Abstract
In classical eyeblink conditioning a subject learns to blink to a previously neutral stimulus. This conditional response is timed to occur just before an air puff to the eye. The learning is known to depend on the cerebellar cortex where Purkinje cells respond with adaptively timed pauses in their spontaneous firing. The pauses in the inhibitory Purkinje cells cause disinhibition of the cerebellar nuclei, which elicit the overt blinks. The timing of a Purkinje cell response was previously thought to require a temporal code in the input signal but recent work suggests that the Purkinje cells can learn to time their responses through an intrinsic mechanism that is activated by metabotropic glutamate receptors (mGluR7).
Keywords: Timing, cerebellum, Purkinje cells, learning, eyeblink conditioning
Background
It has long been suspected that the cerebellum has an important role in movement timing [1, 2] and this has become increasingly clear in the context of eyeblink conditioning. Classical eyeblink conditioning has been a widely used experimental model of associative learning and has also proven to be a good model of motor timing [2]. When a conditional stimulus (CS), such as a tone, is repeatedly succeeded by an unconditional stimulus (US) that elicits a blink reflex, the subject learns to emit a conditioned blink in response to the CS. This conditioned response (CR) not only precedes the US so that it protects the eye, it does so with a high degree of temporal precision, reaching its maximum amplitude close to the time of the (expected) US onset. This holds for CS-US intervals from about 100 ms to about a second [3-5], and for many animal species including mice [3, 6-8].
Work by several groups, using lesions, pharmacological inactivation and electrophysiology, has shown that the main mechanisms underlying eyeblink conditioning reside in the cerebellum (reviewed in [8-10]). For a long time there were conflicting views about the relative importance of the cerebellar cortex and the nuclei. A series of papers by Yeo et al. showed that the critical learning site was most likely in the cortex [11, 12] while others argued that the associative memory trace was in the deep cerebellar nuclei (DCN) [13, 14], or in both sites [15]. However, there was consensus that CRs would not be adaptively timed without the cerebellar cortex; even if CRs remain after inactivation or lesions of the cerebellar cortex, they lose their characteristic temporal profile [16, 17].
Pauses in Purkinje cell activity
Models of motor learning in the cerebellum have focused on the role of the Purkinje cells, the output neurons of the cerebellar cortex. These are inhibitory projection neurons that are driven by intrinsic mechanisms to fire at high rates of 50-100 Hz in vivo [18]. Influential theories of Marr and Albus [19, 20], proposed that the synapses between parallel fibers (carrying the CS signal, Fig. 1A) and Purkinje cells would undergo plastic change when activated in conjunction with climbing fiber input carrying the US signal. It was generally assumed that as a result of conditioning, the CS would come to elicit a pause in the Purkinje cell around the time of the US, and that this pause could, by disinhibiting downstream neurons of the deep cerebellar nuclei, cause a well-timed conditioned blink (Fig. 1B).
Figure 1.
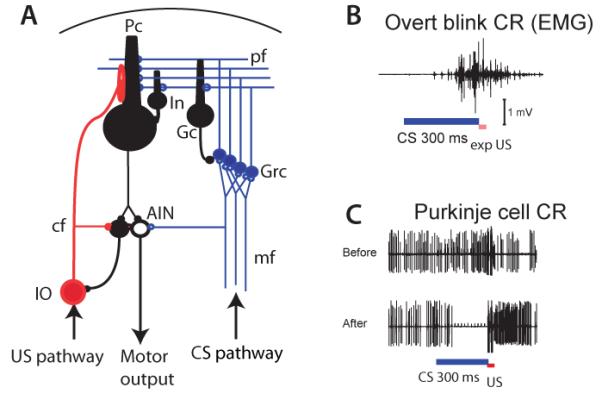
Pauses in Purkinje cell activity (A) Cerebellar circuit. Pc: Purkinje cell. pf: Parallel fiber. cf: climbing fiber. mf: mossy fiber. In: inhibitory interneuron. Gc: Golgi cell. Grc: granule cell. AIN: anterior interpositus nucleus. IO: inferior olive. (B) EMG record of blink CR. (C) Upper panel, Purkinje cell record before training, lower panel: typical Purkinje cell CR.
