Abstract
Purpose
To determine the risk of infection with Chlamydia trachomatis in children who are migrants to communities who are undergoing mass drug administration (MDA), and if their neighborhoods have higher rates of infection over time.
Methods
In 4 communities in Kongwa, Tanzania, all children were enrolled in a longitudinal study of infection and trachoma. New children were identified at census updates as having not been in the community at the previous census. Within communities, neighborhoods were defined as spatially close groups of households, or “balozi”. All children in the communities were invited to be examined for trachoma, and have ocular swabs taken for evidence of infection. Trachoma was graded using the World Health Organization simplified grading scheme, and swabs were processed using Amplicor.
Results
Children who were migrants were more likely to be infected and to have trachoma than children who were resident in the community, which was significant by the time of the survey following the third year of MDA (odds ratio, OR, 2.49, 95% confidence interval, CI, 1.03–6.05). The neighborhoods where newcomers resided were more likely to have infection a year later than neighborhoods with no migrants, which was most pronounced following the third year of MDA (OR 2.86, 95% CI 1.07–7.65)
Conclusion
Migrants to communities may be an important source of re-emergent infection, especially as MDA lowers infection among residents. Highly migrant populations may need a special surveillance and treatment program to avoid slowing progress in communities under MDA.
Keywords: trachoma, ocular Chlamydia trachomatis, Tanzania, prospective study, population
Introduction
The leading infectious cause of blindness worldwide, trachoma, is caused by repeated episodes of ocular infection with Chlamydia trachomatis 1. Communities with trachoma are often those with the fewest resources to take on health issues, and trachoma strikes the most vulnerable members of those communities, women and children. The World Health Organization (WHO) has recommended a multifaceted “SAFE” strategy for trachoma control programs2. This approach includes Surgery for trichiasis cases, and A, F, and E to control active trachoma: Antibiotics to treat the community pool of infection, Face washing and Environmental change to sustain reduction in transmission. The WHO recommends mass treatment with antibiotics, preferably azithromycin, when the prevalence of follicular trachoma (TF) is more than 10% in children aged 1 to 9 years at the district or sub-district level3.
Mass treatment with azithromycin has a strong effect on decreasing chlamydial load following mass treatment4,5; however, the hope that 1–2 rounds of annual mass drug administration (MDA) would be sufficient to eliminate trachoma or infection in most communities has not been realized. WHO guidelines suggest that mass treatment be provided for all communities in a district for at least 3 years, and that when the starting prevalence of TF in a district is high, for example 30%, at least 5 years of annual mass antibiotic administration will be needed before any expectation of significant change6.
But the source of infection following mass treatment is unknown. Non-compliance with treatment was originally a predictor of infection and disease at follow-up in studies in 3 different settings7. However, in another study even with very high compliance of over 95% of children, the prevalence of infection with C. trachomatis at 6 months post-mass treatment was 42% of the pre-treatment levels. There were too few untreated children to suggest non-compliance was the issue8.
Some have argued that infants are one source of re-emergent infection because they are treated (likely unevenly) with topical tetracycline. Infants <6 months of age are not eligible for azithromycin, and may have very high chlamydial burdens9. However, a study in Tanzania which addressed this issue found that infections were few in this age group and that households with infants were not at increased risk of infection following mass treatment10.
Another source of infection may be the reintroduction of C. trachomatis with in-migration of individuals who can bring in infection and from returning community members who acquire infection outside. It was the primary explanation for re-emergence of infection in 2 communities in The Gambia11. Travel by families outside the village where interactions occur that might re-infect children was a source of infection in Ethiopia12. The issue of in-migration has not been addressed in trachoma control programs. In part, the thought is that these persons will be treated during the next annual MDA. However, once infection rates in the villages are lowered, these sources could become a significant component of the residual infection and influence the results of impact surveys as well as be a possible reason why the trajectory of decline is less steep than expected. If migrants are in fact shown to impede progress towards elimination, then a local strategy that addresses new families and a nationwide strategy that addresses intra- and international migration will be needed.
The purpose of this study was to quantify the effect of migrants on the prevalence of infection and clinical trachoma in communities.
