monitored by measuring the nitrite level in the culture medium. This was performed by mixing the medium with Griess reagent system. The optical densities were measured at 540 nm after incubation for 10 min. The nitrite concentration was calculated with reference to a standard curve of sodium nitrite.
Enzyme-linked immunosorbent assay (ELISA)
RAW 264.7 cells were seeded in 12-well plates (5×105 cells/mL) and incubated at 37°C for 24 h. The cells were pre-treated with PRE for 1 h and then stimulated with 1 μg/mL of LPS for 24 h. Levels of TNF-α, IL-6, IL-1β, and PGE2 in the supernatant were measured by ELISA kit according to the procedure provided by the manufacturer. Absorbance at 450 nm was measured in an ELISA plate-reader.
Western blot analysis
Protein expression was evaluated by Western blot analysis according to standard procedures. The cells were pre-treated with PRE and stimulated with LPS for indicated periods at 37°C. After treatment, RAW 264.7 cells were collected and lysed in ice-cold lysis buffer. The protein concentration was estimated with the Bio-Rad DC protein assay using bovine serum albumin (BSA) as a standard. Total protein was separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 10% polyacrylamide gel. The proteins in the gel were transferred to PVDF membranes. The membranes were blocked with blocking buffer for 30 min, incubated with primary antibody at 4°C overnight, and subsequently incubated with secondary antibody for 1 h. Membranes were washed three times with PBST for 10 min each step. The signal was detected using the Amersham ECL system (GE Healthcare, Piscataway, NJ, China). Relative protein expression was quantified using the software ImageJ (NIH) and calculated according to the reference band of β-actin.
High-Performance Liquid Chromatography (HPLC) analysis of PRE
HPLC analysis was performed using a Dionex (Sunnyvale, CA, USA) system comprising automated sample injector (ASI-100), pump (P680), thermostat column compartment (TCC-100), ultra-violet detector (UVD-170U) running Chromeleon 6.80 software. The effluent was monitored by UV detection at 254 nm. Separation column: YMC C18 column (4.6 mm I.D.× 250 mm, 5 μm); column temperature: 32°C; the mobile phase: methanol (A), and 0.4% phosphoric acid (B) that followed: A-B in 50 min. Flow rate: 1.0 mL/min. To prepare analytical samples, PRE powder was accurately weighed and dissolved in 50% ethanol at a concentration of 50 mg/mL. Prior to analysis, sample was filtered through a 0.45 μm filter.
Statistical analysis
Data are expressed as the means ± standard error of the mean (SEM) for the number of experiments. Statistical significance was compared between each treated group and the control, and determined by Student’s t tests. Each experiment was repeated at least three times to yield comparable results. Values with p<0.05 and p<0.01 were considered significant.
RESULTS
Effect of PRE on RAW 264.7 cell viability
The cytotoxicity of PRE was examined using MTT assay. RAW 264.7 cells were treated with PRE (10, 25, 50, and 100 μg/mL). PRE did not affect the viability of the RAW 264.7 cells (Fig. 1).
Fig. 1.
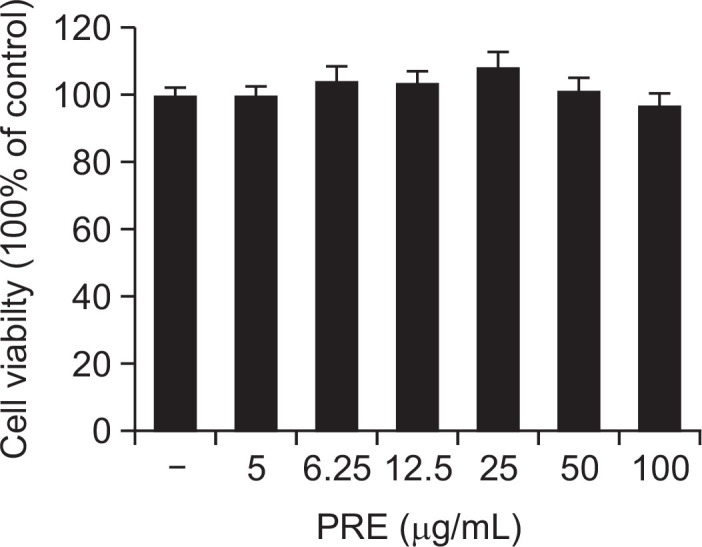
Effect of the PRE on the cell viability of RAW 264.7 macrophages. Cells were treated with different concentrations of PRE for 24 h, and viability determined with the MTT assay. Results of independent experiments were averaged as percentage cell viability, compared with untreated control. Data are presented as means ± SEM of triple determinations.
