Abstract
The present study retrospectively analyzed the utility of topoisomerase IIα expression as a prognostic marker to predict the neoadjuvant chemotherapeutic response and survival among different breast cancer subtypes. The patients were subtyped and the expression of topoisomerase IIα was determined using immunohistochemistry. All patients (n=147) received an anthracycline-containing regimen preoperatively, and 139 (95%) patients also received docetaxel. Of the 147 patients, 25 (17%) were triple-negative and 20 (17%) were human epidermal growth factor receptor 2 (HER2)-positive. Among these subtypes, a significantly higher a rate (P<0.0001) and higher incidence of topoisomerase IIα expression (P=0.036) were observed compared with that in the hormone receptor-positive and HER2-negative breast cancer types. However, the expression of topoisomerase IIα revealed no correlation with the treatment response or survival in any of the subtypes. Therefore, these results indicated that the favorable response to anthracycline-containing chemotherapy among triple-negative and HER2-positive breast cancer was independent of the expression of topoisomerase IIα.
Keywords: triple-negative breast cancer, topoisomerase IIα
Introduction
Breast cancer is the most common malignant disease among women in the Western world and Japan. However, advances in the systemic treatment of breast cancer, particularly in chemotherapy, have contributed to declines in the breast cancer mortality rates (1). Anthracycline-containing regimens are the most widely used in the adjuvant and neoadjuvant settings for patients with breast cancer (2). Several previous clinical investigations have revealed that the use of neoadjuvant chemotherapy for patients with locally advanced breast cancer increased the surgical resectability rates and that the response to therapy correlated with the patients' ultimate disease-free survival (3–5). In addition, significant tumor volume reduction following neoadjuvant chemotherapy may permit subsequent breast-conserving surgical treatment (6,7) and a unique advantage of neoadjuvant chemotherapy is the possibility to take serial measurements of the primary tumor, therefore, allowing in vivo assessment of factors predictive of the sensitivity to the treatment (8).
Anthracyclines act via several mechanisms, however, the interaction with the nuclear enzyme topoisomerase IIα appears to be the most prominent mechanism (9). Topoisomerase IIα, which is a critical nuclear DNA binding enzyme, functions by reducing DNA twisting and supercoiling by cutting both strands of the DNA helix simultaneously, allowing selected regions of the DNA to untangle and to consequently engage in transcription, replication or repair processes. Disruption of topoisomerase IIα has been demonstrated to lead to double-stranded DNA breaks and cell death, and topoisomerase IIα is, therefore, also a proliferation marker of tumor cells, in addition to a target of anthracycline-based chemotherapy (10).
However, previous studies have reported variable expression levels of topoisomerase IIα and responses to anthracycline-containing chemotherapy in breast cancer, and while in vivo and in vitro studies each demonstrate that there is indeed an association between the expression levels of topoisomerase IIα and chemosensitivity to anthracyclines, these results remain controversial (11–14).
Gene expression profiling has identified distinct breast cancer molecular subtypes associated with different clinical outcomes. Breast cancer is a molecularly heterogeneous disease, which can be divided into ≥4 or 5 groups based on the expression profiles, including luminal A and B, normal breast-like, human epidermal growth factor receptor 2 (HER2)-positive, and basal-like (predominantly triple negative) breast cancer (15,16). Previous studies, including our previous study, revealed that triple negative breast cancer is associated with an improved pathological complete response rate compared with the other subtypes (17–19).
In addition, several biomarkers and intrinsic subtypes have been reported as predictors of the neoadjuvant response (20,21). However, no basis for selecting the optimal chemotherapy for individual patients has been determined, and the association between the expression of topoisomerase IIα and the different subtypes remains to be elucidated.
With this in mind, the present study aimed to retrospectively analyze whether the protein expression levels of topoisomerase IIα assisted in predicting the response to anthracycline-containing neoadjuvant chemotherapy among each breast cancer subtype and whether it is a prognostic marker of survival.