Methods
As part of a randomized trial of enhanced coverage of MDA, we enrolled 4 communities for 3 rounds of MDA after completing censusus and surveys at baseline and annually. For the surveys, all children aged under 10 years were included8,13. For this study, we used the annual censuses to identify new families, as defined below. All methods and protocols were approved by the Johns Hopkins Institutional Review Board and the National Institute for Medical Research, and written informed consent was provided by all guardians of participants.
The methods and primary results of the trial have been published elsewhere13 and summarized here. The surveys were done on a complete sample of children younger than 10 years in each community at each time point. Participants were assessed by an experienced trachoma grader for clinical trachoma using 2.5 loupes and the WHO simplified grading scheme for the presence of TF and inflammatory trachoma (TI)14. A random sample of children had photographs taken for quality assurance purposes and the grades compared to those of a master grader for each survey; kappa statistics for agreement were above 0.8 for reach survey round.
A conjunctival swab was also taken at each survey for the determination of evidence of infection with C. trachomatis. Following strict adherence to protocols for avoiding field contamination, a swab was taken of the right upper conjunctiva of each child, inserted dry into a tube which is stored cold in the field and transferred to a refrigerator and shipped within 30 days to the International Chlamydial Laboratory at Johns Hopkins University. Gloves were changed between each child examined by the person who flipped the lid and took the specimen. A 5% sample of air control swabs was also taken to monitor for field contamination. No evidence of field contamination was observed throughout the project.
All ocular specimens were processed for detection of C. trachomatis in the laboratory using the Amplicor CT/NG test (Roche Molecular Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s specifications. Using a known positive sample, 2 positive and 2 negative processing controls were run with each batch of specimens. Optical density (OD) for each specimen was measured. Samples with ODs >0.7 were recorded as positive for C. trachomatis and evidence for infection, samples with ODs <0.2 were recorded as negative, while samples with ODs between 0.2 and 0.7 were considered equivocal. Samples with equivocal results were retested in duplicate; samples that retested equivocal or repeated as negative on 2 occasions were considered negative. Less than 0.1% of specimens were equivocal.
After surveys at baseline and at 12 months and 24 months, annual treatment was offered to all community residents, and comprised a single dose of oral azithromycin at 20mg/kg for adults and children over 6 months old. A 1% topical tetracycline eye ointment was provided for children under 6 months old with instructions to apply twice daily for 4–6 weeks. Antibiotic coverage exceeded 80% for children aged under 10 years in all communities at each MDA15.
Statistical analysis
Migrant families were defined as not present at the previous census and not treated in the previous MDA and present at the current census for years 1, 2, and 3. We defined neighborhoods as those living in the same balozi (a governmental neighborhood grouping within communities). Households in close proximity to households of migrants were defined as those living in the same balozi. All statistical analyses were conducted using SAS 9.2 software (SAS Institute Inc, Cary, NC, USA). Contingency table analysis was used to examine the relationship between migrant status and infection and trachoma (TF). We also examined the relationship across time at years 1, 2, and 3 to see the change in the estimate of prevalence as infection declined. We calculated the weighted estimate of prevalence and compared it to the prevalence if the migrants had not been present at the time of the survey, and determined the proportion of the estimate contributed by the migrants at each time point. Random effects logistic models with infection as the outcome were used to account for clustering of infection within neighborhoods and within communities.
Results
The overall characteristics of the 84 neighborhoods within the 4 communities at baseline and over the 3 years are shown in Table 1. Each year the neighborhoods experienced anywhere from none up to 7 new families, and the average was <1.
Table 1.