Effect of PRE on production of NO and PGE2, and expression of iNOS, and COX-2 in LPS-induced RAW 264.7 cells
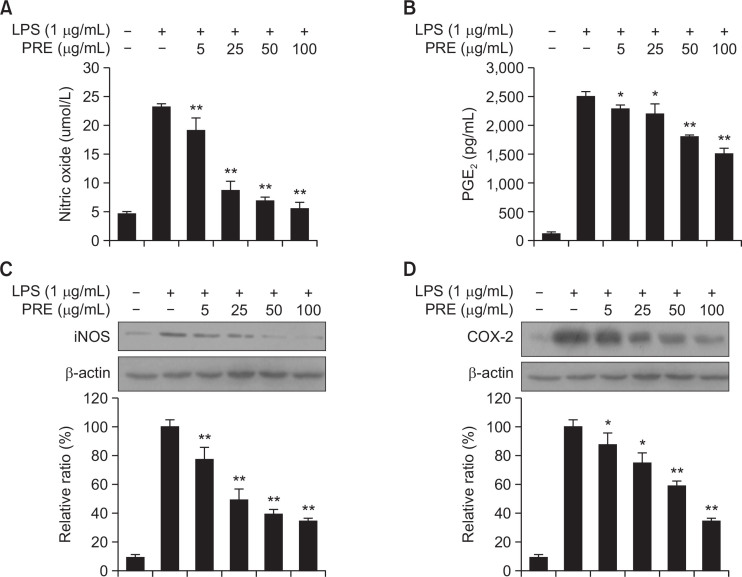
NO is a signaling molecule that plays a key role in the pathogenesis of inflammation, and is considered as a pro-inflammatory mediator due to its overexpression in abnormal situations (Hortelano et al., 2002). In this study, NO production was assessed by measuring the accumulation of nitrites, a stable metabolite of NO, in the media by colorimetric assay based on the Griess reaction. As presented in Fig. 2A, PRE dose-dependently repressed NO generation. In particular, PRE at a dose of 100 μg/mL reduced NO production by 76% in comparison with the LPS-treated control cells. In addition, PRE significantly reduced iNOS protein expression in a dose-dependent manner (Fig. 2C). PGE2 also functions as a mediator of inflammation, which is produced by metabolism of arachidonic acid by COX enzymes at inflammatory sites (Nahar et al., 2014). The inhibitory effects of PRE on PGE2 levels were evaluated in LPS-stimulated macrophages. As shown in Fig. 2B, PGE2 production increased following LPS treatment, whereas in the absence of LPS, PGE2 levels were similar to baseline levels. Treatment with 100 μg/mL of PRE reduced PGE2 levels 43.2% in LPS-stimulated RAW 264.7 cells. In order to assess whether the inhibitory effects of PRE on PGE2 was related to the alteration of COX-2 enzymes, we examined COX-2 protein expression by Western blot analysis. As shown in Fig. 2D, PRE significantly reduced COX-2 protein expression in a dose-dependent manner. Combined, our results indicate that the respective inhibitory effect of PRE on NO and PGE2 production is related with its suppressive on iNOS COX-2 protein expression, respectively (Fig. 2).
Fig. 2.
Effects of PRE on LPS-induced production of NO and PGE2, and protein expression of iNOS, COX-2 in RAW264.7 cells. Cells were pre-treated with different concentrations of PRE for 1 h, followed by stimulation with LPS (1 μg/mL) for 24 h. The culture supernatant was analyzed for NO (A), PGE2 (B) production. Lysates were analyzed for iNOS (C), COX-2 (D) protein expression using Western blotting. As a control, the cells were incubated with vehicle alone. Data shows mean ± SEM values of duplicate determination from three independent experiments. *p<0.05 and **p<0.01 were calculated from comparing with LPS-stimulation value.