A recent study using transient optogenetic inhibition of Purkinje cell firing provides strong support for the idea that pauses in Purkinje cell activity can generate overt movements via “disinhibition” of the cerebellar nuclei [21]. The kinematics of eyelid movement were tightly controlled by the number of Purkinje cells that were inhibited, as well as the intensity and duration of inhibition. The resulting increase in DCN activity showed a strong linear correlation between both the size and speed of eyelid movements, such that there was a direct mapping between graded suppression of Purkinje cell firing, the consequent increases in DCN firing and the regulation of movement kinematics.
The idea that a pause in Purkinje cell activity drove the CR was originally met with resistance. Indeed, some of the early recording studies appeared to directly contradict it. Both increases and decreases in Purkinje cell activity were reported during CR generation [22-24]. This heterogeneity of Purkinje cell responses in previous studies could stem from sampling from cerebellar microzones with different physiological properties [25] or different functional connectivity [26-28]. The results become much more uniform if we consider the activity of Purkinje cells in a part of a zone within the cerebellar cortex, the C3, that has been shown to control eyeblink movements and be critical for conditioning [12, 21, 29-31]. Virtually all Purkinje cells in this area pause or strongly suppress their firing during the CR (Fig. 1C) [27, 28, 32-34].
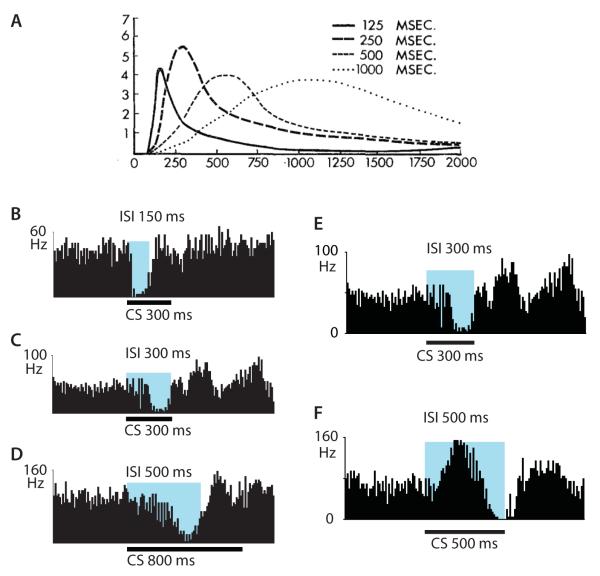
The pause response of Purkinje cells mimics most of the salient features of the overt behavioral CR, including adaptive timing (Fig. 2). For instance, this “Purkinje cell CR” is acquired during paired CS-US presentations, extinguished during CS alone presentations and is very quickly re-acquired during paired presentations after extinction [27]. Furthermore, Purkinje cell firing reaches its minimum about 50 ms before the expected onset of the US for a range of CS-US intervals and returns to baseline just after the US even if the CS continues (Fig. 2B-D) [28, 34, 35]. Like the behavioral CR, the Purkinje cell CR changes its timing if the interval between the CS and US is changed (Fig. 2E-F) [35]. When the expected delays in the motor pathway to the eyelid are taken into account [36], these results provide strong support for the hypothesis that a properly-timed suppression of Purkinje cell firing is the main determinant of the timing of the CR[26].
Figure 2.
daptive timing of blink CRs and Purkinje cell CRs. (A) Average time courses of conditional blinks (nictitating membrane responses) in rabbits trained with different interstimulus intervals (125 – 1000 ms) adapted from [65]. (B-D) Purkinje cell CRs after conditioning with interstimulus intervals between CS and US onsets (ISI, blue shading) of 150 ms, 300 ms or 500 ms. (E-F) Time course of Purkinje cell CRs in the same cell after training with interstimulus intervals (ISI) of 300 ms (E) and after switching to 500 ms (F). Adapted from [27].
How do Purkinje cells learn to pause at the right time?