Characteristics of neighborhoods within communities present at baseline of trachoma infection study, Kongwa, Tanzania
| Characteristic | Year 1 (N=84) | Year 2 (N=100) | Year 3 (N=103) |
|---|---|---|---|
| Population size, median n (IQR) | 69 (54–96) | 65 (51–81) | 62 (54–77) |
| Families, median n (IQR) | 14 (12–18) | 14 (12–18) | 13 (10–16) |
| Children aged <10 years, median n (IQR) | 29 (20–37) | 24 (20–33) | 33 (28–42) |
| Migrant families, % | 38 | 44 | 21 |
| MDA coverage in age group 0–9 years, median % (IQR) | 96 (89–100) | 95 (85–100) | 90 (85–96) |
IQR, interquartile range; MDA, mass drug administration
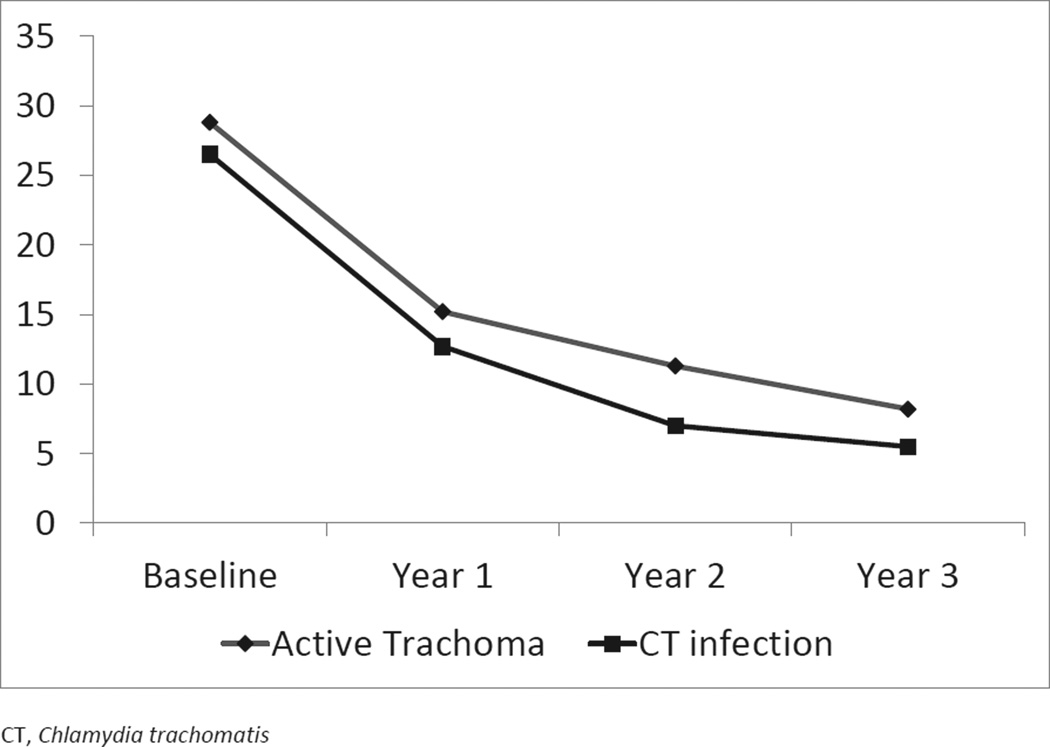
Coverage with MDA was above 80% at all rounds of MDA, as measured in children aged under 10 years, and the rate of trachoma and infection steadily declined over the course of the study (Figure 1).
Figure 1.
Infection and trachoma rates over 3 years in 4 communities in Kongwa, Tanzania
The presence of infection and clinical trachoma (TF) in children by whether they were in the community during the previous MDA is shown in Table 2. Children who were new were more likely to be infected at each time point and to have clinical trachoma, and these differences were statistically significant in the third year.
Table 2.
