Abstract
Naphthalene (NA) is a ubiquitous pollutant to which humans are widely exposed. 1,2-Dihydro-1,2-dihydroxynaphthalene (NA-dihydrodiol) is a major metabolite of NA generated by microsomal epoxide hydrolase (mEH). To investigate the role of the NA-dihydrodiol and subsequent metabolites (ie 1,2-naphthoquinone) in cytotoxicity, we exposed both male and female wild type (WT) and mEH null mice (KO) to NA by inhalation (5, 10, 20 ppm for 4 hours). NA-dihydrodiol was ablated in the KO mice. High-resolution histopathology was used to study site-specific cytotoxicity, and formation of naphthalene metabolites was measured by HPLC in microdissected airways. Swollen and vacuolated airway epithelial cells were observed in the intra- and extrapulmonary airways of all mice at and below the current OSHA standard (10 ppm). Female mice may be more susceptible to this acute cytotoxicity. In the extrapulmonary airways, WT mice were more susceptible to damage than KO mice, indicating that the metabolites associated with mEH-mediated metabolism could be partially responsible for cytotoxicity at this site. The level of cytotoxicity in the mEH KO mice at all airway levels suggests that non-mEH metabolites are contributing to NA cellular damage in the lung. Our results indicate that the apparent contribution of mEH-dependent metabolites to toxicity differs by location in the lung. These studies suggest that metabolites generated through the mEH pathway may be of minor importance in distal airway toxicity and subsequent carcinogenesis from NA exposure.
Keywords: cytochrome P450, cell injury/cell death, carcinogen metabolism, microsomal epoxide hydrolase, HPLC, lung/pulmonary/olfactory, toxicology
INTRODUCTION
Naphthalene (NA) is a widespread pollutant that is currently classified as a possible human carcinogen, though this classification is under review (IARC, 2002; USEPA, 1998). The results of the NTP (National Toxicology Program) chronic rodent bioassays for NA found significant, dose-dependent increases in alveolar adenomas in the lungs of female mice, and in adenomas and neuroblastomas of the nasal epithelium in rats (Abdo et al. 1992; 2001). Primary sources of NA include biomass burning (Kakareka and Kukharchyk, 2003), automobile emissions (USEPA, 1986) and tobacco smoke (Schmeltz et al., 1976; Witschi et al., 1997); however, NA has also been found in food (Kobayashi et al., 2008) and ground water. In the National Human Adipose Tissue Survey, 40% of the study population had detectable levels of NA in adipose tissue (Stanley, 1986). Another study found that 75% of a lactating female population had measurable levels of NA in milk (Pellizzari et al., 1982).
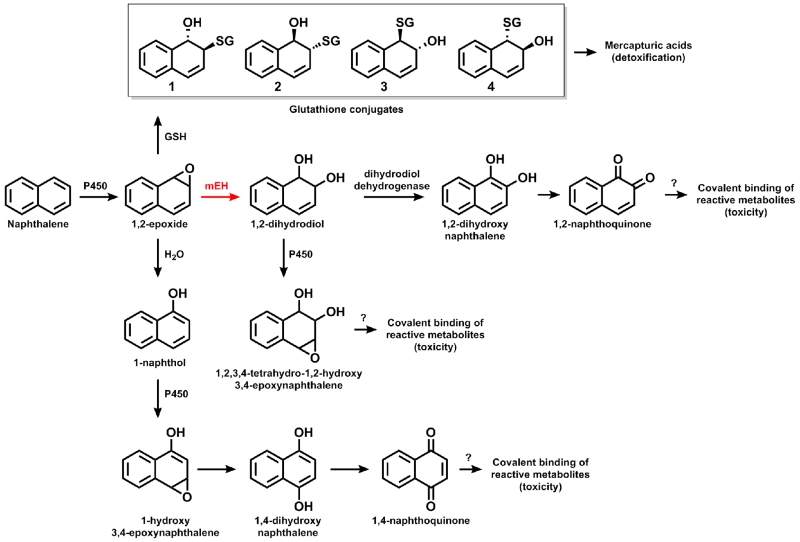
The toxicity of NA is cytochrome P450 dependent, with reactive metabolites produced by enzymes in both the lung and the liver (Buckpitt and Warren, 1983). In the lung, nonciliated bronchiolar Club (formerly known as Clara) cells express the highest amounts of P450 (Plopper et al., 1987) and are severely affected by NA toxicity (Buckpitt et al., 1995). Recurrent cycles of cytotoxicity and proliferation are thought to be the driving force behind formation of mouse lung tumors (National Toxicology Program, 1992; West et al., 2001) and rat nasal tumors (Long et al., 2003; 2000) following chronic exposure to NA because tumors form in respiratory tissues that exhibit high acute toxicity. However, it is unknown which metabolite(s) drive(s) this toxicity. A summary of NA metabolism to potentially toxic metabolites is diagrammed in Fig. 1.
Fig. 1. Naphthalene metabolism to plausible toxic metabolites.
Quantifiable metabolites are the GSH conjugates, 1,2 NA-dihydrodiol, and 1-naphthol. GSH conjugates are usually associated with detoxification while the mEH pathway is associated with toxicity.