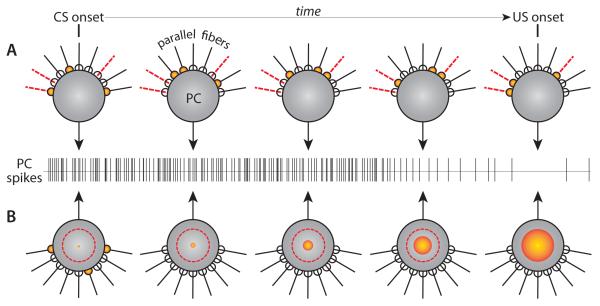
Contemporary theory is dominated by the assumption that during the interstimulus interval, granule cells of the cerebellar cortex have time-varying activity that transmits a temporal code to the post-synaptic Purkinje cell [2, 37, 38]. In these models, a unique and highly reproducible set of granule cells becomes active at each time step after the CS is presented, and it is the evolving granule cell population activity vector that represents the passage of time (Fig. 3A). A number of computational models have been proposed in which these time-varying patterns of granule cell activity during the CS could be driven by delay lines [39, 40], oscillations [41], variability in response kinetics [42] or network interactions [43, 44]. The key in all these models is that because each Purkinje cell receives parallel fiber inputs from many granule cells whose activity is varying in time (Fig. 3A, orange), it can learn to pause at the “right” time by selectively modifying the strength of those parallel fiber synapses that are active around the time of the US (Fig. 3A, dashed red lines),
Figure 3.
Schematic representation summarizing the features of two types of timing models. (A) timing mechanisms based on time-varying patterns of granule cell activity. Active parallel fibers (orange), and parallel fibers active around the time of the US (red dashed lines), are shown at 5 different times during the CS-US interval (B) timing mechanism intrinsic to the Purkinje cell. The onset of the CS activates a subset of parallel fibers (left, orange) that trigger a biochemical cascade (expanding orange sphere) whose time course can be adjusted as a result of learning to reach a target threshold (red dashed circle) at the time of the US.
Because granule cells are very small and densely packed, it has been difficult to record their spike activity in behaving animals and test if the population generates time-varying patterns of activity in response to a CS. Recent work suggests that unipolar brush cells (UBCs), which provide excitatory input to granule cells and other UBCs, can respond with a range of latencies after stimulation [45], raising the possibility that they could be used as delay lines to generate time-varying patterns of activity[39, 40]. However, in vivo experiments have shown that the latency of granule cells to sensory stimulation is very uniform [46, 47].
Another possibility, suggested by a number of computational models [37, 38, 43, 44], is that inhibitory inputs from Golgi cells could be used to sculpt temporal patterns in granule cells. Recently, it has been shown that the main time constant over which the spontaneous firing of Golgi cells has an effect in vivo is on the order of seconds [48]. This time constant could allow Golgi cell activity to set excitability levels and increase the fidelity of granule cells on long time scales [49-51]. However, this scale is probably too long for generating temporal patterns in the 10-100 millisecond range. Sensory-driven activation of Golgi cells also causes a phasic inhibition of granule cells [52] that may be important for performing sparse encoding without loss of information [53], and for modulating the size and reproducibility of sensory responses [54]. Whether this phasic inhibition is able to generate time-varying activity of granule cells in response to sensory stimulation is currently unknown.
Given all these considerations, it is important to ask whether Purkinje cells are capable of achieving motor timing without time-varying patterns of granule cell inputs [55].
Is there a timing mechanism inside the Purkinje cell?
A recent study provides evidence for a radically new view of the mechanisms for motor timing in the cerebellum [56]. Contrary to the predictions of existing models, pairing a CS consisting of direct stimulation of the parallel fibers (Fig. 4A) with a US consisting of direct climbing fiber stimulation led to a Purkinje cell CR that was adaptively timed. That is, the cell reached maximum suppression <75 ms before the onset of the US regardless of CS-US interval (Fig. 4B). This was an unexpected finding because with direct stimulation of parallel fibers, any temporal patterns of granule cell activity are presumably bypassed. Although stimulation-driven antidromic activation of granule cells could generate time-varying patterns of activity, these patterns are likely to be overridden by the regular repetition provided by the train of parallel fiber stimuli. In addition, parallel fiber stimulation will activate inhibitory interneurons of the cerebellar cortex, including Golgi, stellate and basket cells. However, these cells are not necessary for the observed timing because blocking GABA-ergic inhibition had no effect on the Purkinje cell CRs (Fig. 4F-G).