Effect of PRE on TNF-α, IL-6, and IL-1β production in LPS-induced RAW264.7 cells
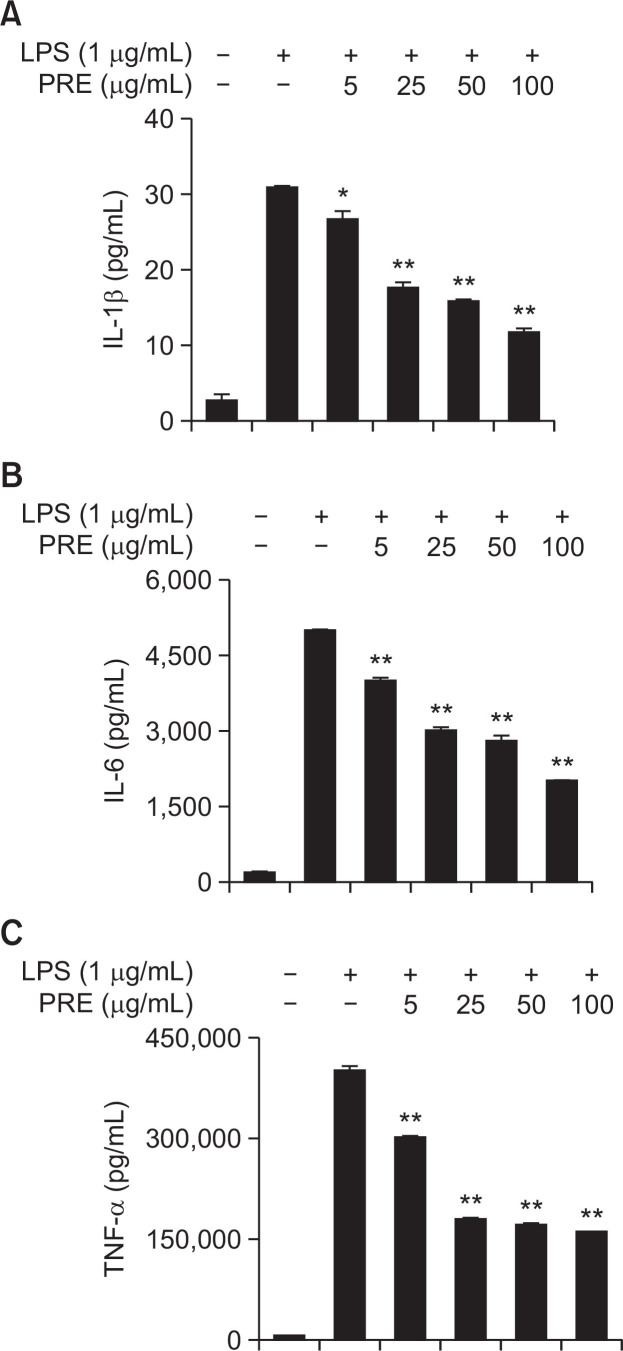
The pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β have several roles in the inflammatory response, including activation of the endothelium and leukocytes and induction of the acute-phase response (Lee et al., 2011). The inhibition of these pro-inflammatory cytokines has been identified as targets for anti-inflammatory therapies (Park et al., 2012). As a central mediator of inflammation, TNF-α is induced by a wide range of pathogenic stimuli and performs a key role in the pathogenesis of chronic inflammatory diseases. Likewise, IL-1β is an important pro-inflammatory cytokine that regulates multiple aspects of immune and inflammatory responses (Lee et al., 2011). To determine the effects of PRE on the production of the pro-inflammatory cytokines, TNF-α, IL-6, and IL-1β were evaluated by ELISA kit according to manufacturer’s protocols. As shown in Fig. 3, the levels of TNF-α, IL-6, and IL-1β were significantly increased in the LPS-treated cells when compared with the untreated cells. However, the levels of TNF-α, IL-6, and IL-1β were significantly reduced in the cells pre-treated with PRE in a dose-dependent manner. These results suggested that PRE inhibited the production of TNF-α, IL-6, and IL-1β.
Fig. 3.
