Abstract
The aim of the present study was to investigate the association between histopathological subtypes, epidermal growth factor receptor (EGFR) mutations and 18F-fluorodeoxyglucose (FDG) uptake in patients with lung adenocarcinoma (ADC). The cases of 97 patients with lung ADC who underwent 18F-FDG positron emission tomography-computed tomography prior to surgical resection were retrospectively reviewed. The patients were stratified according to the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) classification, and graded using a histopathological scoring system. EGFR mutations were identified. Clinicopathological characteristics associated with EGFR mutation status were evaluated using univariate and multivariate analyses. EGFR mutation was identified in 45.4% of the patients and was associated with gender, smoking history, maximum standardized uptake value (SUVmax) and histopathological score. ADC patients with a low SUVmax were more likely to exhibit EGFR mutations compared with patients with a high SUVmax (P=0.018). Patients with a lower histopathological score possessed a significantly lower SUVmax compared with patients with a higher score (P<0.001). Furthermore, the histopathological score and smoking history of the patients were identified to be independent predictors for EGFR mutations, according to multivariate logistic regression analysis. In conclusion, SUVmax and EGFR mutations were associated with lung ADC patients stratified according to the IASLC/ATS/ERS classification. Overall, SUVmax has the potential to be a useful marker in stratifying pre-operative patients with lung ADC and identifying EGFR mutations.
Keywords: epidermal growth factor receptor, histological subtype, adenocarcinoma, maximum standardized uptake value
Introduction
Lung cancer is the leading cause of cancer-associated mortality worldwide, and adenocarcinoma (ADC) is the most common histological type of lung cancer (1). Among males, ADC was the most common type in the United States (31%), Canada (31%), Sweden (30%) and Australia (29%). Among females, ADC made up the greatest proportion of lung cancers, ranging between 38% in the United States and 69% in Japan (2).
Lung ADC is generally heterogeneous, consisting of cells of two or more histological subtypes. In total, 80–90% of surgically resected lung ADCs consist of a mixture of histopathological subtypes (3). In 2011, the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS) and European Respiratory Society (ERS) proposed a novel international multidisciplinary classification system for lung ADC, which classifies patients according to the predominant structural morphology observed in ADC (4). Regarding early-stage invasive adenocarcinomas, the lepidic predominant subtype is associated with better disease free survival (DFS) rates, ranging between 75–85% at 5 years. The acinar and papillary subtypes have intermediate prognosis, with 5-year DFS ranging between 50–70%. The micropapillary and solid predominant subtypes have the poorest prognoses, with 5-year survival rates of 30–40% (5–9). During the past 10 years, targeting the epidermal growth factor receptor (EGFR) pathway has become the mainstream of treatment for advanced lung ADC. The identification of the association between histology and EGFR status becomes clinically relevant when choosing which patients may receive properly targeted therapies. Fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is a metabolic imaging technique that accurately detects the increased trapping of glucose in cancer cells. High FDG uptake may be associated with a poor prognosis of lung cancer (10).
Increasing evidence demonstrates that the histopathological subtype of ADC is closely associated with EGFR mutations and 18F-FDG uptake, which may be identified using PET-CT (11–13). However, to the best of our knowledge, the association between lung ADC histopathological subtypes, EGFR mutations and 18F-FDG uptake remains unclear. Therefore, the present study retrospectively reviewed and reclassified surgically resected lung ADC according to the novel IASLC/ATS/ERS classification, in order to elucidate the association between lung ADC subtype, EGFR mutation status and 18F-FDG uptake. In addition, the association between the EGFR mutations and clinicopathological characteristics of the patients was investigated to determine independent predictors for EGFR mutations.
Patients and methods
Patients
The protocol of the present study was reviewed and approved by the Institutional Review Board of China-Japan Friendship Hospital (Beijing, China). A total of 97 patients with lung ADC who underwent 18F-FDG PET-CT prior to surgical resection between January 2013 and September 2014 were enrolled in the current retrospective study. The resected tumor specimens were pathologically confirmed as primary lung ADC, according to the 2004 World Health Organization (WHO) classification (14). The clinical data of the patients, including age, gender, smoking history, pre-operative serum carcinoembryonic antigen (CEA) level and tumor site, were obtained and are summarized in Table I.
Table I.
Association between the clinicopathological characteristics of 97 patients with lung adenocarcinoma and EGFR mutations in the tumors of these patients.