Figure 4.
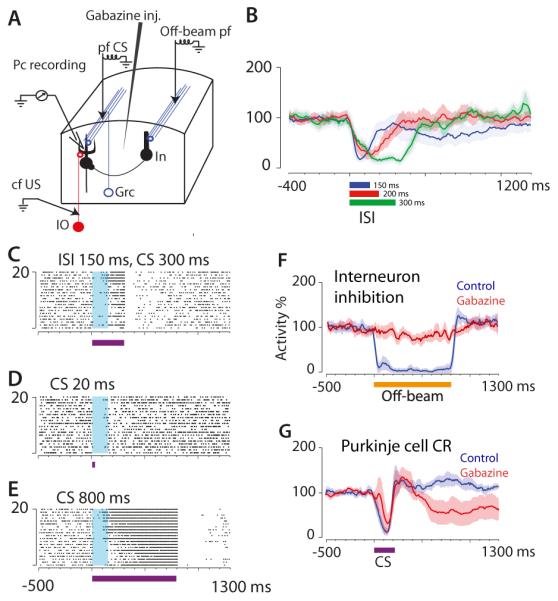
Adaptive timing of Purkinje cell CRs with parallel fiber CS. (A) Experimental setup. CS was electrical stimulation of parallel fibers lying on a beam terminating on the recorded Purkinje cell (pf CS). Off-beam pf stimulation activated inhibitory interneurons (In). (B) Purkinje cell activity after conditioning with three different ISIs, 150, 200 and 300 ms. Curves and shading show mean and SEM (adapted from [56]), C-E) raster plots showing time course of Purkinje cell firing using a pf CS of 20, 300 or 800 ms in a cell previously trained with ISI = 150 ms. (F-G) Effect of GABA antagonist Gabazine on suppression of Purkinje cell firing elicited by off-beam pf stimulation (F), and lack of effect on Purkinje cell CR elicited by the CS (G).
The timing of the Purkinje cell CR exhibited a key property that provides some clues about the underlying mechanisms. After training with a parallel fiber CS of 300 ms in duration, the Purkinje cell CR had the same time course on probe trials, whether the CS lasted for 20 ms or 800 ms [56] (Fig. 4 C-E). These results echo the findings of previous reports indicating that the time course of both the Purkinje cell CR [57] and the behavioral CR [58] is determined by the initial part (<20 ms) of the CS, and therefore is insensitive to any temporally patterned CS input. In addition, both interneuron inhibition [56] (Fig. 3 F-G) and AMPA/kainate receptors in the parallel fiber to Purkinje cell synapses can be blocked without disrupting the Purkinje cell CR [59]. These data strongly suggest that when parallel fiber stimulation is used as the CS, the main mechanism underlying the adaptive timing of the Purkinje cell CR is within the cell itself.
What kind of intrinsic cellular mechanisms might underlie the adaptive timing of the Purkinje cell CR? Fiala and colleagues [60] proposed a spectral timing model in which each Purkinje cell has a fixed number of metabotropic receptors (mGluR1), but this number varies across Purkinje cells. A particular receptor density is assumed to produce a rise in the intracellular Ca2+ concentration with a particular latency, and when the concentration reaches a threshold level, it turns on a hyperpolarizing current through Ca2+-activated K+ channels that results in properly timed pause in Purkinje cell firing. In this spectral model, each individual Purkinje cell is predisposed to learn a particular interstimulus interval that is given by the fixed number of mGluR1 receptors on its membrane, and timing would thus be accomplished by selecting the right set of Purkinje cells. However, this model is inconsistent with previous data showing that any Purkinje cell can learn any interstimulus interval [35, 56] (Fig. 2E-F).
Steuber & Willshaw improved the original spectral model of Purkinje cell timing by suggesting that the number of mGluR1s could be adaptively regulated during learning [61]. With paired CS-US presentations the learning mechanism adjusts the mGluR1 density, and hence the onset latency of the rise in Ca2+, to match the interstimulus interval. However, this model cannot explain double peaked responses observed in single Purkinje cells trained with multiple intervals [28, 35, 56]. This model is also inconsistent with recent findings showing that the mGluR1 can be blocked in vivo without disrupting the Purkinje cell CR [59].