PRE diminishes IL-1β (A), IL-6 (B), and TNF-α (C) production in RAW264.7 macrophages stimulated with LPS. Cells were pre-treated for 1 h with different concentrations of PRE, followed by stimulation with LPS (1 μg/mL) for 24 h. The concentrations of IL-1β, IL-6, and TNF-α were determined as described in Methods. Shown are the mean values of three experiments ± SEM. *p<0.05 and **p<0.01 were calculated from comparing with LPS-stimulation value.
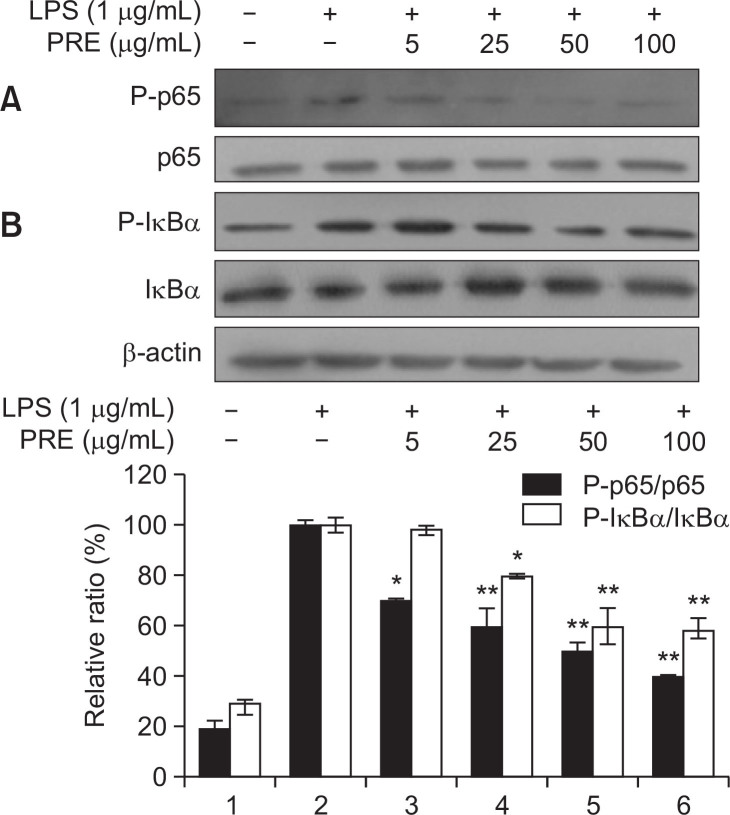
Inhibitory effect of PRE on LPS-induced phosphorylation of NF-κB and IκBα
The above data showed that PRE downregulated pro-inflammatory mediators as well as cytokines production. Expression of these cytokines and their genes can be regulated by the activation of the transcription factor NF-κB, which is crucially involved in several aspects of the pathogenesis of rheumatism and other chronic inflammatory diseases (Redington et al., 2001). Most anti-inflammatory drugs have been shown to repress the expression of these genes by inhibiting the NF-κB activation pathway (Yun et al., 2008). We therefore investigated whether PRE suppressed the phosphorylation of IκB and NF-κB p65 (a component of the heterodimer of NF-κB). RAW264.7 cells were pre-treated with various concentrations of PRE and then stimulated with LPS for 30 min. As shown in Fig. 4A, PRE obviously inhibited the LPS-induced phosphorylation of NF-κB/p65 as well as phosphorylation and degradation of IκBα. Upon stimulation of cells with LPS, IκB becomes phosphorylated and triggers its own proteolytic degradation, following which NF-κB translocates to the nucleus where it can activate certain genes by binding to transcription-regulatory elements in a nucleotide sequence-specific manner (Mikanagi et al., 1994). Our results demonstrated that PRE could inhibit LPS-induced activation of NF-κB by suppression of IκBα and NF-κB/p65 phosphorylation.
Fig. 4.
Effects of PRE on LPS-stimulated NF-κB p65 (A) and IκBα phosphorylation (B) in RAW 264.7 cells. Cells were pre-treated for 1 h with different concentrations of PRE and then the cells were incubated for an additional 30 min with LPS (1 μg/mL). Total cellular proteins of cells were harvested for measurements of total or phosphorylated p-p65, p65, p-IκB-α, and IκB-α protein by Western blotting. β-actin was used as an internal control. The results from three separate experiments are represented, and data shown represent the mean values of three experiments ± SEM, *p<0.05, **p<0.01 indicates significant differences from the LPS-treated group.