In mice, the bioactivation of NA by P450 enzymes (CYP2A5 and CYP2F2) generates a 1,2-epoxide (Li et al., 2011). Glutathione (GSH) conjugates can form from the epoxide, and are eliminated in urine as mercapturic acids (Pakenham et al., 2002). NA 1,2-epoxide can also be metabolized by microsomal epoxide hydrolase (mEH) to NA-dihydrodiol (Kitteringham et al., 1996). When GSH is depleted, it is possible that the mEH pathway may be favored. Previous studies demonstrated that severe GSH depletion increases NA toxicity in the respiratory tract (Phimister et al., 2004; Plopper et al., 2001). It has been speculated that this increased toxicity and the resulting tumorigenesis of NA is mediated by the formation of a 1,2-naphthoquinone (NQ) through the mEH pathway. In fact, Bogen et al. suggests that the most recent EPA evaluation of NA hinged on the interpretation of bioassays that suggested that 1,2- NQ is genotoxic (Bogen et al., 2008). However, the toxicity could also result from increased levels of NA epoxide. 1,2-NA epoxide can form adducts with protein (Waidyanatha et al., 2002). In animal models, the extent to which specific metabolic pathways (to epoxides, quinones, and diols) contribute to cytotoxicity is unknown. Studies indicating the potential genotoxicity of NQs are either in vitro (Flowers-Geary et al., 1996; National Toxicology Program, 1992) or have not evaluated critical tumor sites, such as the lung. Recent studies (Saeed et al. 2007; Saeed et al. 2009) demonstrated the formation of both depurinated and stable NA DNA adducts in vitro and in skin painting studies but the relevance of the painting studies is uncertain because skin is not a known target tissue for NA cytotoxicity. NA metabolite-derived DNA adducts have not been demonstrated in the respiratory system of any species yet. There is a current need for information relating specific in vivo NA metabolism pathways to NA toxicity so that risk from exposure can be understood.
In the current study, we investigated the role of mEH-mediated metabolites (ie 1,2-NQ) in cytotoxicity. To do this, we used mEH knockout (KO) mice (Miyata et al., 1999). These mice have been used previously to investigate the role of mEH in benzene-induced toxicity (Bauer et al., 2003) and 7, 12-dimethylbenz[a]anthracene-induced tumorigenicity (Miyata et al., 1999). The aims of this study were to determine (1) if mEH metabolites are of importance in the overall toxicity of NA at known tumor sites, (2) if cytotoxicity of NA is dose-dependent in mEH KO mice, and (3) if NA toxicity differs between intra- and extrapulmonary airways in mEH KO mice. To explore these questions, we exposed both male and female wild type (WT) and mEH null mice (KO) to NA by inhalation (5, 10, 20 ppm; 4 hrs). These data will inform future studies of NA risk assessment.
METHODS
Animals
For these experiments, adult (8-10 week old) male and female B6:129 wild type (WT) and mEH1 null mice (KO) were obtained from Jackson labs and re-derived and maintained by the mouse biology program at UC Davis from a breeding stock initially created by Frank J. Gonzales at the National Institute of Health (Miyata et al., 1999). All mice were maintained in a barrier facility, housed in a high efficiency particle air (HEPA)-filtered cage rack in AAALAC approved facilities on a 12-hour light/dark cycle with food and water ad libitum. All animal experiments were performed under protocols approved by the University of California Davis IACUC in accordance with National Institutes of Health guidelines.
Ex vivo metabolite formation in airways
Lungs from WT and KO naïve male (nWT =5, nKO = 6) and female (nWT =5, nKO = 5) mice were cannulated, removed en bloc, inflated with agarose and microdissected (Plopper et al., 1991). Trachea and lobar bronchi were separated and defined as extrapulmonary airways. Airways microdissected from the lobes of the lung were defined as intrapulmonary airways. Extra- and intrapulmonary airways were then placed in 1 ml Waymouth’s medium on ice with 5-10 CDNB units glutathione transferase (GST), 5 mM GSH, 250 μM NA dissolved in 5 μl HPLC grade methanol. Reaction vessels were capped tightly and transferred to a shaking water bath at 37° C for 120 min. Following the incubation, samples were transferred to an ice bath and 1 ml ice-cold HPLC grade methanol was added to quench the reaction. Samples were homogenized and then centrifuged at 16000 × G for 10 min to remove precipitated proteins. The supernatant was transferred to a clean tube and evaporated under vacuum to complete dryness. Dried samples were stored at −80°C until HPLC analysis. The protein pellet was dissolved in 1 N NaOH and an aliquot was removed for determination of protein, using a bovine serum albumin standard for protein determinations (Lowry et al., 1951).
HPLC separation of NA metabolites
Samples were reconstituted in 100 μl water and centrifuged to remove particulates. NA-dihydrodiol and glutathione conjugates of 1,2-epoxide were separated on a Phase Sep C18 ODS2 column (250 × 4.6 mm i.d.; 5-μm particle) (Waters, Milford, MA). The eluates were monitored by UV absorbance at 260 nm as described previously (Buckpitt et al., 1987). Metabolites, prepared synthetically, were used to generate standard curves.