An alternative intrinsic mechanism for cerebellar motor timing is based on the discovery that activation of mGluR7 receptors is necessary for generating the Purkinje cell CR [59]. In this model (Fig. 3B), activation of mGluR7 initiates a biochemical signaling cascade whose latency to on- and offset of the voltage response is adjustable. This time course is under the control of a learning mechanism that selects a subset of molecular components for synthesis or activation [62]. If the onset of the CS initiates a predictable and evolving biochemical process (Fig. 3B, orange sphere), US onset at different time points in this process could signal the induction of interval-specific changes to the cascade from receptor activation to voltage response. In this way, different molecular components with particular properties (e.g. that adapt the duration of ion channel open states) are selected so that the time course of the Purkinje cell CR matches the CS-US interval (Fig. 3B, “PC spikes”). In a sense, this could be seen as a molecular version of a delay-line mechanism. The theoretical difference from published delay-line mechanisms is that distinct lines being “tapped” by the US are not potentiated or depressed. Instead, they prompt synthesis (or activation) of distinct molecules.
Conclusions
During conditioning, the Purkinje cells controlling a particular muscle learn to generate a movement in response to the CS and to adjust the timing so that it reaches its maximum just before the onset of the US. Recent work indicates that to do this, Purkinje cells do not require time-varying patterns of granule cell activity. Instead, individual Purkinje cells appear to be equipped with an intrinsic cellular mechanism that allows them to create a memory of the time between the CS and US in the absence of any temporal code in the parallel fiber input. Whether time-varying patterns of granule cell inputs may also contribute to cerebellar timing, particularly in situations where dynamically modulated sensory and proprioceptive information is readily available [63], remains an open question. It is also likely that circuit-level interactions at the level of the inferior olive could play an important role in adjusting cerebellar-dependent motor timing [37, 64] but a full discussion of this topic is beyond the scope of this review.
The work summarized above provides a plausible account of how the cerebellar cortex learns to emit an adaptively timed movement. We would like to suggest that analogous mechanisms might explain how the Purkinje cells can learn to send signals to the motor and premotor cortex and that such signals can either initiate movements or correct ongoing movement. In this way, the cerebellar cortex can also fine-tune the timing of movements generated by the forebrain.
Highlights.
Timing of conditional eyeblinks determined by pauses in cerebellar Purkinje cells
Blink-controlling Purkinje cells learn to pause during eyeblink conditoning
Conditional Purkinje cell pause responses are adaptively timed
Timing of Purkinje cell pauses determined by intrinsic cellular mechanisms
Conditional pause responses elicited by glutamate via mGluR7 activation
Acknowledgements
This work was supported by a grant to J.F.M. from the US National Institutes of Health (R01 MH093727) and by grants to G.H. from the Swedish Research Council (349-2007-8695 and 09899).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ivry R. Cerebellar Timing Systems. International Review of Neurobiology. 1997;41:555–573. [PubMed] [Google Scholar]
- 2.Mauk MD, Buonomano DV. The neural basis of temporal processing. Annual Review of Neuroscience. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 3.Kehoe EJ, Macrae M. Fundamental Behavioral Methods and Findings in Classical Conditioning. In: Moore JW, editor. A Neuroscientist's Guide to Classical Conditioning. Springer-Verlag; New York: 2002. pp. 171–231. [Google Scholar]
- 4.Gallistel C. The Organization of Learning. Bradford Books/MIT Press; Cambridge, MA: 1990. [Google Scholar]
- 5.Gormezano I, Moore JW. Classical conditioning. In: Marx MH, editor. Learning: Processes. Macmillan; New York: 1969. [Google Scholar]
- 6.Chettih SN, et al. Adaptive timing of motor output in the mouse: the role of movement oscillations in eyelid conditioning. Frontiers in Integrative Neuroscience. 2011;5:1–11. doi: 10.3389/fnint.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Heiney SA, et al. Cerebellar-dependent expression of motor learning during eyeblink conditioning in head-fixed mice. J Neurosci. 2014;34(45):14845–53. doi: 10.1523/JNEUROSCI.2820-14.2014. First to demonstrate that expression and proper timing of a previously conditioned eyeblink response are entirely dependent on an intact cerebellum in mice.