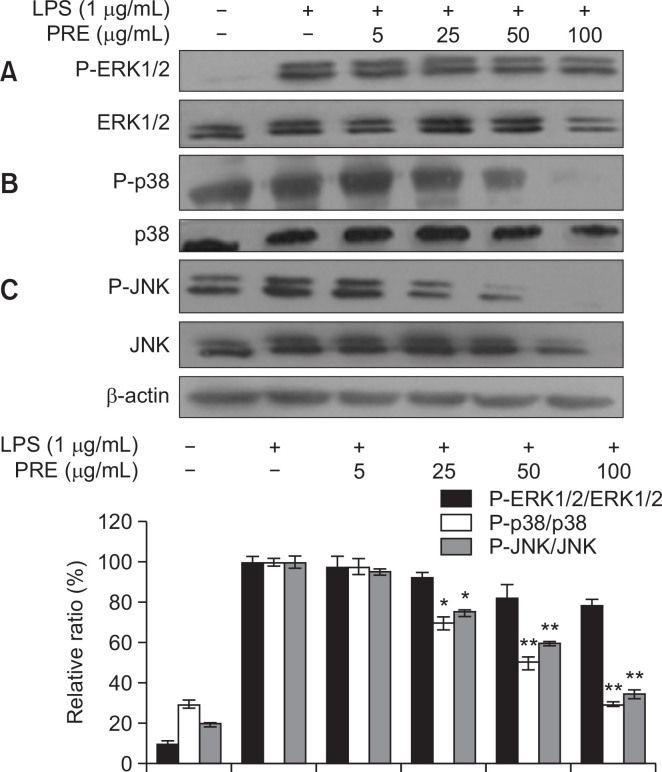
Effect of PRE on LPS-induced activation of ERK1/2, JNK, and p38 MAPK in RAW 264.7 macrophages
MAPKs signal transduction pathways are classified into at least three components: p38, ERK1/2 and JNK. These pathways are ubiquitous and highly evolutionarily conserved mechanisms of eukaryotic cell regulation (Chen et al., 2014). Here, we explored the effects of PRE on phosphorylation levels of p38, ERK1/2, and JNK in LPS-induced RAW 264.7 cells. As shown in Fig. 5, the results indicated that PRE suppressed LPS-induced p38 and JNK phosphorylation levels but had no effect on phosphorylation of ERK1/2.
Fig. 5.
Effects of PRE on LPS-stimulated phosphorylation of MAPKs in RAW 264.7cells. Cells were pre-treated for 1 h with different concentrations of PRE and then the cells were incubated for an additional 30 min with LPS (1 μg/mL). Total cellular proteins of cells were harvested for measurements of total or phosphorylated ERK1/2, JNK, and p38 by Western blotting. β-actin was used as an internal control. The results from three separate experiments are represented, and data shown represents the mean values of three experiments ± SEM, *p<0.05, **p<0.01 indicates significant differences from the LPS-treated group.
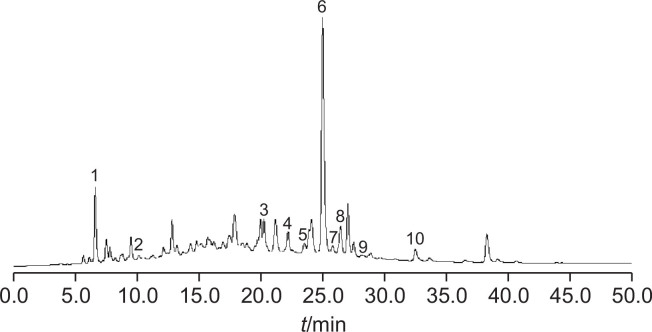
HPLC analysis of PRE
The main component profile of PRE was analyzed via HPLC. The representative chromatogram is shown in Fig. 6. We extracted and purified ten compounds from PRE and the structural identifications of all the ten compounds from PRE were carried out using 1H-NMR, 13C-NMR, and by comparing with our previous report on LC-MSn data (Gu et al., 2013). We confirmed that PRE is a complex mixture mainly containing phenolic compounds and flavonoids. Ellagic acid which was the most important compound of PRE observed in PRE chromatograms and recorded at peak 6.