| Characteristic | Patients, n (%) | Wild-type EGFR | Mutated EGFR | P-value |
|---|---|---|---|---|
| Total, n (%) | 97 (100.0) | 53 (54.6) | 44 (45.4) | |
| Gender, n (%) | 0.020 | |||
| Male | 50 (51.5) | 33 (66.0) | 17 (34.0) | |
| Female | 47 (48.5) | 20 (42.6) | 27 (57.4) | |
| Age, n (%) | 0.461 | |||
| <65 years | 38 (39.2) | 19 (50.0) | 19 (50.0) | |
| ≥65 years | 59 (60.8) | 34 (57.6) | 25 (42.4) | |
| Smoking status, n (%) | 0.001 | |||
| No | 46 (47.4) | 17 (37.0) | 29 (63.0) | |
| Yes | 51 (52.6) | 36 (70.6) | 15 (29.4) | |
| CEA level, n (%) | 0.176 | |||
| <5 ng/ml | 50 (51.5) | 24 (48.0) | 26 (52.0) | |
| ≥5 ng/ml | 47 (48.5) | 29 (61.7) | 18 (38.3) | |
| Tumor site, n (%) | 0.199 | |||
| Left upper lobe | 21 (21.6) | 12 (57.1) | 9 (42.9) | |
| Left lower lobe | 13 (13.4) | 6 (46.2) | 7 (53.8) | |
| Right upper lobe | 34 (35.1) | 23 (67.6) | 11 (32.4) | |
| Right middle lobe | 8 (8.2) | 2 (25.0) | 6 (75.0) | |
| Right lower lobe | 21 (21.6) | 10 (47.6) | 11 (52.4) | |
| TNM stage, n (%) | 0.034 | |||
| I | 58 (59.8) | 32 (55.2) | 26 (44.8) | |
| II | 15 (15.5) | 12 (80.0) | 3 (20.0) | |
| III | 24 (24.7) | 9 (37.5) | 15 (62.5) | |
| T factor, n (%) | 0.270 | |||
| 1 | 36 (37.1) | 16 (44.4) | 20 (55.6) | |
| 2 | 49 (50.5) | 29 (59.2) | 20 (40.8) | |
| 3 | 12 (12.4) | 8 (66.7) | 4 (33.3) | |
| N factor, n (%) | 0.231 | |||
| 0 | 64 (66.0) | 38 (59.4) | 26 (40.6) | |
| 1 | 10 (10.3) | 6 (60.0) | 4 (40.0) | |
| 2 | 23 (23.7) | 9 (39.1) | 14 (60.9) | |
| LVI, n (%) | 0.469 | |||
| Negative | 48 (49.5) | 28 (58.3) | 20 (41.7) | |
| Positive | 49 (50.5) | 25 (51.0) | 24 (49.0) | |
| Histopathological score, n (%) | 0.002 | |||
| 2 | 3 (3.1) | 1 (33.3) | 2 (66.7) | |
| 3 | 37 (38.1) | 13 (35.1) | 24 (64.9) | |
| 4 | 39 (40.2) | 26 (66.7) | 13 (33.3) | |
| 5 | 10 (10.3) | 6 (60.0) | 4 (40.0) | |
| 6 | 8 (8.2) | 7 (87.5) | 0 (0.0) | |
| Tumor size median (IQR), mm | 25.0 (18.0–40.0) | 24.0 (16.5–34.8) | 0.334 | |
| SUVmax median (IQR) | 5.7 (3.6–12.8) | 3.8 (2.5–8.9) | 0.034 |
EGFR, epidermal growth factor receptor; CEA, carcinoembryonic antigen; TNM, tumor-node-metastasis; LVI, lymphovascular invasion; SUVmax, maximum standardized uptake value; IQR, interquartile range.
Integrated 18F-FDG PET-CT
18F-FDG PET-CT was performed using an integrated PET-CT scanner (GE Discovery ST; GE Healthcare Life Sciences, Chalfont, UK). All patients with a pre-scan glucose level of >200 mg/dl fasted for 6 h prior to the PET/CT scan, followed by intravenous administration of 7.4 MBq/kg 18F-FDG (Atom Hi-Tech Co., Ltd., Beijing, China). PET scans between the skull base and mid-thigh levels were performed 1 h after 18F-FDG injection. Concomitant CT data were used for attenuation correction of PET images and anatomical localization of PET abnormalities. All PET-CT images were evaluated and reviewed by two experienced nuclear medicine physicians from the China-Japan Friendship Hospital. The region of interest (ROI) was manually drawn around the primary tumor and the activity concentration of 18F-FDG in the ROI was determined and expressed as the standardized uptake value (SUV). The SUV was adjusted for the injected dose of 18F-FDG and the body weight of the patient using the standard software tools provided with the PET-CT scanner. In order to minimize variation according to the size of the ROIs and assure reproducibility, the maximum SUV (SUVmax) was defined as the peak SUV of the pixel with the highest counts in the sequential transaxial scans through the ROI.
Histopathological evaluation
Hematoxylin and eosin-stained slides for the lung ADC of each patient (mean, 4 slides/patient; range, 1–13 slides/patient) were assessed in a blinded and independent manner for the histopathological subtype of lung ADC by two pathologists from the China-Japan Friendship Hospital. If there was a difference in opinion, the slides were discussed until a consensus was reached. The slides were assessed according to the novel IASLC/ATS/ERS classification (4). The tumors were graded according to a three-tier grading system as follows: Low-grade, including ADC in situ (AIS), minimally invasive ADC (MIA) and the lepidic pattern of invasive ADC; intermediate-grade, including papillary and acinar patterns; and high-grade, including micropapillary, solid patterns and variants of invasive ADC (5,15). Finally, the histopathological scores of the patients were calculated by adding the two most predominant grades observed for each patient (15). Tumors composed of a pure histological subtype were scored by double tumor grading.
The following histopathological characteristics were also investigated: Tumor size, which was defined as the maximum tumor diameter; pathological stage according to 2010 American Joint Committee for Cancer tumor-node-metastasis (TNM) staging manual (16); and lymphovascular invasion (LVI), tumor cells that were observed in the lymphatic and vascular lumen.
Detection of EGFR mutations
EGFR mutations were detected by the amplification refractory mutation system (ARMS), as previously described (17). ARMS analysis was conducted using an AmoyDx® EGFR Mutations Test kit (Amoyx Diagnostics, Co., Ltd., Xiamen, China), which has received China Food and Drug Administration approval for clinical use in mainland China. The EGFR mutations kit covered 29 EGFR mutation hotspots between exons 18 and 21. The assay was performed according to the manufacturer's protocols, with the Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The end result for the analysis was positive or negative, which was determined using the criteria that was defined by the manufacturer's protocol. Briefly, DNA was extracted from 2–20 mg tumor tissue. The DNeasy Tissue Kit (Qiagen, Hilden, Germany) was used to extract DNA according to the manufacturer's protocol. The concentration and purity of DNA were determined by the NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Inc.). DNA extracted from the tumor tissue was standardized to 1 ng/µl. All reactions were performed in 25 µl volumes, including 4.7 µl of template DNA, 0.3 µl of Taq polymerase and 20 µl of reaction buffer mix, and all primers and reagents were included in the AmoyDx® EGFR Mutations Test kit. PCR thermal cycling was as follows: 5 min incubation at 95°C, followed by 15 cycles of 95°C for 25 sec, 64°C for 20 sec and 72°C for 20 sec, and then 31 cycles of 93°C for 25 sec, 60°C for 35 sec and 72°C for 20 sec. Fluorescent signal was collected from the FAM and HEX channels. Data analysis was performed with MxPro version 4.10 (Stratagene, La Jolla, CA, USA). The cycle threshold (Cq) accounts for the threshold at which the signal was detected above background fluorescence. Normal human genomic DNA was used as a control. ΔCq values were calculated as the difference between the mutation Cq and control Cq (18). Positive results were defined as follows: i) Cq is <26; ii) Cq is >26 and ΔCq is lower compared with the cut-off ΔCq value (11 for 19Del and L858R, 7 for T790M). The analysis of each sample was performed in duplicate and the entire test process required 90 min.