- 8.Hesslow G, Yeo CH. The Functional Anatomy of Skeletal Conditioning. In: Moore JW, editor. A Neuroscientist's Guide to Classical Conditioning. Springer-Verlag; New York: 2002. pp. 86–146. [Google Scholar]
- 9.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning & Memory. 2003;10(6):427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- 10.Freeman JH. Cerebellar learning mechanisms. Brain Research. 2014 doi: 10.1016/j.brainres.2014.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longley M, Yeo CH. Distribution of neural plasticity in cerebellum-dependent motor learning. Prog Brain Res. 2014;210:79–101. doi: 10.1016/B978-0-444-63356-9.00004-2. [DOI] [PubMed] [Google Scholar]
- 12.Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Experimental Brain Research. 1985;60(1):99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]
- 13.Lavond DG, et al. Reacquisition of classical conditioning after removal of cerebellar cortex. Experimental Brain Research. 1987;67(3):569–593. doi: 10.1007/BF00247289. [DOI] [PubMed] [Google Scholar]
- 14.Lavond DG. Role of the nuclei in eyeblink conditioning. Cerebellum: Recent Developments in Cerebellar Research. 2002;978:93–105. doi: 10.1111/j.1749-6632.2002.tb07558.x. [DOI] [PubMed] [Google Scholar]
- 15.Medina JF, et al. Mechanisms of cerebellar learning suggested by eyelid conditioning. Current Opinion in Neurobiology. 2000;10(6):717–724. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 16.Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. Journal of Neuroscience. 1993;13(4):1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia KS, Mauk MD. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology. 1998;37(4-5):471–480. doi: 10.1016/s0028-3908(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 18.Cerminara NL, Rawson JA. Evidence that climbing fibers control an intrinsic spike generator in cerebellar Purkinje cells. Journal of Neuroscience. 2004;24(19):4510–4517. doi: 10.1523/JNEUROSCI.4530-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albus J. A theory of cerebellar function. Math.Biosci. 1971;10:25–61. [Google Scholar]
- 20.Marr D. A theory of cerebellar cortex. Journal of Physiology (London) 1969;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Heiney SA, et al. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J Neurosci. 2014;34(6):2321–30. doi: 10.1523/JNEUROSCI.4547-13.2014. Suppression of Purkinje cell firing is sufficient to control the speed and amplitude of eyelid movements via graded disinhibition of cells in the anterior interpositus nucleus of the cerebellum.
- 22.Berthier NE, Moore JW. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Experimental Brain Research. 1986;63(2):341–350. doi: 10.1007/BF00236851. [DOI] [PubMed] [Google Scholar]
- 23.Green JT, Steinmetz JE. Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learning & Memory. 2005;12(3):260–269. doi: 10.1101/lm.89505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotani S, Kawahara S, Kirino Y. Purkinje cell activity during learning a new timing in classical eyeblink conditioning. Brain Research. 2003;994(2):193–202. doi: 10.1016/j.brainres.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Cerminara NL, et al. Redefining the cerebellar cortex as an assembly of non uniform Purkinje cell microcircuits. Nat Rev Neurosci. 2015;16(2):79–93. doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jirenhed D-A, Hesslow G. Are Purkinje cell pauses drivers of classically conditioned blink responses? Cerebellum. 2015 doi: 10.1007/s12311-015-0722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. Journal of Neuroscience. 2007;27(10):2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Halverson HE, Khilkevich A, Mauk MD. Relating cerebellar purkinje cell activity to the timing and amplitude of conditioned eyelid responses. J Neurosci. 2015;35(20):7813–32. doi: 10.1523/JNEUROSCI.3663-14.2015. Suppression of firing in identified blink-controlling Purkinje cells is correlated with the kinematic properties of the simultaneously recorded conditioned eyelid responses.