Fig. 6.
HPLC Chromatogram of PRE. 1. Gallic acid, 2. Protocatechuic acid, 3.Quercetin-3-O-(2″-O-β-D-glucopyranosyl)-β-D-galactopyranoside, 4. Kaempferol-3-O-(2″-O-β-D-glucopyranosyl)-β-D-glucopyranoside, 5. Hyperoside, 6. Ellagic acid, 7. Avicularin, 8. Astragalin, 9. Juglanin, 10. Kaempferol.
DISCUSSION
The present study was undertaken to evaluate the potential anti-inflammatory activities of PRE, using the accepted LPS-induced RAW 264.7 model system. The results showed that the evaluated polyphenolic-rich extracts of PRE successfully reduced the expression of inflammatory enzymes, iNOS and COX-2 in LPS-activated RAW 264.7 macrophages, and this reduction was accompanied by the decrease of NO and PGE2 production levels. Suppression of NO production via inhibition of iNOS expression (Moon et al., 2008) as well as PGE2 production via inhibition of COX-2 expression are potentially attractive therapeutic targets for the treatment of inflammation (Redington et al., 2001). Our results suggest that PRE contains strong inhibitory activity on pro-inflammatory mediators. Several inflammatory cytokines, particularly TNF-α, IL-1β, and IL-6, are known to play key roles in the induction and perpetuation of inflammation in macrophages (Yun et al., 2008). In the present study, LPS could directly stimulate the output of TNF-α, IL-1β, and IL-6 by activating RAW 264.7cells. However, the levels of TNF-α, IL-6, and IL-1β were significantly reduced in the cells pre-treated with PRE. These findings suggest that PRE, which acts as an anti-inflammatory agent, reduces the production of inflammatory molecules stimulated by LPS.
The heterodimeric NF-κB complex is sequestered in the cytoplasm as an inactive precursor, complexed with an inhibitory IκB-like protein. Activation of NF-κB, an important transcription factor in the inflammatory response, occurs following the phosphorylation, ubiquitination, and proteolytic degradation of IκBα (Park et al., 2012). IκBα degradation and subsequent NF-κB p65 translocation from cytosol to nucleus by various stimuli are essential in NF-κB activation (Shao et al., 2013). In this study, we found that PRE significantly inhibited LPS-induced phosphorylation and degradation of IκBa, as well as the phosphorylation of NF-κB p65. The phosphorylation and activation of three major MAPKs have been shown to initiate inflammatory gene expression in LPS-induced macrophages. We also investigated the effect of PRE on the MAPKs phosphorylation in RAW 264.7 macrophages. In this study, we found that PRE inhibited the LPS-induced phosphorylation of p-38 and JNK but had no effect on phosphorylation of ERK1/2, which suggested that p38 and JNK pathway may be involved in the PRE-mediated inhibition of LPS-induced inflammation effect.
Phenolic compounds are plant secondary metabolites known for their anti-oxidative and anti-inflammatory properties (Dussossoy et al., 2011). Our research showed PRE is enriched in phenolic compounds and flavonoids. Among these compounds, gallic acid and ellagic acid were the major compounds in PRE extract. Ellagic acid exhibited pronounced anti-inflammatory activities both in vitro (Kassim et al., 2010; Sugimoto et al., 2011) and in vivo levels (Angeles Rosillo et al., 2012; Anitha et al., 2013). To a certain extent, ellagic acid is the major component of PRE involved in suppressing inflammation, and may therefore be responsible for the anti-inflammatory activities of PRE.
In summary, the current study is the first report to investigate the mechanisms underlying the anti-inflammatory activity of PRE fraction from the flowers of Rosa rugosa. This study showed that PRE significantly reduced the production of inflammatory mediators such as, NO, PGE2, and inflammatory cytokines such as, TNF-α, IL-6, and IL-1β, as well as suppressed the protein expression of iNOS and COX-2. These effects may be mediated through suppression of NF-κB and MAPKs activation. All these evidences confirmed that flower extract of Rosa rugosa may be a beneficial anti-inflammatory agent.
Supplemental Instructions
Acknowledgments
This work was supported by the Joint Funds of the National Natural Science Foundation of China (Grant No. U1203203) and the Key Research Program of the Chinese Academy of Sciences (Grant No. KSZD-EW-Z-004).