Statistical analysis
Continuous variables were examined for normality and skewness. Non-normally distributed data were expressed as the median and interquartile range. The χ2 test and Fisher's exact test were used to compare categorical variables between groups. Mann-Whitney and Kruskal-Wallis tests were used to compare the non-normally distributed variables between groups. Univariate and multivariate logistic regression analyses were performed to investigate the association between clinical characteristics and EGFR mutations. SPSS version 20.0 software (IBM SPSS, Armonk, NY, USA) was used for all statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
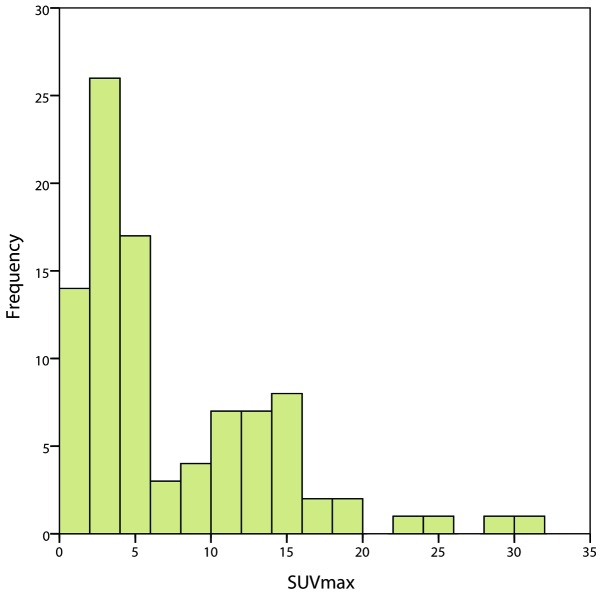
The clinicopathological characteristics of the 97 patients with lung ADC are summarized in Table I. The patient cohort consisted of 50 men (51.5%) and 47 women (48.5%). The median age of the patients was 66 years (range, 34–86 years). The pathological TNM stages of the patients were as follows: Stage I, 58 patients; stage II, 15 patients; and stage III, 24 patients. The range of the SUVmax of the primary tumors was 0.9–31.0, with a median of 4.4 [interquartile range (IQR), 3.1–12.4] (Fig. 1). EGFR mutations were identified in 44 out of the 97 patients (45.4%). The patients identified had mutations in exon 19 in 21 patients (47.7%) and in exon 21 in 23 patients (52.3%).
Figure 1.
Frequency distribution of the SUVmax in 97 patients with lung adenocarcinoma. SUVmax, maximal standardized uptake value.
Lung ADC histopathological subtypes and histopathological scores
The histopathological subtypes of lung ADC, identified according to the IASLC/ATS/ERS classification, were AIS in 2 patients (2.1%), MIA in 3 patients (3.1%), lepidic predominant in 14 patients (14.4%), acinar predominant in 28 patients (28.9%), papillary predominant in 38 patients (39.2%), solid predominant in 9 patients (9.3%), micropapillary predominant in 2 patients (2.1%) and variants of invasive ADC in 1 patient (1.0%). In total, 77 patients (79.4%) were classified as a mixed subtype according to the 2004 WHO classification. The histopathological scores were calculated and are shown in Table I.
Association between EGFR mutations and clinicopathological characteristics
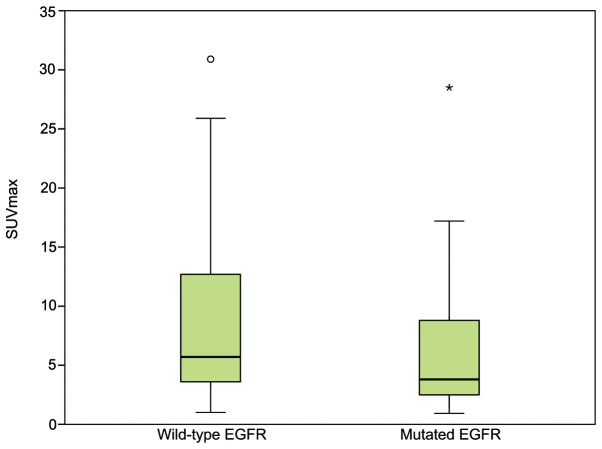
The association between the EGFR mutations and clinicopathological characteristics of the patients were evaluated using univariate analysis (Table I). EGFR mutations were more frequently identified in women compared with men (57.4 vs. 34.0%; P=0.020) and in non-smokers compared with former or current smokers (63.0 vs. 29.4%; P=0.001). Patients with mutant EGFR in their primary tumors possessed a lower histopathological score compared with patients with wild-type EGFR (P=0.002). Patients with mutant EGFR possessed significantly lower SUVmax compared with patients with wild-type EGFR (P=0.034; Fig. 2). The pathological stage of the patients was associated with EGFR mutations (P=0.034). Age and serum CEA level of the patients, tumor location, tumor size, pathological T factor, pathological N factor and LVI demonstrated no significant associations with the frequency of EGFR mutations.