High-resolution light microscopy
Male and female mice (n=3) were exposed to filtered air +/− NA vapor at 0, 5, 10 or 20 ppm for 4 hours as described previously (West et al., 2001). All animals were necropsied 24 hrs after the whole body exposure. At necropsy, animals were euthanized with an overdose of pentobarbital and the trachea was cannulated. The lungs were removed en bloc and fixed via tracheal cannula at 30 cm of pressure with Karnovsky’s fixative (0.9% glutaraldehyde/0.7% paraformaldehyde in cacodylate buffer, adjusted to pH 7.4, 330 mOsmol/kgH2O) for 1 hr. Lungs were stored in fixative until use. Karnovsky’s fixed tissue samples were embedded separately in Araldite 502 epoxy resin. Specimens were sectioned at 1 μm (Leica Ultracut UCT ultramicrotome with glass knives) and stained with Methylene Blue/Azure II. High magnification images were captured using a 20× objective lens using an Olympus BH-2 microscope in bright field mode.
Tissues were evaluated using a damage score matrix without knowledge of exposure groups. Terminal bronchioles were scored for intrapulmonary toxicity, and trachea was scored for extrapulmonary toxicity. Extrapulmonary airway damage scores were as follows: 0 – no damage, 1 – few swollen/vacuolated cells, 2 – swollen cells and cells with vacuoles throughout cytoplasm, 3 – all cells have vacuoles throughout cytoplasm. Intrapulmonary scores were: 0 – no damage, 1 – damage only in the proximal airway, 2 - damage in proximal airway and a few swollen cells in terminal bronchioles, 3 – damage in proximal airway and many swollen/vacuolated cells in terminal bronchioles.
Gene Expression
The trachea was cannulated and the lungs were removed and inflated to capacity with RNAlater (Ambion/Applied Biosystems; Foster City, CA). The lungs (n =5) were stored at 4°C for 24 hr and then moved to 22°C until microdissection and RNA isolation could be performed. Intrapulmonary airways were microdissected from the surrounding parenchyma. RNA was reverse transcribed into single-stranded cDNA using the High-Capacity RNA-to-cDNA kit (Applied Biosystems) according to the manufacturer’s suggested protocol. qRT-PCR was carried out in the StepOnePlus Real-Time PCR System.
Statistics
Data are reported as mean ± standard deviation unless otherwise stated. Parametric data analysis was performed using Student’s unpaired t-test. Values of p < 0.05 were considered statistically significant. Statistical analysis was performed using StatView 5.0.1 (SAS Institute Inc., 1999).
RESULTS AND DISCUSSION
Naphthalene metabolism
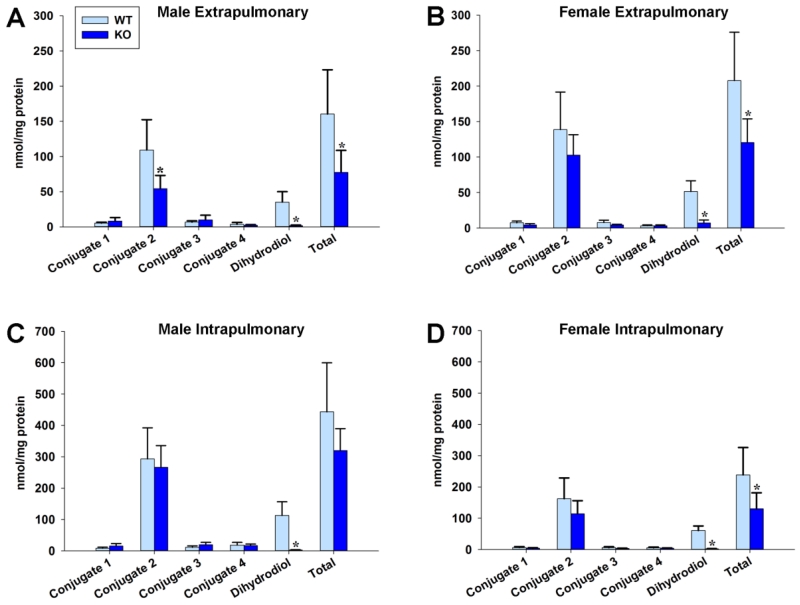
The first step of NA metabolism is the conversion to 1,2-epoxide by cytochrome P450 monooxygenase (Fig. 1). The majority of 1,2-epoxide is detoxified by conjugation to GSH and eliminated as mercapturic acids in urine (Habig et al., 1974), converted to 1,2-dihydrodiol by mEH, or rearranges to 1-naphthol which is eliminated as glucuronide or sulfate conjugates in the urine. NA-GSH conjugates can be measured by HPLC, but 1,2-NQ and 1,2-epoxide are unstable and difficult to measure directly. Therefore, we assessed the P450 mediated metabolism of NA to the 1,2-epoxide by measuring the four glutathione conjugates and the 1,2-dihydrodiol, the measurable precursor to 1,2-NQ, as in previous studies of NA metabolism (Buckpitt et al., 1995). We confirmed that NA-dihydrodiol was ablated in the KO mice for both intrapulmonary and extrapulmonary airways, for both sexes (Fig. 2). Total metabolism is defined in Fig. 2 as the sum of the five measured metabolites. The significant decrease in dihydrodiol resulted in a significant decrease in total metabolism for both airways and sexes in the KO mice.