Patients and methods
Patients
A prospective database of 147 Japanese women with stage II or III breast cancer who received neoadjuvant chemotherapy between May 1985 and January 2008 was analyzed. All patients received standard anthracycline-containing neoadjuvant chemotherapy. Adjuvant endocrine therapy for 5 years was prescribed for patients with hormone receptor (HR) -positive tumors, whereas adjuvant trastuzumab for 1 year was prescribed for patients with HER2-amplified/overexpressed tumors from 2001 onwards. Systemic and breast examinations were performed prior to neoadjuvant chemotherapy, prior to surgery, and every 12 months postoperatively using chest and abdominal computed tomography, mammograms, breast ultrasonography and bone scans. The present study was approved by the Ethics Committee of the Jikei University School of Medicine and written informed consent was obtained from the patients.
Immunohistochemistry (IHC) and defining breast cancer subtypes
IHC was performed, according to the standard protocol using 3 µm sections of paraffin-embedded tissues and the rabbit monoclonal antibody, anti-estrogen receptor (ER; SP1; Roche Diagnostics, Ltd., West Sussex, UK), for ER staining, and the rabbit monoclonal antibody, anti-progesterone receptor (PgR; 1E2; Roche Diagnostics, Ltd.), for PgR staining. Nuclear staining of ≥10% was considered positive. Tumors with ER and/or PgR positive expression were considered hormone receptor (HR)-positive. The expression of HER2 was determined using IHC with a rabbit polyclonal antibody (Dako, Glostrup, Denmark) on 4 µm sections of paraffin-embedded tissue. A staining score of 3+, according to the HercepTest criteria (22), was considered positive and a 2+ result was only considered positive if confirmed by fluorescence in situ hybridization with an amplification ratio of ≥2.0. The expression of topoisomerase IIα was determined by IHC using a mouse monoclonal antibody (M7186; 1:100; Dako) on 3 µm sections of paraffin-embedded tissue. The topoisomerase IIα staining was considered positive if nuclear staining ≥20% was observed (Fig. 1). Immunohistochemical proxies were used for subtyping and the tumors were classified into three subtypes, HR−/HER2−(triple-negative), any HR/HER2+(HER2-positive) and HR+/HER2−.
Figure 1.
Positive immunostaining for topoisomerase IIα.
Statistics
The response to chemotherapy was assessed, according to the Response Evaluation Criteria in Solid Tumors guidelines. The overall survival was measured from the date of diagnosis to the date of mortality, or the last follow-up. Disease-free survival was measured from the date of operation until the date of recurrence or the last follow-up. The association between each subtype and the age of the patients was evaluated using the Kruskal-Wallis test. The association between each subtype and the clinical factors, response rate to neoadjuvant chemotherapy and topoisomerase IIα expression in the patients, were evaluated using the Fisher's exact test. Cumulative survival probabilities were calculated using the Kaplan-Meier method, and differences between the survival rates were tested using the log-rank test. Logistic regression analyses were performed to evaluate the association between the expression of topoisomerase IIα, and the response to chemotherapy and survival among each breast cancer subtype. All statistical analyses were performed using Stata® software (Version 13; StataCorp LP, College Station, TX, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Patients and tumor characteristics
The performed chemotherapeutic regimens, which have changed over time since the first cases were obtained in 1985, were as follows: 6 cycles of doxorubicin (50 mg/m2), 5-fluorouracil (500 mg/m2) and cyclophosphamide (500 mg/m2) in 8 patients (5%); 6 cycles of alternate administration of epirubicin (60 mg/m2), 5-fluorouracil (500 mg/m2) and cyclophosphamide (500 mg/m2) with docetaxel (75 mg/m2) in 6 patients (4%); 6 cycles of concurrent administration of doxorubicin (50 mg/m2) and docetaxel (60 mg/m2) in 41 patients (28%); 4 cycles of epirubicin (100 mg/m2), 5-fluorouracil (500 mg/m2) and cyclophosphamide (500 mg/m2), followed by 4 cycles of docetaxel (100 mg/m2) in 92 patients (63%). Therefore, all patients received an anthracycline-based regimen and 139 patients (95%) also received docetaxel. The regimens did not differ according to the subtype. The median patient age was 51 years (range, 27–71 years). Table I lists the demographic, tumor characteristics, and the results of the Fisher's exact and Kruskal-Wallis tests among each subtype. The age of the patients with HR/HER2+ tumors was significantly higher compared with that of patients with HR−/HER2− (P=0.04) and HR+/HER2− tumors (P=0.03), and the menopausal status significantly differed between patients with any HR/HER2+ and the other two subtypes (P=0.02). By contrast, the tumor size and nodal status were similar among the three subtypes (Table I).