Figure 2.
Distribution of SUVmax in patients with wild-type or mutated EGFR status. Patients with mutated EGFR have a significantly lower SUVmax compared with patients with wild-type EGFR (P=0.018). Open circle represents mild outliers, asterisk represents extreme outliers. SUVmax, maximum standardized uptake value; EGFR, epidermal growth factor receptor.
Variables with P<0.05 in the univariate analysis were investigated with a logistic regression model. In the univariate logistic regression analysis, the gender, smoking history and histopathological score of the patients were significantly associated with the presence of EGFR mutations (P=0.022, P=0.001 and P=0.004, respectively). To eliminate the potential confounding effect, multivariate logistic regression analysis was used to identify independent factors for EGFR mutations. The present results demonstrated that smoking history (OR, 0.287; P=0.030) and histopathological score (OR, 0.508; P=0.027) were independent factors associated with the presence of EGFR mutations (Table II). The present results indicated that gender and SUVmax were confounding factors for EGFR mutations.
Table II.
Logistic regression analysis of clinicopathological characteristics of patients with lung adenocarcinoma as predictors of epidermal growth factor receptor mutations.
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Gender (male vs. female) | 0.382 | 0.168–0.869 | 0.022 | 1.142 | 0.355–3.667 | 0.824 |
| Smoking history (yes vs. no) | 0.244 | 0.104–0.571 | 0.001 | 0.287 | 0.093–0.884 | 0.030 |
| Histopathological score | 0.472 | 0.284–0.785 | 0.004 | 0.508 | 0.278–0.928 | 0.027 |
| SUVmax | 0.943 | 0.881–1.010 | 0.092 | 0.973 | 0.893–1.061 | 0.538 |
| Tumor stage (II and III vs. I) | 1.290 | 0.805–2.067 | 0.290 | 1.552 | 0.864–2.788 | 0.141 |
Histopathological score and SUVmax were treated as continous variables. OR, odds ratio; CI, confidence interval; SUVmax, maximum standardized uptake value.
Comparison of characteristics between patients stratified by histopathological score
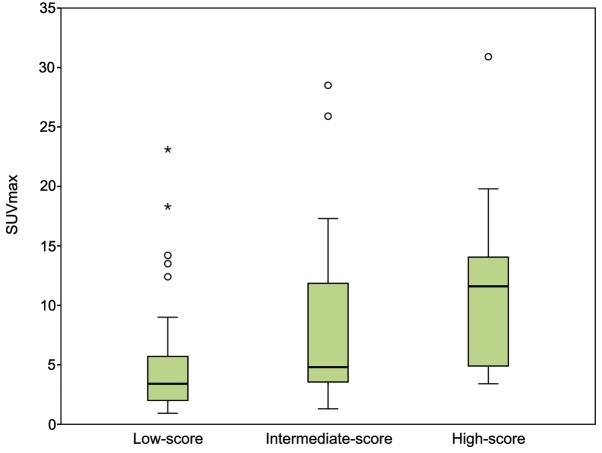
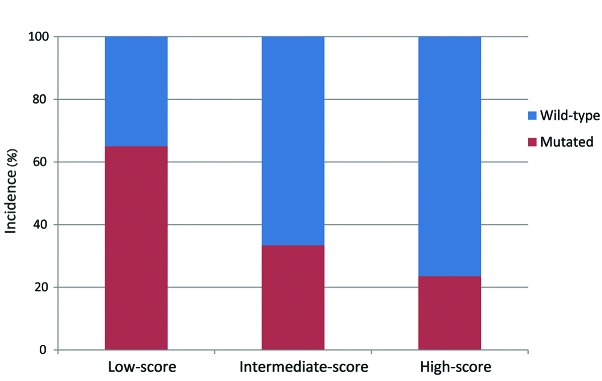
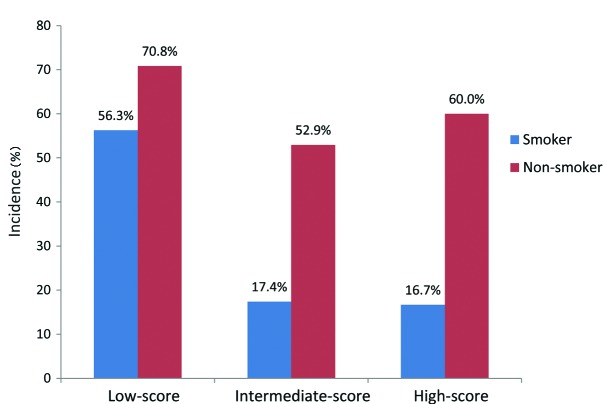
Patients were stratified into the following groups according to their histopathological score: Low-score, 2 or 3 points (40 patients); intermediate-score, 4 points (40 patients); and high-score, 5 or 6 points (17 patients). The association between SUVmax, LVI, EGFR mutation status and the histopathological patient groups are summarized in Table III. The high-score group had the highest SUVmax (median, 11.7; IQR, 4.9–15.1), followed by the intermediate-score (median, 4.8; IQR, 3.5–12.7) and low-score (median, 3.4; IQR, 1.9–5.7) groups (Fig. 3). A high histopathological score was associated with a higher probability of LVI (P=0.008). By contrast, the incidence of EGFR mutations appeared to be significantly decreased when associated with a higher histopathological score (P=0.005; Fig. 4). Furthermore, when the patients were stratified by smoking history and histopathological score, the incidence of EGFR mutations was highest in non-smokers from the low-score group, and the incidence of EGFR mutations was extremely low in smokers of the intermediate- and high-score groups (Fig. 5).
Table III.