Fig. 2. Metabolism to the 1,2-dihydrodiol is ablated in mEH KO mice.
This verifies the KO mouse is not metabolizing significant epoxide through the mEH pathway in these airways: (A) male extrapulmonary, (B) female extrapulmonary, (C) male intrapulmonary, (D) female intrapulmonary. * denotes significant difference (p < 0.05) from WT
As seen in these figures, GSH conjugates are not formed in equal amounts. In WT and KO mice, conjugate 2 is the primary NA metabolite, followed in abundance by the dihydrodiol. In KO mice, GSH conjugate 2 was significantly decreased for male mice (p = 0.015) in extrapulmonary airways but not in intrapulmonary airways, or for female mice. Besides the clear differences in dihydrodiol generation, differences in the rates of GSH conjugate 2 formation was the only difference detected between the WT and KO mice, and this was only noted in male mice. Reasons for this decrease are unclear, but this decrease in the detoxifying GSH conjugate does not appear to correlate with increased susceptibility to cytotoxicity in males.
In the extrapulmonary airways, WT mice had significantly total higher metabolism than KO mice for both males (p = 0.016) and females (p = 0.017). The only observed sex difference was with conjugate 3, which was higher in male KO mice than in female KO mice (p = 0.034). In the intrapulmonary airways, the males had higher metabolism for many of the measured metabolites (Table 1). These sex differences in intrapulmonary metabolism are likely not a result of our KO model, as the majority of statistically significant sex differences also exist in the WT mice. We measured statistically higher total metabolism for males than females in intrapulmonary airways, for both KO (p < 0.001) and WT (p = 0.023).
Table 1. Sex differences in intrapulmonary NA metabolism are not caused by mEH KO.
We measured statistically higher metabolism in control male intrapulmonary airways for most endpoints (p < 0.05). Data are expressed as the mean ± SD for n =5-6 as nmoles metabolite/mg protein/120 minutes.
| Wildtype | Knockout | |||||
|---|---|---|---|---|---|---|
| Male | Female | P-value | Male | Female | P-value | |
| Conjugate 1 | 8.10 ± 3.38 | 6.30 ± 3.02 | NS | 15.2 ± 7.91 | 3.70 ± 1.83 | 0.006 |
| Conjugate 2 | 293 ± 99.2 | 162 ± 66.3 | 0.022 | 267 ± 69.2 | 114 ± 41.7 | 0.001 |
| Conjugate 3 | 11.3 ± 4.50 | 5.69 ± 3.04 | 0.028 | 19.1 ± 7.72 | 3.49 ± 0.690 | 0.002 |
| Conjugate 4 | 18.2 ± 8.43 | 5.47 ±2.33 | 0.019 | 16.7 ± 4.79 | 4.20 ± 0.830 | < 0.001 |
| Dihydrodiol | 113 ± 43.5 | 60.4 ± 14.9 | 0.025 | 2.43 ± 0.890 | 2.04 ± 0.580 | NS |
| Total | 443 ± 156 | 239 ± 87.5 | 0.023 | 320 ± 70.0 | 130 ± 51.2 | < 0.001 |
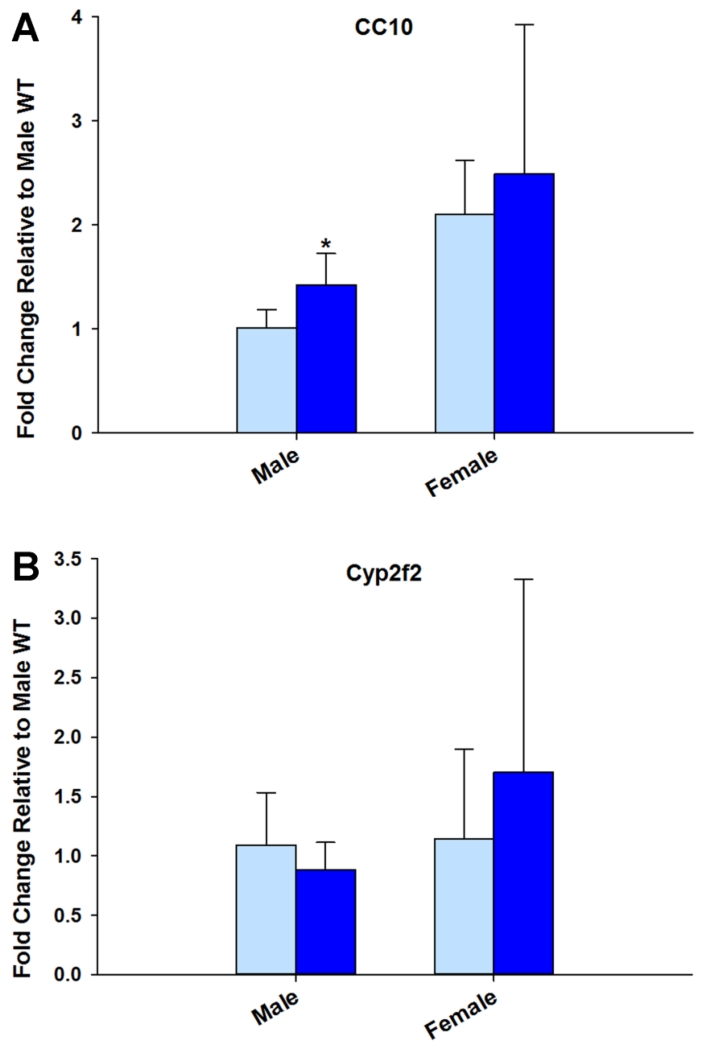
In the lung, NA activation to a 1,2-epoxide is driven by the high levels of Cyp2f2 in nonciliated bronchiolar Club cells (Plopper et al., 1987). To confirm that the differences in intrapulmonary metabolism were not a result of altered Cyp2f2 expression, we measured gene expression in microdissected airways. Cyp2f2 expression was not altered in the KO mouse, however the expression of Cyp2f2 is higher in female mice relative to male mice (Fig. 3B). CC10 (club cell 10-kDa protein) expression was increased in the male KO mouse, indicating male KO mice might have more club cells than WT mice (p = 0.036, Fig. 3A).