REFERENCES
- An HJ, Kim IT, Park HJ, Kim HM, Choi JH, Lee KT. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-alpha expression through inactivation of the nuclear factor-kappa b pathway in RAW 264.7 macrophages. Int Immunopharmacol. 2011;11:504–510. doi: 10.1016/j.intimp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Angeles Rosillo M, Sanchez-Hidalgo M, Cardeno A, Aparicio-Soto M, Sanchez-Fidalgo S, Villegas I, Alarcon de la Lastra C. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol Res. 2012;66:235–242. doi: 10.1016/j.phrs.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Anitha P, Priyadarsini RV, Kavitha K, Thiyagarajan P, Nagini S. Ellagic acid coordinately attenuates Wnt/beta-catenin and NF-kappa B signaling pathways to induce intrinsic apoptosis in an animal model of oral oncogenesis. Eur J Nutr. 2013;52:75–84. doi: 10.1007/s00394-011-0288-y. [DOI] [PubMed] [Google Scholar]
- Boje KMK. Nitric oxide neurotoxicity in neurodegenerative diseases. Front Biosci. 2004;9:763–776. doi: 10.2741/1268. [DOI] [PubMed] [Google Scholar]
- Chen X, Zong C, Gao Y, Cai R, Fang L, Lu J, Liu F, Qi Y. Curcumol exhibits anti-inflammatory properties by interfering with the JNK-mediated AP-1 pathway in lipopolysaccharide-activated RAW264.7 cells. Eur J Pharmacol. 2014;723:339–345. doi: 10.1016/j.ejphar.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Dussossoy E, Brat P, Bony E, Boudard F, Poucheret P, Mertz C, Giaimis J, Michel A. Characterization, anti-oxidative and anti-inflammatory effects of Costa Rican noni juice (Morinda citrifolia L.) J Ethnopharmacol. 2011;133:108–115. doi: 10.1016/j.jep.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Fu M, Ng TB, Jiang Y, Pi ZF, Liu ZK, Li L, Liu F. Compounds from rose (Rosa rugosa) flowers with human immunodeficiency virus type 1 reverse transcriptase inhibitory activity. J Pharm Pharmacol. 2006;58:1275–1280. doi: 10.1211/jpp.58.9.0015. [DOI] [PubMed] [Google Scholar]
- Gu D, Yang Y, Bakri M, Chen Q, Xin X, Aisa HA. A LC/QTOF-MS/MS Application to Investigate Chemical Compositions in a Fraction with Protein Tyrosine Phosphatase 1B Inhibitory Activity from Rosa rugosa Flowers. Phytochem Anal. 2013;24:661–670. doi: 10.1002/pca.2451. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/S0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Hortelano S, Zeini M, Bosca L. Nitric oxide and resolution of inflammation. Methods Enzymol. 2002;359:459–465. doi: 10.1016/S0076-6879(02)59208-9. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Fang SC, Yen GC. Anti-inflammatory effects of phenolic compounds isolated from the flowers of Nymphaea mexicana Zucc. Food Funct. 2013;4:1216–1222. doi: 10.1039/c3fo60041f. [DOI] [PubMed] [Google Scholar]
- Kassim M, Achoui M, Mustafa MR, Mohd MA, Yusoff KM. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr Res. 2010;30:650–659. doi: 10.1016/j.nutres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Kim KS, Cui X, Lee DS, Sohn JH, Yim JH, Kim YC, Oh H. Anti-Inflammatory Effect of Neoechinulin A from the Marine Fungus Eurotium sp SF-5989 through the Suppression of NF-kappa B and p38 MAPK Pathways in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. Molecules. 2013a;18:13245–13259. doi: 10.3390/molecules181113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Lee DS, Bae GS, Park SJ, Kang DG, Lee HS, Oh H, Kim YC. The inhibition of JNK MAPK and NF-kappa B signaling by tenuifoliside A isolated from Polygala tenuifolia in lipopolysaccharide-induced macrophages is associated with its anti-inflammatory effect. Eur J Pharmacol. 2013b;721:267–276. doi: 10.1016/j.ejphar.