Table I.
Demographic and tumor characteristics.
| Characteristic | All patients n=147 | HR−/HER2− n=25 | Any HR/HER2+ n=20 | HR+/HER2− n=102 | P-value |
|---|---|---|---|---|---|
| Age (years) | |||||
| Median | 51.0 | 49.5a | 55.4 | 50.5b | 0.04a, 0.03b |
| Range | 27–71 | 34–68 | 39–70 | 27–71 | |
| Menopause, n (%) | |||||
| Pre | 83 (57) | 13 (52) | 6 (30) | 64 (63) | 0.02 |
| Post | 64 (43) | 12 (48) | 14 (70) | 38 (37) | |
| Pretreatment tumor size, n (%) | |||||
| ≤5 cm | 90 (61) | 18 (72) | 13 (65) | 59 (58) | NS |
| >5 cm | 57 (39) | 7 (28) | 7 (35) | 43 (42) | |
| Pretreatment lymph node status, n (%) | |||||
| Negative | 84 (57) | 13 (52) | 9 (45) | 62 (61) | NS |
| Positive | 63 (43) | 12 (48) | 11 (55) | 40 (39) |
Chi-square and Fisher's exact tests; NS, not statistically significant; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
P-value between any HR/HER2+ and HR−/HER2−
P-value between any HR/HER2+ and HR+/HER2−.
Response rate to neoadjuvant chemotherapy
The clinical and pathological response rates did not differ among the regimes (data not shown). Table II lists the clinical and pathological response rates to neoadjuvant chemotherapy. A total of 132 patients (90%) showed an objective clinical response. The objective clinical response rate revealed no difference among the subtypes. A total of 26 patients (18%) achieved a pathological complete response; 10 patients (40%) with HR−/HER2− tumors and 8 patients (40%) with any HR/HER2+ tumors achieved favorable pathological complete response rates, and these rates were significantly higher compared with the response rate of patients with HR+/HER2− tumors (8%; P<0.0001).
Table II.
Responses to chemotherapy according to the breast cancer subtypes.
| Response | All patients n=147 | HR−/HER2− n=25 | Any HR/HER2+ n=20 | HR+/HER2− n=102 | P-value |
|---|---|---|---|---|---|
| Clinical response, n (%) | |||||
| Complete/partial response | 132 (90) | 22 (88) | 19 (95) | 91 (89) | NS |
| Stable disease | 15 (10) | 3 (12) | 1 (5) | 11 (11) | |
| Pathological response, n (%) | |||||
| Complete response | 26 (18) | 10 (40) | 8 (40) | 8 (8) | <0.0001 |
| Residual disease | 121 (82) | 15 (60) | 12 (60) | 94 (92) |
NS, not statistically significant; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Expression levels of topoisomerase IIα in the subtypes
Table III shows the expression levels of topoisomerase IIα among the subtypes. It was demonstrated that 88/147 tumors (60%), including 19/25 (76%) HR−/HER2− tumors, 15/20 (75%) any HR/HER2+ tumors and 54/102 (52%) HR+/HER2− tumors, overexpressed topoisomerase IIα. The frequency of topoisomerase IIα overexpression was significantly higher in any HR/HER2+ and HR−/HER2− tumors compared with in the HR+/HER2− tumors (P=0.036).