Fig. 3. Differences in airway metabolism were not a result of decreased Cyp2f2 or CC10 expression.
Male CC10 fold change is greater for KO than WT. Fold change expressed relative to WT. * denotes significant difference (p < 0.05) from WT
Cytotoxicity
The dose and route of exposure are important in predicting NA toxicity. Previous studies have found that inhalation of NA vapor below the current OSHA standard of 10 ppm causes proximal airway Club cell injury in mice (West et al., 2001). However, when a similar dose is administered intraperitoneally, injury is primarily observed in the distal airways, including terminal bronchioles (West et al., 2001). At intraperitoneal doses up to 200 mg/kg, the bronchi and trachea can contain affected cells, although the extent of injury varies by mouse strain (Lawson et al., 2002) and sex (Van Winkle et al., 2002).
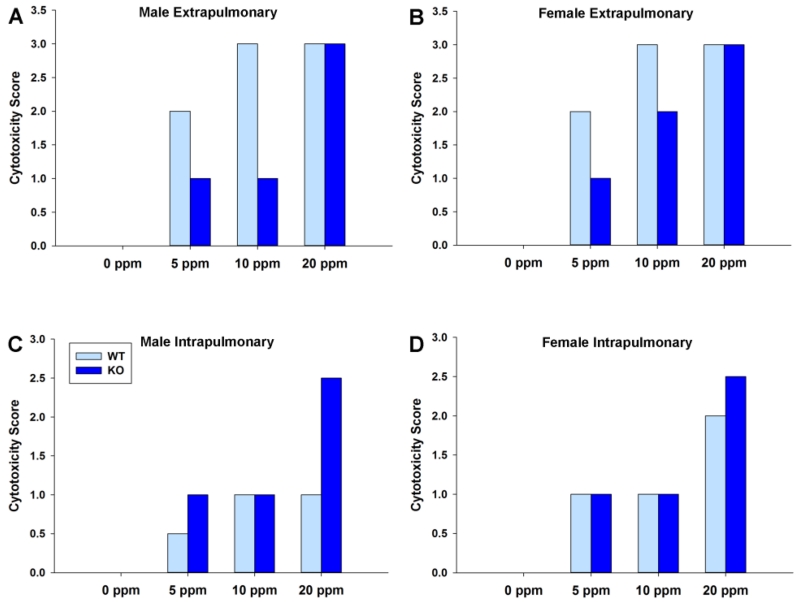
All mice in this experiment were sensitive to damage from NA exposure; however, the extent of cytotoxicity varied by airway generation and exposure concentration (Fig. 4). Smaller intrapulmonary airways experienced slight cytotoxicity at and below the OSHA standard of 10 ppm. Our results indicate that damage to the large extrapulmonary airways can occur at exposure concentrations as low as 5 ppm when exposed acutely to NA. For the extrapulmonary airways, WT cells appear to be more susceptible than KO at 5 and 10 ppm. We observed severe WT tissue damage at 10 ppm, with a greater degree of vacuolization and swelling in females. This same pattern exists for the KO mice at 10 ppm, however the overall damage is less severe than in WT mice. At 20 ppm, swollen/vacuolated airway epithelial cells were observed in the extra- and intrapulmonary airways of all mice at 20 ppm, though this effect was minimal in WT male extrapulmonary airways.
Fig. 4. KO mice had more severely damaged airways at sites of known NA-driven tumor formation.
Male and female differences appear to be present in KO extrapulmonary airways at 10 ppm and in WT intrapulmonary airways at 20 ppm.
In the extrapulmonary airways, cytotoxicity may be partially a result of mEH downstream metabolites (Fig. 4AB). However, these results do indicate that non-mEH metabolites are contributing to NA cellular damage in the lung. There appears to be a dose-dependent response to NA exposure in KO mice, while WT mice are very sensitive to damage even at 5 ppm. In the critical tumor site, intrapulmonary airways were more severely damaged in KO than WT for 20 ppm (Fig. 4CD). Toxicity cannot be from the 1,2-NQ in the intrapulmonary airways, as that pathway has been ablated in the KO animals.