Presence of trachoma infection in children according to status as belonging to a migrant family or present during the previous MDA, Kongwa, Tanzania
| Year | Migrant or resident for previous year |
Children, n |
Infected, % | Odds ratio (95% CI) |
Trachoma (TF), % |
Odds ratio (95% CI) |
|---|---|---|---|---|---|---|
| 1 | Resident | 2,275 | 12.7 | 1.00 | 15.7 | 1.00 |
| Migrant | 92 | 19.6 | 1.76 (0.93–3.34) | 18.5 | 1.23 (0.66–2.28) | |
| 2 | Resident | 2,444 | 7.0 | 1.00 | 11.5 | 1.00 |
| Migrant | 153 | 11.8 | 1.56 (0.81–2.99) | 12.4 | 1.09 (0.60–2.99) | |
| 3 | Resident | 2,640 | 5.8 | 1.00 | 8.6 | 1.00 |
| Migrant | 71 | 15.5 | 2.49 (1.03–6.05) | 15.5 | 2.07 (1.09–3.97) |
CI, confidence interval; MDA, mass drug administration, TF, follicular trachoma
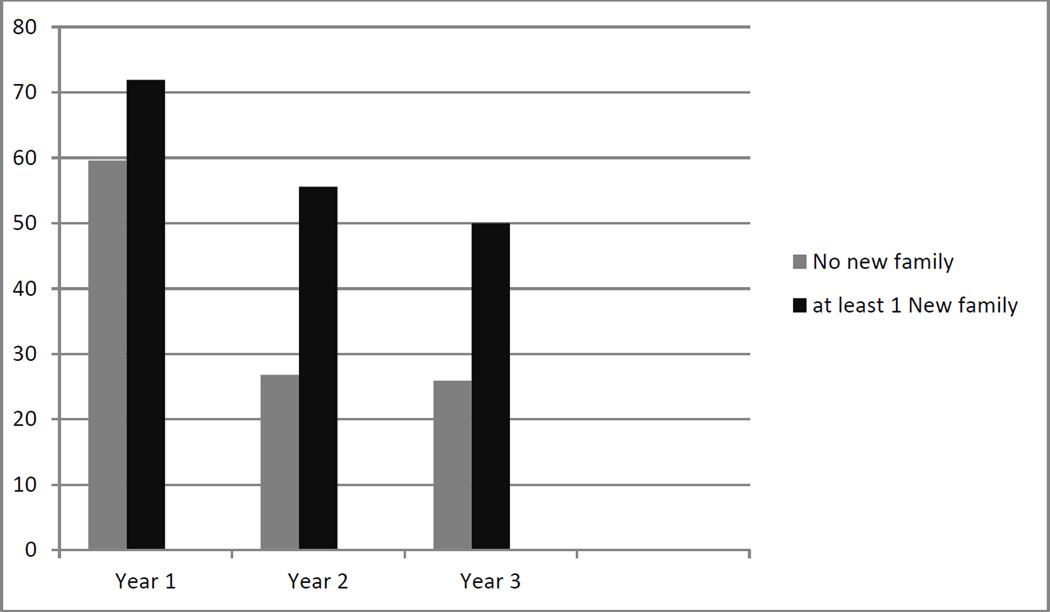
Since the data suggest that newly arrived children were more likely to be infected, we examined the proportion of neighborhoods that had at least 2 or more children infected, to enable us to see infection in children other than the newly arrived children. Figure 2 shows that in each year, the neighborhoods with newly arrived families with children were more likely to have 2 or more infected children than the neighborhoods with no new families, and the difference was statistically significant in years 2 and 3. The odds of a neighborhood having 2 or more children infected if there were newly arrived families was 1.76 in year 1, 2.68 in year 2, and 2.86 in year 3 (Table 3).
Figure 2.
Proportion of neighborhoods with 2 or more children infected with trachoma for each of 3 years, by immigration of new families, Kongwa, Tanzania.
Table 3.
Odds of having 2+ children with trachoma infection if the neighborhood has migrant families or does not have migrant families since the last MDA, Kongwa, Tanzania
| Year | Neighborhood type |
Neighborhoods, n | ≥2 infected children, % |
Odds ratio (95% CI) |
|---|---|---|---|---|
| 1 | Without migrant families | 52 | 59.6 | 1.00 |
| With migrant families | 32 | 71.9 | 1.73 (0.66–4.54) | |
| 2 | Without migrant families | 56 | 26.8 | 1.00 |
| With migrant families | 44 | 55.6 | 2.68 (1.10–6.50) | |
| 3 | Without migrant families | 81 | 25.9 | 1.00 |
| With migrant families | 22 | 50.0 | 2.86 (1.07–7.65) |
CI, confidence interval; MDA, mass drug administration
The weighted prevalence of infection overall was 13.0%, 7.3%, and 6.1% in each year of the study, respectively. The absolute contribution of migrant children to prevalence was about 0.3–0.4% in each year, but as the prevalence of infection dropped in the resident community, the percentage contribution from migrants to the estimate of community prevalence increased, from 2.3% at baseline to almost 7% at the third year.