Association between 97 patients with lung adenocarcinoma stratified according to histopathological score and the SUVmax, LVI and EGFR mutation status of these patients.
| Hisbottomathological score, n (%) | ||||
|---|---|---|---|---|
| Characteristic | Low (n=40) | Intermediate (n=40) | High (n=17) | P-value |
| SUVmax | 0.005 | |||
| <4.4 | 27 (67.5) | 17 (42.5) | 4 (23.5) | |
| ≥4.4 | 13 (32.5) | 23 (57.5) | 13 (76.5) | |
| LVI | 0.008 | |||
| Negative | 25 (62.5) | 20 (50.0) | 3 (17.6) | |
| Positive | 15 (37.5) | 20 (50.0) | 14 (82.4) | |
| EGFR mutation | 0.005 | |||
| Negative | 14 (35.0) | 27 (67.5) | 12 (70.6) | |
| Positive | 26 (65.0) | 13 (32.5) | 5 (29.4) | |
SUVmax, maximum standardized uptake value; LVI, lymphovascular invasion; EGFR, epidermal growth factor receptor.
Figure 3.
SUVmax in patients with lung adenocarcinoma stratified according to the histopathological score (P<0.001). The high-score group of patients has the highest SUVmax, followed by the intermediate-score group and the low-score group. Open circle represents mild outliers, asterisk represents extreme outliers. SUVmax, maximal standardized uptake value.
Figure 4.
Frequency of mutated and wild-type EGFR in patients with lung adenocarcinoma stratified according to histopathological score (P=0.005). The low-score group has the highest incidence of EGFR mutations, followed by the intermediate- and high-score groups. EGFR, epidermal growth factor receptor.
Figure 5.
Frequency of mutated EGFR in patients with lung adenocarcinoma stratified according to histopathological score and smoking status. The incidence of EGFR mutations was highest in patients who were non-smokers and were of the histopathological low-score group, while the incidence of EGFR mutations was extremely low in smokers of the intermediate- and high-score groups of patients. EGFR, epidermal growth factor receptor.
Discussion
The present study performed an extensive analysis based on several previously described associations in lung ADC between clinicopathological patient characteristics, histopathological subtypes and EGFR mutations (11–13). Histopathological subtypes, diagnosed according to IASLC/ATS/ERS classification, were observed to be associated with SUVmax and EGFR mutations in lung ADC. The tumors of patients with higher histopathological scores were more likely to have a higher SUVmax and wild-type EGFR status. Therefore, the present study hypothesized that the IASLC/ATS/ERS classification reflects not only morphological heterogeneities, but also functional characteristics and genetic alterations. The present findings would be beneficial for determining therapeutic strategies for patients with lung ADC.
Previous studies have revealed that EGFR mutations are predominantly identified in Asian populations, women, individuals that have never smoked and ADC patients (19–21). In the current study, the frequency of EGFR mutations was 45.7%, and a high frequency of these EGFR mutations was associated with women, patients that had never smoked and a lower SUVmax and histopathological score. Furthermore, the present study revealed that the smoking history and histopathological score of the patients were independent factors for the presence of EGFR mutations, following the adjustment for confounding variables using multivariate analysis. Similar results have been reported in a study by Hsiao et al (22), which found that gender was a confounding factor, whereas the smoking history and histological subtype of patients were independently and significantly associated with EGFR mutations in non-small cell lung cancer.
In addition, the present study demonstrated that there was an association between EGFR mutations and the histopathological score of the patients. EGFR mutations occurred more frequently in patients with lower histopathological scores. Several studies have investigated the association between EGFR mutations and the IASLC/ATS/ERS classification; however, the results remain inconsistent, as summarized in Table IV (12,13,23–31). The variations between EGFR mutations and histological subtypes in these previous results may be due to the cohort size, molecular analytical methods employed and ethnicity of the study cohort. Notably, smoking history, which the present study demonstrated is an independent predictor for the presence of EGFR mutations, may possibly contribute to inconsistent results. The dose of tobacco smoke exposure is hypothesized to be inversely associated with the rate of EGFR mutations (32,33). In addition, the majority of ADCs have various combinations of two or more histological subtypes. Therefore, evaluating the association between EGFR mutations and histology according to the most predominant histological subtype may not be accurate.
Table IV.
Previous studies of correlation between EGFR mutation and predominant subtype.
| First author, year | Patients, n | Freq. EGFR mutations, % | Freq. distribution EGFR mutations in various predominant tumor subtypes, % | Ref. |
|---|---|---|---|---|
| Shim et al, 2011 | 107 | 50.5 | Micropapillary (83.3) > lepidic (62.5) > acinar (50.0) = papillary (50.0) > solid (28.6) | (23) |
| Sun et al, 2012 | 382 | 51.3 | Papillary (70.0) > acinar (56.6) > micropapillary (50.0) > lepidic (42.9) > solid (38.9) | (24) |
| Zhang et al, 2012 | 349 | 76.2 | AIS (100.0) = MIA (100.0) > micropapillary (83.3) > acinar (83.1) > lepidic (82.4) > papillary (72.2) > solid (60.9) > invasive mucinous adenocarcinoma (28.6) | (25) |
| Russell et al, 2013 | 59 | 29.0 | Acinar (44.0) > micropapillary (38.0) > solid (5.0) > papillary (0.0) | (26) |
| Song et al, 2013 | 161 | 41.6 | Micropapillary (73.9) > lepidic > (72.2) > acinar (42.1) > papillary (40.5) > MIA (16.7) = solid (16.7) > variants of invasive adenocarcinoma (0.0) | (27) |
| Yoshizawa et al, 2013 | 167 | 53.9 | AIS (85.7) > MIA (83.3) > lepidic (71.4) > papillary (68.5) > micropapillary (40.1%) > acinar (38.4%) > solid (14.3 %) > invasive mucinous adenocarcinoma (0.0) | (12) |
| Tsuta et al, 2013 | 880 | 40.5 | Papillary (56.0) > lepidic (44.6) > micropapillary (39.7) >AIS + MIA (39.0) > acinar (32.3)> solid (15.8) > invasive mucinous predominant (0.0) | (28) |
| Yanagawa et al, 2014 | 131 | 54.4 | Lepidic (77.0) > AIS (62.0) > MIA (60.0) > papillary (50.0) > acinar (49.0) > micropapillary (43.0) > solid (28.0) > invasive mucinous adenocarcinoma (0.0) | (13) |
| Villa et al, 2014 | 200 | 20.5 | Lepidic (44.0) > acinar (42.0) > solid (10.0) > papillary (5.0) > micropapillary (0.0) | (29) |
| Sun et al, 2014 | 102 | 38.2 | Micropapillary (70.6) > lepidic (50.0) > papillary (33.3) > acinar (30.0) > solid (8.3) > variants of invasive adenocarcinoma (0.0) | (30) |
| Kim et al, 2014 | 135 | 37.6 | Papillary (81.3)> lepidic (70.4)> acinar (58.1) > solid (28.3) | (31) |
EGFR, epidermal growth factor receptor; freq., frequency; ref., reference; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma.