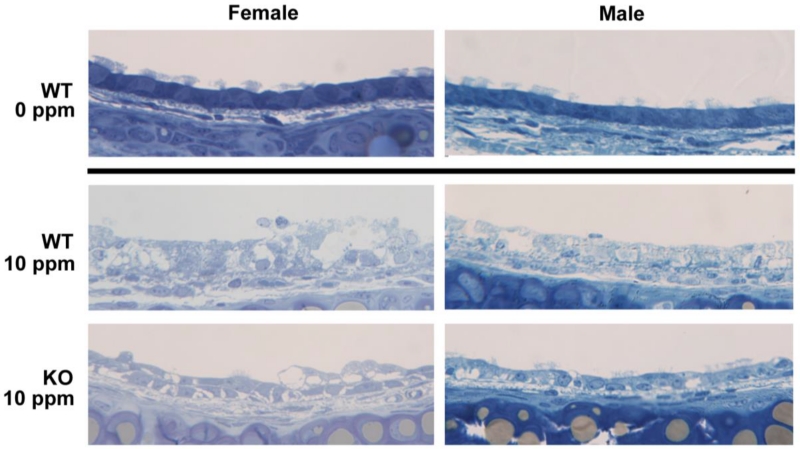
We observed a possible increase in intrapulmonary cytotoxicity at 20 ppm in WT female relative to WT males. In the extrapulmonary airways, we observed a possible increase in cytotoxicity to KO females relative to KO males at 10 ppm. Representative images from female and male extrapulmonary airways after acute 10 ppm exposure are shown in Fig. 5. In both extrapulmonary and intrapulmonary airways, the pattern of cytotoxicity suggests increased susceptibility to acute NA exposure in females. This is consistent with previous studies. The NTP chronic bioassay showed that female mice—but not male mice—will develop lung tumors following chronic NA exposure (National Toxicology Program, 1992).
Fig. 5. Extrapulmonary toxicity may be more severe for females than males at 10 ppm.
Representative trachea sections illustrating severe WT tissue damage at 10 ppm, with a greater degree of vacuolization and swelling in the female section. There is no damage at 0 ppm for WT or KO mice (only WT shown).
Our results indicate that the apparent contribution of mEH-dependent metabolites to toxicity differs by location in the lung. For the extrapulmonary airways, WT cells are more susceptible than KO at 5 and 10 ppm. This indicates that cytotoxicity in this region may be partially a result of mEH downstream metabolites, including the 1,2-NQ. However, because the KO animals are still susceptible to extrapulmonary damage from NA exposure, mEH is not the only enzyme driving this toxicity. In the intrapulmonary airways, where mouse lung tumors are known to form following inhalation of NA vapor (Abdo et al., 2001), cells from KO mice are more susceptible to NA injury than WT at 20 ppm. This observed toxicity cannot be from the 1,2-NQ, as that pathway has been ablated in the KO animals. Further investigation is needed to explore the in vivo toxicity of the mEH pathway, to determine if either (1) metabolites generated through the mEH pathway are of minor importance in the overall toxicity and subsequent distal airway carcinogenesis from NA exposure or that (2) compensatory alterations in gene expression associated with mEH KO may alter other pathways in the activation and detoxification of NA.
Conclusions
Sex, airway generation and the mEH pathway affect susceptibility to cytotoxicity in mice, following acute exposure to NA. Females may be more susceptible to NA toxicity than males, and in the intrapulmonary airways, this may be due in part to sex differences in GSH conjugate formation. In the extrapulmonary airways, WT mice were more susceptible to damage than KO mice, indicating mEH could be partially responsible for cytotoxicity at this site. In the intrapulmonary airways, metabolites generated through the mEH pathway may be of minor importance in distal airway toxicity and subsequent carcinogenesis from NA exposure. The pattern of toxicity observed in the KO mice suggests that mEH metabolites may not be driving the overall toxicity of NA for known tumor sites. The 1,2-NQ is often suspected of being the toxic metabolite of NA; however, our results suggest that other metabolites, perhaps a NA-epoxide or the 1,4-NQ, or a pathway that is currently unknown, may be contribute to intrapulmonary toxicity and subsequent carcinogenesis.
Highlights.
mEH metabolites may not be driving the overall toxicity of NA for known tumor sites
The mEH pathway may be partially responsible for cytotoxicity in extrapulmonary airways
Acute exposure to naphthalene causes cytotoxicity in all airway generations at 5 ppm
Female mice may be more susceptible to naphthalene acute toxicity than males
ACKNOWLEDGEMENTS
We are grateful to Judy Shimizu, Jason Thornton, Paige Spiess, and Katie Sutherland for their technical assistance during sample collection and processing. We also thank the mouse biology program at UC Davis for maintaining our transgenic mice colony. Imaging was conducted at the UC Davis Cellular and Molecular Imaging core. Research supported by R01 ES004311, R01 ES020867, P30-ES023513. Sarah Carratt received partial Superfund trainee support (P42 ES04699) and is currently supported by a fellowship on NIEHS T32 ES007059 for Advanced Training in Environmental Health Sciences. Transgenic mouse colony support also from TRDRP 14RT-0132.