Discussion
Our data suggest that not only are newly arrived children more likely to have infection, but the neighborhoods into which they move are more likely to have 2 or more children infected by the next census. The findings were statistically significant and the odds increased after each successive round of MDA in the community. These data also support our hypothesis that as infection and trachoma in the community goes down with each successive MDA, the contribution of migrants to the cases of TF and thus to the prevalence estimate for the community increases. Moreover, the risk to the neighborhood of having multiple infected children increases as well.
The contribution to the estimate of prevalence of infection or trachoma in the community from migrant children depends a great deal on how many new families move into the neighborhoods. As can be seen in Table 2, about 6% of the children in the community in the last year were from new families, according to our definition. If the proportion of children who were from migrant families was greater, then their proportionate contribution to the estimate of the prevalence of infection would be concomitantly greater. For example at 3 years if, instead of 71 migrant children of the 2,640, there were 200 migrant children, then their contribution to the estimate of prevalence of infection in the communities would increase from 7% to 11%.
Because of our strict definition of “migrant” it is possible that we have missed migrant families into these communities. First, it is possible that families have moved into existing households, and we did not capture that scenario in these analyses. It is also possible that families moved in and left within a year, and we would have missed capturing them in the census update. However, short stays in the community are unlikely to have caused spread across neighbors, as our previous study found that it took at least 6 months to see clustering around households with infection after MDA16.
It is interesting to note that with each successive year of MDA, the infection in the newly arrived children, while still higher than the resident children, appeared to decrease. While we did not capture data on the origins of the newly arrived families, the fact that the entire district of Kongwa was being treated with MDA suggests that some of the new arrivals had also been treated for trachoma before. In Kongwa, anecdotal information suggests that influx to the villages comes from movement around the villages within the district but also from other districts when families seek new farmland.
Our data on migrants is similar to data found on travelers, as reported by Burton et al11 in The Gambia and Gaynor et al [AU: please provide missing reference details] in Ethiopia. However, in The Gambia, the newly arrived cases of infection did not appear to lead to re-emergence in the community even though MDA had ceased. This may reflect the very low prevalence of trachoma in The Gambia and that environmental conditions may not favor re-establishing ongoing transmission. In Ethiopia, the authors concluded that these infections may play a role in re-introducing infection into communities. In the early trials of azithromycin which evaluated entire communities for infection following MDA, the authors noted a higher prevalence of infection in those newly arrived in the communities7. The effect on neighborhoods was not assessed.
There are some limitations to our study. First, as noted, we did not capture all the newly arrived families, as some unknown fraction may have left the community before our next census. To the extent they resided in neighborhoods that we may have categorized as not having new families, we may have underestimated the effect of migrants. Second, we examined the effect on neighborhoods, which was defined using the “balozi” structure in Tanzania. These are geographically grouped households who decide on a leader for purposes of information exchange with their community leadership. However, it is possible that persons could live outside the geographical grouping and still affiliate with a balozi, or that, due to the spread of households within a village, none of the households in a balozi are close enough together to allow much spread of infection. We are still in the process of collecting global positioning system (GPS) data to allow us to group households more exactly, but do not have the data at present. In our current study, we assigned households by balozi for monitoring purposes to Community Monitors, and it is seldom they trade off households because they are not geographically part of the balozi, so we do not feel this is a major issue.
In summary, this study adds to the knowledge of the importance that in-migration to communities undergoing MDA may have on maintaining infection and trachoma rates. In areas where in-migration is considerable, the timing of MDA may be helpful in helping to control re-emergence if migration has a known seasonality, or devising a system to offer migrant families access to treatment in the interim may be advisable. Further research on the effect of treating migrants for trachoma and infection in communities undergoing MDA is warranted.
Acknowledgments
This was supported by a grant EY022584 from the National Institutes of Health, National Eye Institute, and in part by the Division of Intramural Research, NIAID, NIH
Footnotes
None of the authors have any proprietary interests or conflicts of interest related to this submission.
This submission has not been published anywhere previously and that it is not simultaneously being considered for any other publication.