Despite the controversial results, ADCs with lower-grade predominant subtypes generally have a higher frequency of EGFR mutations (23–31). The mechanism for the association between EGFR mutations and histological subtypes remains unclear. It has been hypothesized that the origin of the majority of lung ADCs are type 2 pneumocytes, Clara cells and precursor cells, which are common in the terminal respiratory unit. The activation of the EGFR signaling pathway, including the activation of downstream mediators, is an early and essential event for ADC oncogenesis (34,35); however, the EGFR pathway plays a decreased role in developed lung cancer. Additionally, Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations, which are associated with a solid growth pattern, usually occur in a mutually-exclusive manner with EGFR mutations. Therefore, EGFR mutations are less frequent in the solid predominant subtype of lung ADC (36,37).
The present study revealed that there is an association between the SUVmax and the histopathological score of patients. Lung ADC with a lepidic subtype is well known to possess a lower SUVmax compared with lung ADC without a lepidic subtype (38). Chiu et al (11) demonstrated that the SUVmax was significantly higher in solid predominant ADC compared with ADC with other predominant histology. In addition, Kadota et al (39) observed that tumors with high-grade histology exhibited the highest SUVmax [mean ± standard deviation (SD), 6.2±2.8], followed by those with intermediate-grade (3.7±2.5) and low-grade (2.5±1.6) histology. 18F-FDG uptake reflects the proliferation and aggressiveness of lung cancer cells, which may explain why the SUVmax varies dramatically depending on histological components (40). AIS, MIA and lepidic growth patterns of ADC, which often present with ground-glass opacity lesions, have a much longer tumor volume doubling time compared with semi-solid and solid lesions, and the SUVmax is associated with the tumor volume doubling time and proliferation rate (41–43). Consequently, the SUVmax is lower in tumors that possess a lower histopathological score.
Since the SUVmax and EGFR mutations are closely associated with the histopathological score, the SUVmax is more likely to be associated with EGFR mutations. Usuda et al (44) reported that tumors with a mutant EGFR status possessed a significantly lower SUVmax (mean ± SD, 3.66±4.53) compared with tumors with a wild-type EGFR status (8.26±6.11; P<0.0001). In addition, Na et al (45) demonstrated that patients with a low SUVmax were more likely to possess EGFR mutations compared with patients with a high SUVmax (low vs. high SUVmax, 40 vs. 11%; P=0.001). Mak et al (46) also reported that a high SUVmax was associated with wild-type EGFR. In the present study, tumors with mutant EGFR exhibited a significantly lower SUVmax, which is consistent with previous studies. In terms of the molecular mechanism underlying the association between SUVmax and EGFR mutation, the SUVmax is significantly associated with the expression of glucose transporter-1 (GLUT-1) in lung cancer, and overexpressed GLUT-1 is indicative of the increasing proliferative activity, energy requirements and aggressive behavior of cells (47–49). Yun et al (50) observed that an increase in GLUT-1 expression and glucose uptake was critically dependent on KRAS mutations in colorectal cancer cells, and Sasaki et al (51) identified that GLUT-1 overexpression was significantly associated with the gene mutation status of tumors, including EGFR (mutant vs. wild-type, 23.4 vs. 58.3%; P<0.0001) and KRAS (mutant vs. wild-type, 66.7 vs. 46.6%; P=0.038). These results support the fact that the SUVmax is decreased in tumors that possess mutant EGFR compared with tumors with wild-type EGFR.
The present results have potential practical implications. Firstly, although the IASLC/ATS/ERS classification and EGFR mutation status are important factors for determining therapeutic strategies for patients, they are occasionally not feasible due to the inoperability of the patients, insufficient tissue materials available or the high cost of the molecular examination. Since pre-operative SUVmax is possibly a predictor for the aggressiveness and EGFR mutation status of ADC, the present study identified patients who were at a high-risk of post-operative tumor relapse and patients who were sensitive to EGFR-targeted therapy without examining a surgical tumor specimen. Patients with a high SUVmax should be considered for intensive treatment in order to reduce recurrence and improve survival outcomes, including anatomical pulmonary resection instead of limited resection, and may not benefit from EGFR-targeted therapy. Secondly, the smoking history combined with the histopathological score of patients provides a potential predictor for EGFR mutations, which would be useful if molecular examination is not available.