Abbreviations used
- P450
cytochrome P450
- NA-dihydrodiol
1,2-dihydro-1,2 dihydroxynaphthalene
- GSH
glutathione
- GST
glutathione transferase
- KO
knockout
- mEH
microsomal epoxide hydrolase
- NA
naphthalene
- NQ
naphthoquinone
- OSHA
Occupational Safety and Health Administration
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP CONTRIBUTIONS
Participated in research design: Morin, Buckpitt, and Van Winkle
Conducted experiments: Carratt, Morin, Buckpitt, Edwards, Van Winkle
Performed data analysis: Carratt and Morin
Wrote or contributed to the writing of the manuscript: Carratt, Buckpitt, and Van Winkle
REFERENCES
- Abdo KM, Grumbein S, Chou BJ, Herbert R. Toxicity and carcinogenicity study in F344 rats following 2 years of whole-body exposure to naphthalene vapors. Inhal Toxicol. 2001;13:931–950. doi: 10.1080/089583701752378179. [DOI] [PubMed] [Google Scholar]
- Bauer AK, Faiola B, Abernethy DJ, Marchan R, Pluta LJ, Wong VA, Gonzalez FJ, Butterworth BE, Borghoff SJ, Everitt JI, Recio L. Male mice deficient in microsomal epoxide hydrolase are not susceptible to benzene-induced toxicity. Toxicological sciences: an official journal of the Society of Toxicology. 2003;72:201–209. doi: 10.1093/toxsci/kfg024. [DOI] [PubMed] [Google Scholar]
- Bogen KT, Benson JM, Yost GS, Morris JB, Dahl AR, Clewell HJ, 3rd, Krishnan K, Omiecinski CJ. Naphthalene metabolism in relation to target tissue anatomy, physiology, cytotoxicity and tumorigenic mechanism of action. Regulatory toxicology and pharmacology: RTP. 2008;51:S27–36. doi: 10.1016/j.yrtph.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckpitt A, Chang AM, Weir A, Van Winkle L, Duan X, Philpot R, Plopper C. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Molecular pharmacology. 1995;47:74–81. [PubMed] [Google Scholar]
- Buckpitt AR, Castagnoli N, Jr., Nelson SD, Jones AD, Bahnson LS. Stereoselectivity of naphthalene epoxidation by mouse, rat, and hamster pulmonary, hepatic, and renal microsomal enzymes. Drug metabolism and disposition: the biological fate of chemicals. 1987;15:491–498. [PubMed] [Google Scholar]
- Buckpitt AR, Warren DL. Evidence for hepatic formation, export and covalent binding of reactive naphthalene metabolites in extrahepatic tissues in vivo. The Journal of pharmacology and experimental therapeutics. 1983;225:8–16. [PubMed] [Google Scholar]
- Flowers-Geary L, Bleczinki W, Harvey RG, Penning TM. Cytotoxicity and mutagenicity of polycyclic aromatic hydrocarbon ortho-quinones produced by dihydrodiol dehydrogenase. Chemico-biological interactions. 1996;99:55–72. doi: 10.1016/0009-2797(95)03660-1. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. The Journal of biological chemistry. 1974;249:7130–7139. [PubMed] [Google Scholar]
- IARC . Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. 2002. [PMC free article] [PubMed] [Google Scholar]
- Kakareka SV, Kukharchyk TI. PAH emission from the open burning of agricultural debris. The Science of the total environment. 2003;308:257–261. doi: 10.1016/S0048-9697(02)00650-2. [DOI] [PubMed] [Google Scholar]
- Kitteringham NR, Davis C, Howard N, Pirmohamed M, Park BK. Interindividual and interspecies variation in hepatic microsomal epoxide hydrolase activity: studies with cis-stilbene oxide, carbamazepine 10, 11-epoxide and naphthalene. The Journal of pharmacology and experimental therapeutics. 1996;278:1018–1027. [PubMed] [Google Scholar]
- Kobayashi R, Okamoto RA, Maddalena RL, Kado NY. Polycyclic aromatic hydrocarbons in edible grain: a pilot study of agricultural crops as a human exposure pathway for environmental contaminants using wheat as a model crop. Environmental research. 2008;107:145–151. doi: 10.1016/j.envres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Lawson GW, Van Winkle LS, Toskala E, Senior RM, Parks WC, Plopper CG. Mouse strain modulates the role of the ciliated cell in acute tracheobronchial airway injury-distal airways. Am J Pathol. 2002;160:315–327. doi: 10.1016/S0002-9440(10)64375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wei Y, Van Winkle L, Zhang QY, Zhou X, Hu J, Xie F, Kluetzman K, Ding X. Generation and characterization of a Cyp2f2-null mouse and studies on the role of CYP2F2 in naphthalene-induced toxicity in the lung and nasal olfactory mucosa. The Journal of pharmacology and experimental therapeutics. 2011;339:62–71. doi: 10.1124/jpet.111.184671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long PH, Herbert RA, Peckham JC, Grumbein SL, Shackelford CC, Abdo K. Morphology of nasal lesions in F344/N rats following chronic inhalation exposure to naphthalene vapors. Toxicologic pathology. 2003;31:655–664. doi: 10.1080/01926230390242016. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of biological chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Miyata M, Kudo G, Lee YH, Yang TJ, Gelboin HV, Fernandez-Salguero P, Kimura S, Gonzalez FJ. Targeted disruption of the microsomal epoxide hydrolase gene. Microsomal epoxide hydrolase is required for the carcinogenic activity of 7,12-dimethylbenz[a]anthracene. The Journal of biological chemistry. 1999;274:23963–23968. doi: 10.1074/jbc.274.34.23963. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program . Toxicology and Carcinogenesis Studies of Naphthalene (CAS No. 91-20-3) in B6C3F1 Mice (Inhalation Studies) 1992. pp. 1–172. National Toxicology Program technical report series 410. [PubMed] [Google Scholar]