- 29.Hesslow G. Inhibition of classically conditioned eyeblink responses by stimulation of the cerebellar cortex in the decerebrate cat. Journal of Physiology (London) 1994;476(2):245–256. doi: 10.1113/jphysiol.1994.sp020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. Journal of Physiology (London) 1994;476(2):229–244. doi: 10.1113/jphysiol.1994.sp020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mostofi A, et al. Electrophysiological Localization of Eyeblink-Related Microzones in Rabbit Cerebellar Cortex. Journal of Neuroscience. 2010;30:8920–8934. doi: 10.1523/JNEUROSCI.6117-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hesslow G, Ivarsson M. Suppression of cerebellar Purkinje cells during conditioned responses in ferrets. Neuroreport. 1994;5(5):649–652. doi: 10.1097/00001756-199401000-00030. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen A, Jirenhed D-A, Hesslow G. Simple and Complex Spike Firing Patterns in Purkinje cells During Classical Conditioning. Cerebelllum. 2008;7:563–566. doi: 10.1007/s12311-008-0068-2. [DOI] [PubMed] [Google Scholar]
- *34.Ten Brinke MM, et al. Evolving Models of Pavlovian Conditioning: Cerebellar Cortical Dynamics in Awake Behaving Mice. Cell Reports. 2015;13:1977–1988. doi: 10.1016/j.celrep.2015.10.057. Suppression of firing in identified blink-controlling Purkinje cells correlates trial by trial to conditioned eyelid behavior.
- 35.Jirenhed DA, Hesslow G. Learning Stimulus Intervals – Adaptive Timing of Conditioned Purkinje Cell Responses. Cerebellum. 2011;10:523–535. doi: 10.1007/s12311-011-0264-3. [DOI] [PubMed] [Google Scholar]
- 36.Lepora NF, et al. Sensory prediction or motor control? Application of marr albus type models of cerebellar function to classical conditioning. Front Comput Neurosci. 2010;4:140. doi: 10.3389/fncom.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medina JF, Mauk MD. Computer simulation of cerebellar information processing. Nature Neuroscience. 2000;3:1205–1211. doi: 10.1038/81486. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki T, Tanaka S. Computational models of timing mechanisms in the cerebellar granular layer. Cerebellum. 2009;8:423–32. doi: 10.1007/s12311-009-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore JW, Choi JS. Conditioned response timing and integration in the cerebellum. Learning and Memory. 1997;4(1):116–129. doi: 10.1101/lm.4.1.116. [DOI] [PubMed] [Google Scholar]
- 40.Moore JW, Desmond JE, Berthier NE. Adaptively timed conditioned responses and the cerebellum: a neural network approach. Biol.Cybern. 1989;62(1):17–28. doi: 10.1007/BF00217657. [DOI] [PubMed] [Google Scholar]
- 41.Gluck M, Reifsnider E, Thompson R. Adaptive signal processing and the cerebellum: models of classical conditioning and VOR adaptation. In: Gluck M, Rumelhart D, editors. Neuroscience and connectionist theory. Erlbaum; Hillsdale, New Jersey: 1990. pp. 131–186. [Google Scholar]
- 42.Bullock D, Fiala J, Grossberg S. A neural model of timed response learning in the cerebellum. Neural Netw. 1994;7:1101–1114. [Google Scholar]
- 43.Buonomano DV, Mauk BD. Neural network model of the cerebellum: temporal discrimination and the timing of motor responses. Neural Computation. 1994;6:38–55. [Google Scholar]
- 44.Medina JF, et al. Timing mechanisms in the cerebellum: testing predictions of a large- scale computer simulation. Journal of Neuroscience. 2000;20(14):5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.van Dorp S, De Zeeuw CI. Variable timing of synaptic transmission in cerebellar unipolar brush cells. Proceedings of the National Academy of Sciences. 2014;111:5403–5408. doi: 10.1073/pnas.1314219111. Unipolar brush cells respond to presynaptic stimulation in cerebellar slices with a range of delays that depend on stimulation frequency.