The present study concludes that the smoking history and histopathological score of patients with lung ADC, based on the two most predominant subtypes of lung ADC, are independent factors associated with the EGFR mutation status. Lung ADCs of various histopathological subtypes vary in terms of metabolic activity and EGFR mutations. The present results support the fact that the histopathological score is a powerful tool for stratifying patients with lung ADC according to the biological aggressiveness and molecular alteration of tumors. However, as a retrospective and single-center study, there was inevitably patient selection bias, and there was a relatively small number of patients with a histopathological score of 2 or 6. Additional in-depth analyses may benefit the validation of the association between these radiographical, histopathological and molecular characteristics, which may provide a promising strategy for patient care.
Acknowledgements
The present study was supported by grants from the National Key Clinical Specialty Construction Program of China (grant no. 2011873).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: Geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 3.Kerr KM. Pulmonary adenocarcinomas: Classification and reporting. Histopathology. 2009;54:12–27. doi: 10.1111/j.1365-2559.2008.03176.x. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, Park BJ, Rusch VW, Travis WD. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: Prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 6.Zugazagoitia J, Enguita AB, Nuñez JA, Iglesias L, Ponce S. The new IASLC/ATS/ERS lung adenocarcinoma classification from a clinical perspective: Current concepts and future prospects. J Thorac Dis. 2014;6(Suppl 5):S526–S536. doi: 10.3978/j.issn.2072-1439.2014.01.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung JJ, Jeng WJ, Chou TY, Hsu WH, Wu KJ, Huang BS, Wu YC. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg. 2013;258:1079–1086. doi: 10.1097/SLA.0b013e31828920c0. [DOI] [PubMed] [Google Scholar]
- 8.Warth A, Muley T, Meister M, Stenzinger A, Thomas M, Schirmacher P, Schnabel PA, Budczies J, Hoffmann H, Weichert W. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–1446. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 9.Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Prognostic value of the IASLC/ATS/ERS classification in stage I lung adenocarcinoma patients - based on a hospital study in China. Eur J Surg Oncol. 2013;39:1262–1268. doi: 10.1016/j.ejso.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Paesmans M, Berghmans T, Dusart M, et al. European Lung Cancer Working Party, and on behalf of the IASLC Lung Cancer Staging Project: Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: Update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol. 2010;5:612–619. doi: 10.1097/JTO.0b013e3181d0a4f5. [DOI] [PubMed] [Google Scholar]
- 11.Chiu CH, Yeh YC, Lin KH, Wu YC, Lee YC, Chou TY, Tsai CM. Histological subtypes of lung adenocarcinoma have differential 18F-fluorodeoxyglucose uptakes on the positron emission tomography/computed tomography scan. J Thorac Oncol. 2011;6:1697–1703. doi: 10.1097/JTO.0b013e318226b677. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: Analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 13.Yanagawa N, Shiono S, Abiko M, et al. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg. 2014;98:453–458. doi: 10.1016/j.athoracsur.2014.04.108. [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. World Health Organization Classification of Tumours. [Google Scholar]
- 15.Sica G, Yoshizawa A, Sima CS, Azzoli CG, Downey RJ, Rusch VW, Travis WD, Moreira AL. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34:1155–1162. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 16.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th. New York, NY: Springer-Verlag; 2010. pp. 253–270. [Google Scholar]
- 17.Shaozhang Z, Ming Z, Haiyan P, Aiping Z, Qitao Y, Xiangqun S. Comparison of ARMS and direct sequencing for detection of EGFR mutation and prediction of EGFR-TKI efficacy between surgery and biopsy tumor tissues in NSCLC patients. Med Oncol. 2014;31:926. doi: 10.1007/s12032-014-0926-3. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, et al. Spanish Lung Cancer Group: Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 20.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 21.Toyooka S, Matsuo K, Shigematsu H, Kosaka T, Tokumo M, Yatabe Y, Ichihara S, Inukai M, Suehisa H, Soh J, et al. The impact of sex and smoking status on the mutational spectrum of epidermal growth factor receptor gene in non small cell lung cancer. Clin Cancer Res. 2007;13:5763–5768. doi: 10.1158/1078-0432.CCR-07-0216. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao SH, Lin SE, Chou YT, Wang JL, Chung CL, Yu MC, Fang CL, Lee HL, Chiang LL, Liu HE, Wu CW. Histological subtype and smoking status, but not gender, are associated with epidermal growth factor receptor mutations in non-small-cell lung cancer. Mol Clin Oncol. 2014;2:252–258. doi: 10.3892/mco.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shim HS, Lee H, Park EJ, Kim SH. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med. 2011;135:1329–1334. doi: 10.5858/arpa.2010-0493-OA. [DOI] [PubMed] [Google Scholar]
- 24.Sun PL, Seol H, Lee HJ, Yoo SB, Kim H, Xu X, Jheon S, Lee CT, Lee JS, Chung JH. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: Correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol. 2012;7:323–330. doi: 10.1097/JTO.0b013e3182381515. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res. 2012;18:1947–1953. doi: 10.1158/1078-0432.CCR-11-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell PA, Barnett SA, Walkiewicz M, Wainer Z, Conron M, Wright GM, Gooi J, Knight S, Wynne R, Liew D, John T. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol. 2013;8:461–468. doi: 10.1097/JTO.0b013e3182828fb8. [DOI] [PubMed] [Google Scholar]