Table III.
Expression of topoisomerase IIα according to the breast cancer subtypes.
| Topoisomerase IIα expression | Overall n=127 | HR−/HER2− n=25 | Any HR/HER2+ n=20 | HR+/HER2− n=102 | P-value |
|---|---|---|---|---|---|
| Positivea | 88 (60%) | 19 (76%) | 15 (75%) | 54 (52%) | 0.036 |
| Negative | 59 (40%) | 6 (24%) | 5 (25%) | 48 (47%) |
HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Defined as >20% positive staining.
Correlation between the expression of topoisomerase IIα and the response to neoadjuvant chemotherapy among the subtypes
Table IV shows the association between the expression of topoisomerase IIα and the pathological complete response rates. It was demonstrated that 19/88 (22%) topoisomerase IIα-positive tumors and 7/59 (12%) topoisomerase IIα-negative tumors achieved a pathological complete response. Topoisomerase IIα-positive expression was associated with a favorable response. Additionally, 8/19 (42%) topoisomerase IIα-positive and 2/6 (33%) topoisomerase IIα-negative HR−/HER2− tumors achieved a pathological complete response. Furthermore, 6/15 (40%) topoisomerase IIα-positive and 2/5 (40%) topoisomerase IIα-negative any HR/HER2+ tumors, 5/54 (9%) topoisomerase IIα-positive and 3/48 (6%) topoisomerase IIα-negative HR+/HER2− tumors achieved a pathological complete response. Topoisomerase IIα-positive expression was not significantly associated with a favorable response among all subtypes.
Table IV.
Association between the expression of topoisomerase IIα and the pathological complete response rate.
| Pathological complete response rate | |||||
|---|---|---|---|---|---|
| Topoisomerase IIα expression | Overall | HR−/HER2− | Any HR/HER2+ | HR+/HER2− | P-value |
| Positive | 19/88 (22%) | 8/19 (42%) | 6/5 (40%) | 5/54 (9%) | 0.051 |
| Negative | 7/59 (12%) | 2/6 (33%) | 2/5 (40%) | 3/48 (6%) | 0.019 |
HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Association between the expression of topoisomerase IIα and survival
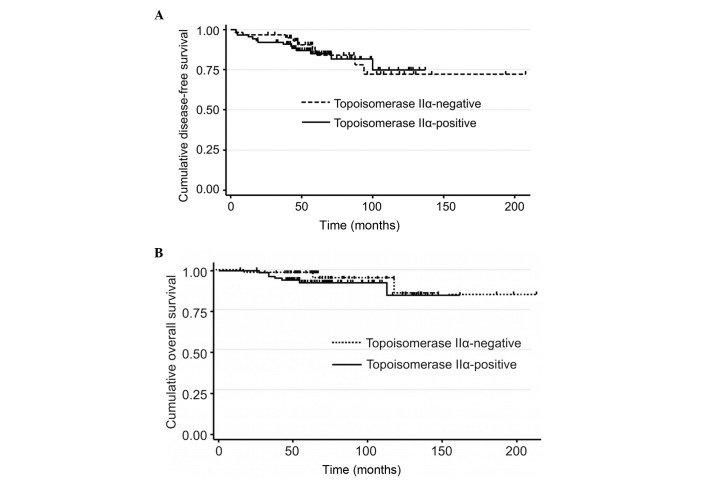
Fig. 2 shows the association between the expression of topoisomerase IIα and survival. It was revealed that 7/88 (8%) patients with topoisomerase IIα-positive and 3/59 (5%) patients with topoisomerase IIα-negative tumors succumbed to mortality, while 14/88 (16%) patients with topoisomerase IIα-positive and 9/59 (15%) patients with topoisomerase IIα-negative tumors exhibited recurrence. The expression of topoisomerase IIα was not associated with the overall and disease-free survival.