- 46.Jörntell H, Ekerot CF. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. Journal of Neuroscience. 2006;26(45):11786–11797. doi: 10.1523/JNEUROSCI.2939-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428(6985):856–60. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- 48.Bengtsson F, Geborek P, Jörntell H. Cross-correlations between pairs of neurons in cerebellar cortex in vivo. Neural Netw. 2013;47:88–94. doi: 10.1016/j.neunet.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(Pt 3):753–9. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duguid I, et al. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. Journal of Neuroscience. 2012;32:11132–43. doi: 10.1523/JNEUROSCI.0460-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33(4):625–33. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- 52.Mapellii L, Solinas S, D'Angelo E. Integration and regulation of glomerular inhibition in the cerebellar granular layer circuit. Frontiers in Cellular Neuroscience. 2014 doi: 10.3389/fncel.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Billings G, et al. Network structure within the cerebellar input layer enables lossless sparse encoding. Neuron. 2014;83:960–74. doi: 10.1016/j.neuron.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duguid I, et al. Control of cerebellar granule cell output by sensory-evoked Golgi cell inhibition. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1510249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Hesslow G, et al. Classical conditioning of motor responses: what is the learning mechanism? Neural Networks. 2013;47:81–87. doi: 10.1016/j.neunet.2013.03.013. Argues that long-term depression of ionotropic glutamate receptors cannot be the main mechanism underlying eyeblink conditioning.
- **56.Johansson F, et al. Memory trace and timing mechanism localized to cerebellar Purkinje cells. Proceedings of the National Academy of Sciences. 2014;111:14930–14934. doi: 10.1073/pnas.1415371111. Recordings from the decerebrate ferrets show that conditional Purkinje cell responses show adaptive timing even when the CS is direct stimulation of parallel fibers. Also shows that Purkinje cell CRs do not depend on GABA-ergic interneuron inhibition.
- 57.Jirenhed DA, Hesslow G. Time Course of Classically Conditioned Purkinje Cell Response is Determined by Initial Part of Conditioned Stimulus. Journal of Neuroscience. 2011;31:9070–9074. doi: 10.1523/JNEUROSCI.1653-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Svensson P, Ivarsson M. Short-lasting conditioned stimulus applied to the middle cerebellar peduncle elicits delayed conditioned eye blink responses in the decerebrate ferret. European Journal of Neuroscience. 1999;11(12):4333–4340. doi: 10.1046/j.1460-9568.1999.00862.x. [DOI] [PubMed] [Google Scholar]
- **59.Johansson F, et al. Activation of a temporal memory in Purkinje cells by the mGluR7 receptor. Cell Reports. 2015;13:1741–1746. doi: 10.1016/j.celrep.2015.10.047. Shows that conditional Purkinje cell responses depend on the mGluR7 receptor but not on mGluR1 or AMPA/Kainate receptors.
- 60.Fiala JC, Grossberg S, Bullock D. Metabotropic glutamate receptor activation in cerebellar Purkinje cells as substrate for adaptive timing of the classically conditioned eye-blink response. Journal of Neuroscience. 1996;16(11):3760–3774. doi: 10.1523/JNEUROSCI.16-11-03760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steuber V, Willshaw D. A Biophysical Model of Synaptic Delay Learning and Temporal Pattern Recognition in a Cerebellar Purkinje Cell. Journal of Computational Neuroscience. 2004;17:149–164. doi: 10.1023/B:JCNS.0000037678.26155.b5. [DOI] [PubMed] [Google Scholar]
- 62.Johansson F, Hesslow G. Theoretical Considerations for Understanding a Purkinje cell Timing Mechanism. Communicative & Integrative Biology. 2014;7(6):e994376. doi: 10.4161/19420889.2014.994376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Medina JF, Carey MR, Lisberger SG. The Representation of Time for Motor Learning. Neuron. 2005;45:157–167. doi: 10.1016/j.neuron.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen A, Hesslow G. Feedback control of learning by the cerebello olivary pathway. Prog Brain Res. 2014;210:103–19. doi: 10.1016/B978-0-444-63356-9.00005-4. [DOI] [PubMed] [Google Scholar]
- 65.Smith MC. CS-US interval and US intensity in classical conditioning of the rabbit's nictitating membrane response. Journal of comparative and physiological psychology. 1968;66:679–687. doi: 10.1037/h0026550. [DOI] [PubMed] [Google Scholar]