- 27.Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol. 2013;30:645. doi: 10.1007/s12032-013-0645-1. [DOI] [PubMed] [Google Scholar]
- 28.Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer. 2013;81:371–376. doi: 10.1016/j.lungcan.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Villa C, Cagle PT, Johnson M, et al. Correlation of EGFR mutation status with predominant histologic subtype of adenocarcinoma according to the new lung adenocarcinoma classification of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society. Arch Pathol Lab Med. 2014;138:1353–1357. doi: 10.5858/arpa.2013-0376-OA. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Yu X, Shi X, Hong W, Zhao J, Shi L. Correlation of survival and EGFR mutation with predominant histologic subtype according to the new lung adenocarcinoma classification in stage IB patients. World J Surg Oncol. 2014;12:148. doi: 10.1186/1477-7819-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Choi EY, Jin HJ, Shin KC. Relationship between EGFR mutations and clinicopathological features of lung adenocarcinomas diagnosed via small biopsies. Anticancer Res. 2014;34:3189–3195. [PubMed] [Google Scholar]
- 32.Tokumo M, Toyooka S, Kiura K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res. 2005;11:1167–1173. [PubMed] [Google Scholar]
- 33.Pham D, Kris MG, Riely GJ, et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol. 2006;24:1700–1704. doi: 10.1200/JCO.2005.04.3224. [DOI] [PubMed] [Google Scholar]
- 34.Otto WR. Lung epithelial stem cells. J Pathol. 2002;197:527–535. doi: 10.1002/path.1160. [DOI] [PubMed] [Google Scholar]
- 35.Okudela K, Suzuki M, Kageyama S, et al. PIK3CA mutation and amplification in human lung cancer. Pathol Int. 2007;57:664–671. doi: 10.1111/j.1440-1827.2007.02155.x. [DOI] [PubMed] [Google Scholar]
- 36.Rekhtman N, Ang DC, Riely GJ, Ladanyi M, Moreira AL. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol. 2013;26:1307–1319. doi: 10.1038/modpathol.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki M, Shigematsu H, Iizasa T, et al. Exclusive mutation in epidermal growth factor receptor gene, HER-2, and KRAS, and synchronous methylation of nonsmall cell lung cancer. Cancer. 2006;106:2200–2207. doi: 10.1002/cncr.21853. [DOI] [PubMed] [Google Scholar]
- 38.Vesselle H, Salskov A, Turcotte E, Wiens L, Schmidt R, Jordan CD, Vallières E, Wood DE. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 2008;3:971–978. doi: 10.1097/JTO.0b013e31818307a7. [DOI] [PubMed] [Google Scholar]
- 39.Kadota K, Colovos C, Suzuki K, Rizk NP, Dunphy MP, Zabor EC, Sima CS, Yoshizawa A, Travis WD, Rusch VW, Adusumilli PS. FDG-PET SUVmax combined with IASLC/ATS/ERS histologic classification improves the prognostic stratification of patients with stage I lung adenocarcinoma. Ann Surg Oncol. 2012;19:3598–3605. doi: 10.1245/s10434-012-2414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higashi K, Ueda Y, Yagishita M, Arisaka Y, Sakurai A, Oguchi M, Seki H, Nambu Y, Tonami H, Yamamoto I. FDG PET measurement of the proliferative potential of non-small cell lung cancer. J Nucl Med. 2000;41:85–92. [PubMed] [Google Scholar]
- 41.Hasegawa M, Sone S, Takashima S, Li F, Yang ZG, Maruyama Y, Watanabe T. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73:1252–1259. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 42.de Geus-Oei LF, van Krieken JH, Aliredjo RP, Krabbe PF, Frielink C, Verhagen AF, Boerman OC, Oyen WJ. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer. 2007;55:79–87. doi: 10.1016/j.lungcan.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Li M, Sun Y, Liu Y, Han A, Zhao S, Ma L, Zheng J, Yu J. Relationship between primary lesion FDG uptake and clinical stage at PET-CT for non-small cell lung cancer patients: An observation. Lung Cancer. 2010;68:394–397. doi: 10.1016/j.lungcan.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, Matoba M, Taniguchi M, Tonami H, Ueda Y, Sakuma T. Relationships between EGFR mutation status of lung cancer and preoperative factors - are they predictive? Asian Pac J Cancer Prev. 2014;15:657–662. doi: 10.7314/APJCP.2014.15.2.657. [DOI] [PubMed] [Google Scholar]
- 45.Na II, Byun BH, Kim KM, Cheon GJ, Choe H, Koh JS, Lee DY, Ryoo BY, Baek H, Lim SM, et al. 18F-FDG uptake and EGFR mutations in patients with non-small cell lung cancer: A single-institution retrospective analysis. Lung Cancer. 2010;67:76–80. doi: 10.1016/j.lungcan.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Mak RH, Digumarthy SR, Muzikansky A, Engelman JA, Shepard JA, Choi NC, Sequist LV. Role of 18F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist. 2011;16:319–326. doi: 10.1634/theoncologist.2010-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Usuda K, Sagawa M, Aikawa H, Ueno M, Tanaka M, Machida Y, Zhao XT, Ueda Y, Higashi K, Sakuma T. Correlation between glucose transporter-1 expression and 18F-fluoro-2-deoxyglucose uptake on positron emission tomography in lung cancer. Gen Thorac Cardiovasc Surg. 2010;58:405–410. doi: 10.1007/s11748-010-0603-1. [DOI] [PubMed] [Google Scholar]
- 48.Mamede M, Higashi T, Kitaichi M, Ishizu K, Ishimori T, Nakamoto Y, Yanagihara K, Li M, Tanaka F, Wada H, et al. [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia. 2005;7:369–379. doi: 10.1593/neo.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen XC, Lee WW, Chung JH, et al. FDG uptake, glucose transporter type 1, and Ki-67 expressions in non-small-cell lung cancer: Correlations and prognostic values. Eur J Radiol. 2007;62:214–219. doi: 10.1016/j.ejrad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Yun J, Rago C, Cheong I, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sasaki H, Shitara M, Yokota K, Hikosaka Y, Moriyama S, Yano M, Fujii Y. Overexpression of GLUT1 correlates with Kras mutations in lung carcinomas. Mol Med Rep. 2012;5:599–602. doi: 10.3892/mmr.2011.736. [DOI] [PubMed] [Google Scholar]