- National Toxicology Program . Toxicology and carcinogenesis studies of naphthalene (cas no. 91-20-3) in F344/N rats (inhalation studies) 2000. pp. 1–173. National Toxicology Program technical report series. [PubMed] [Google Scholar]
- Pakenham G, Lango J, Buonarati M, Morin D, Buckpitt A. Urinary naphthalene mercapturates as biomarkers of exposure and stereoselectivity of naphthalene epoxidation. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:247–253. doi: 10.1124/dmd.30.3.247. [DOI] [PubMed] [Google Scholar]
- Pellizzari ED, Hartwell TD, Harris BS, 3rd, Waddell RD, Whitaker DA, Erickson MD. Purgeable organic compounds in mother’s milk. Bulletin of environmental contamination and toxicology. 1982;28:322–328. doi: 10.1007/BF01608515. [DOI] [PubMed] [Google Scholar]
- Phimister AJ, Lee MG, Morin D, Buckpitt AR, Plopper CG. Glutathione depletion is a major determinant of inhaled naphthalene respiratory toxicity and naphthalene metabolism in mice. Toxicological sciences: an official journal of the Society of Toxicology. 2004;82:268–278. doi: 10.1093/toxsci/kfh258. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Chang AM, Pang A, Buckpitt AR. Use of microdissected airways to define metabolism and cytotoxicity in murine bronchiolar epithelium. Experimental lung research. 1991;17:197–212. doi: 10.3109/01902149109064411. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Cranz DL, Kemp L, Serabjit-Singh CJ, Philpot RM. Immunohistochemical demonstration of cytochrome P-450 monooxygenase in Clara cells throughout the tracheobronchial airways of the rabbit. Experimental lung research. 1987;13:59–68. doi: 10.3109/01902148709064309. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Van Winkle LS, Fanucchi MV, Malburg SR, Nishio SJ, Chang A, Buckpitt AR. Early events in naphthalene-induced acute Clara cell toxicity. II. Comparison of glutathione depletion and histopathology by airway location. American journal of respiratory cell and molecular biology. 2001;24:272–281. doi: 10.1165/ajrcmb.24.3.4247. [DOI] [PubMed] [Google Scholar]
- Saeed M, Higginbotham S, Gaikwad N, Chakravarti D, Rogan E, Cavalieri E. Depurinating naphthalene-DNA adducts in mouse skin related to cancer initiation. Free radical biology & medicine. 2009;47:1075–1081. doi: 10.1016/j.freeradbiomed.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M, Higginbotham S, Rogan E, Cavalieri E. Formation of depurinating N3adenine and N7guanine adducts after reaction of 1,2-naphthoquinone or enzyme-activated 1,2-dihydroxynaphthalene with DNA. Implications for the mechanism of tumor initiation by naphthalene. Chemico-biological interactions. 2007;165:175–188. doi: 10.1016/j.cbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Schmeltz I, Tosk J, Hoffmann D. Formation and determination of naphthalenes in cigarette smoke. Analytical chemistry. 1976;48:645–650. doi: 10.1021/ac60368a031. [DOI] [PubMed] [Google Scholar]
- Stanley J. In: Broadscan analysis of the FY 82 national human adipose tissue survey specimens: Vol.1 - Executive summary. US Environmental Protection Agency, O.o.T.S., editor. Washington DC, USA: 1986. [Google Scholar]
- USEPA . Summary of Emissions Associated with Sources of Naphthalene. 1986. [Google Scholar]
- USEPA . Toxicological Review of Naphthalene. 1998. pp. 1–42. [Google Scholar]
- Van Winkle LS, Gunderson AD, Shimizu JA, Baker GL, Brown CB. Gender differences in both naphthalene metabolism and naphthalene-induced acute lung injury. Am J Physiol: Lung Cell Mol Physiol. 2002;282:L1122–L1134. doi: 10.1152/ajplung.00309.2001. [DOI] [PubMed] [Google Scholar]
- Waidyanatha S, Troester MA, Lindstrom AB, Rappaport SM. Measurement of hemoglobin and albumin adducts of naphthalene-1,2-oxide, 1,2-naphthoquinone and 1,4-naphthoquinone after administration of naphthalene to F344 rats. Chemico-biological interactions. 2002;141:189–210. doi: 10.1016/s0009-2797(02)00048-0. [DOI] [PubMed] [Google Scholar]
- West JA, Pakehham G, Morin D, Fleschner CA, Buckpitt AR, Plopper CG. Inhaled naphthalene causes dose dependent Clara cell cytotoxicity in mice but not in rats. Toxicology and applied pharmacology. 2001;173:114–119. doi: 10.1006/taap.2001.9151. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Maronpot RR, Pinkerton KE, Jones AD. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997;18:2035–2042. doi: 10.1093/carcin/18.11.2035. [DOI] [PubMed] [Google Scholar]