References
- 1.Mariotti SP, Pascolini D, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009 May;93(5):563–568. doi: 10.1136/bjo.2008.148494. [DOI] [PubMed] [Google Scholar]
- 2.Assembly WH. Global elimination of blinding trachoma. 1998. WHA 51.11. [Google Scholar]
- 3.WHO_working_group. Report of the eighth meeting of the WHO Alliance for the Global Elimination of blinding trachoma. Geneva: World Health Organization WHO/PBD/GET/04.2; 2004. [Google Scholar]
- 4.Solomon AW, Holland MJ, Alexander ND, et al. Mass treatment with single-dose azithromycin for trachoma. N.Engl.J.Med. 2004;351(19):1962–1971. doi: 10.1056/NEJMoa040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West ES, Munoz B, Mkocha H, et al. Mass treatment and the effect on the load of Chlamydia trachomatis infection in a trachoma-hyperendemic community. Invest Ophthalmol.Vis.Sci. 2005;46(1):83–87. doi: 10.1167/iovs.04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World_Health_Organization. [Accessed May 9 2013];Third Global Scientific Meeting on Trachoma. 2010. 2013
- 7.Schachter J, West SK, Mabey D, et al. Azithromycin in control of trachoma. Lancet. 1999;354(9179):630–635. doi: 10.1016/S0140-6736(98)12387-5. [DOI] [PubMed] [Google Scholar]
- 8.Cajas-Monson LC, Mkocha H, Munoz B, Quinn TC, Gaydos CA, West SK. Risk factors for ocular infection with Chlamydia trachomatis in children 6 months following mass treatment in Tanzania. PLoS Negl Trop Dis. 2011;5(3):e978. doi: 10.1371/journal.pntd.0000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon AW, Holland MJ, Burton MJ, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet. 2003;362(9379):198–204. doi: 10.1016/S0140-6736(03)13909-8. [DOI] [PubMed] [Google Scholar]
- 10.West SK, Stare D, Mkocha H, Munoz B, Gaydos C, Quinn TC. Do infants increase the risk of re-emergent infection in households after mass drug administration for trachoma? Invest Ophthalmol Vis Sci. 2011 Jul;52(8):6040–6042. doi: 10.1167/iovs.11-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton MJ, Holland MJ, Makalo P, et al. Re-emergence of Chlamydia trachomatis infection after mass antibiotic treatment of a trachoma-endemic Gambian community: a longitudinal study. Lancet. 2005;365(9467):1321–1328. doi: 10.1016/S0140-6736(05)61029-X. [DOI] [PubMed] [Google Scholar]
- 12.Shah NA, House J, Lakew T, et al. Travel and implications for the elimination of trachoma in ethiopia. Ophthalmic Epidemiol. 2010 Mar;17(2):113–117. doi: 10.3109/09286581003624921. [DOI] [PubMed] [Google Scholar]
- 13.Stare D, Harding-Esch E, Munoz B, et al. Design and baseline data of a randomized trial to evaluate coverage and frequency of mass treatment with azithromycin: the Partnership for Rapid Elimination of Trachoma (PRET) in Tanzania and The Gambia. Ophthalmic Epidemiol. 2011 Feb;18(1):20–29. doi: 10.3109/09286586.2010.545500. [DOI] [PubMed] [Google Scholar]
- 14.Harding-Esch EM, Edwards T, Sillah A, et al. Active trachoma and ocular Chlamydia trachomatis infection in two Gambian regions: on course for elimination by 2020? PLoS neglected tropical diseases. 2009;3(12):e573. doi: 10.1371/journal.pntd.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West SK, Bailey R, Munoz B, et al. A randomized trial of two coverage targets for mass treatment with azithromycin for trachoma. PLoS Negl Trop Dis. 2013;7(8):e2415. doi: 10.1371/journal.pntd.0002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broman AT, Shum K, Munoz B, Duncan DD, West SK. Spatial clustering of ocular chlamydial infection over time following treatment, among households in a village in Tanzania. Invest Ophthalmol.Vis.Sci. 2006;47(1):99–104. doi: 10.1167/iovs.05-0326. [DOI] [PubMed] [Google Scholar]