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Lee HS, Ryu DS, Lee GS, Lee DS. Anti-inflammatory effects of dichloromethane fraction from Orostachys japonicus in RAW 264.7 cells: Suppression of NF-kappa B activation and MAPK signaling. J Ethnopharmacol. 2012;140:271–276. doi: 10.1016/j.jep.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son JK, Shin HK. Anti-inflammatory activity of Angelica dahurica ethanolic extract on RAW264.7 cells via upregulation of heme oxygen-ase-1. Food Chem Toxicol. 2011;49:1047–1055. doi: 10.1016/j.fct.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Mikanagi Y, Yokoi M, Saito N, Ueda Y, Hirabayashi H, Suzuki S. Flower flavonoid distribution in rosa-rugosa thunb exmurray and interspecific rosa hybrids. J Jpn Soc Hortic Sci. 1994;62:857–866. doi: 10.2503/jjshs.62.857. [DOI] [Google Scholar]
- Moon DO, Kim MO, Lee HJ, Choi YH, Park YM, Heo MS, Kim GY. Curcumin attenuates ovalbumin-induced airway inflammation by regulating nitric oxide. Biochem Biophys Res Commun. 2008;375:275–279. doi: 10.1016/j.bbrc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and surival-application to proliferation to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nahar PP, Driscoll MV, Li L, Slitt AL, Seeram NP. Phenolic mediated anti-inflammatory properties of a maple syrup extract in RAW 264.7 murine macrophages. J. Funct. Foods. 2014;6:126–136. doi: 10.1016/j.jff.2013.09.026. [DOI] [Google Scholar]
- Oh YC, Cho WK, Jeong YH, Im GY, Lee KJ, Yang HJ, Ma JY. Anti-inflammatory effect of Sosihotang via inhibition of nuclear factor-kappa B and mitogen-activated protein kinases signaling pathways in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Food Chem Toxicol. 2013;53:343–351. doi: 10.1016/j.fct.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Park JW, Kwon OK, Jang Hy, Jeong H, Oh SR, Lee HK, Han SB, Ahn KS. A leaf methanolic extract of wercklea insignis attenuates the lipopolysaccharide-induced inflammatory response by blocking the NF-kappa B signaling pathway in RAW 264.7 Macrophages. Inflammation. 2012;35:321–331. doi: 10.1007/s10753-011-9322-8. [DOI] [PubMed] [Google Scholar]
- Redington AE, Meng QH, Springall DR, Evans TJ, Creminon C, Maclouf J, Holgate ST, Howarth PH, Polak JM. Increased expression of inducible nitric oxide synthase and cyclo-oxygenase-2 in the airway epithelium of asthmatic subjects and regulation by corticosteroid treatment. Thorax. 2001;56:351–357. doi: 10.1136/thorax.56.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Li Y, Wang Z, Xiao M, Yin P, Lu Y, Qian X, Xu Y, Liu J. 7b, a novel naphthalimide derivative, exhibited anti-inflammatory effects via targeted-inhibiting TAK1 following down-regulation of ERK1/2-and p38 MAPK-mediated activation of NF-kappa B in LPS-stimulated RAW264.7 macrophages. Int Immunopharmacol. 2013;17:216–228. doi: 10.1016/j.intimp.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Sakamoto S, Nakagawa K, Hayashi S, Harada N, Yamaji R, Nakano Y, Inui H. Suppression of inducible nitric oxide synthase expression and amelioration of lipopolysaccharide-induced liver injury by polyphenolic compounds in Eucalyptus globulus leaf extract. Food Chem. 2011;125:442–446. doi: 10.1016/j.foodchem.2010.09.026. [DOI] [Google Scholar]
- Xie Y, Zhang W. Antihypertensive activity of Rosa rugosa Thunb. flowers: Angiotensin I converting enzyme inhibitor. J Ethnopharmacol. 2012;144:562–566. doi: 10.1016/j.jep.2012.09.038. [DOI] [PubMed] [Google Scholar]
- Yun KJ, Kim JY, Kim JB, Lee KW, Jeong SY, Park HJ, Jung HJ, Cho YW, Yun K, Lee KT. Inhibition of LPS-induced NO and PGE(2) production by asiatic acid via NF-kappa B inactivation in RAW 264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int Immunopharmacol. 2008;8:431–441. doi: 10.1016/j.intimp.2007.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.