Figure 2.
(A) The disease-free survival according to topoisomerase IIα the status. (B) The overall survival according to the topoisomerase IIα status.
Discussion
The present study used IHC to evaluate the expression levels of topoisomerase IIα, HER2, ER and PgR in tumor samples obtained from the pretreatment biopsies of breast cancer patients receiving an anthracycline-containing regimen as neoadjuvant chemotherapy. This retrospective data analysis suggested that the favorable response to anthracycline-containing neoadjuvant chemotherapy among the triple-negative and HER2-positive subtypes was independent of the expression of topoisomerase IIα.
Anthracyclines, including doxorubicin and epirubicin, which is less cardiotoxic compared with doxorubicin, are extensively used for the treatment of breast cancer, and anthracycline-containing polychemotherapy regimens have reduced breast cancer mortality by ~1/3 (1). The cardiac toxicity of anthracyclines is well described and the most common form, congestive heart failure, is known to be closely associated with the cumulative dose. Although limiting the cumulative dose to ~240–360 mg/m2 doxorubicin has assisted in reducing the incidence of congestive heart failure to ~1.6–2.1%, data from long-term survivors of childhood cancer indicate that there is no true threshold for anthracycline-assoicated cardiotoxicity, and that cardiac damage may become apparent years later. However, studies from adjuvant breast cancer trials have shown that the likelihood of late cardiac effects in women who receive adjuvant anthracycline is low (2). However, since not all patients benefit from anthracyclines, a means of selecting the appropriate patients for the treatment is clearly of great interest. Anthracyclines have three major mechanisms of action: i) Inhibition of DNA and RNA synthesis by intercalating between the base pairs of the DNA/RNA strand; ii) enhancement of catalysis of oxidation-reduction reactions and iii) inhibition of topoisomerase IIα (9). Notably, the first mechanism also appears to be dependent on the inhibition of topoisomerase IIα for cytotoxicity.
Topoisomerase IIα is the only enzyme able to cleave and relegate double-stranded DNA. This enzyme acts during the relaxation of DNA supercoils, which accumulate during gene transcription and along with the progression of the replication fork. In addition, only topoisomerase IIα can perform the decatenation of replicated circular double-stranded DNA, and it is obligatorily involved in the remodeling of chromatin during mitosis. There are two highly homologous isoforms of topoisomerase II in humans, which are encoded by different genes. The gene for topoisomerase IIα is located on chromosome 17q21–22, while the gene for topoisomerase IIβ is located on chromosome 3q24 (10,23).
Drugs that interfere with topoisomerase IIα include anthracyclines (doxorubicin and epirubicin), etoposide, teniposide and amsacrine. These agents act by binding covalently with topoisomerase IIα following the occurrence of double-strand breaks, inducing lethal cellular damage by inhibition of relegation. An increase in the expression of topoisomerase IIα is associated with the sensitivity to these agents as a result of the increased substrate on which the drug may act.
Gene expression profiling has identified distinct breast cancer molecular subtypes (15,16) and previous studies have shown that triple-negative breast cancer is associated with an improved pathological complete response rate compared with the other subtypes (17–19). Nevertheless, the predictive role of topoisomerase IIα in each subtype remains to be elucidated. By contrast, HER2 amplification and overexpression have been reported as predictive markers of the benefit of anthracycline treatment in the adjuvant setting (14). Because of its location in the identical amplicon on chromosome 17, the gene encoding topoisomerase IIα (TOP2A) is frequently co-amplified with that of HER2 (24,25), which in turn leads to the overexpression of its protein product and possibly, to a greater sensitivity to anthracyclines (25–27). In 2011, Di Leo et al (14) performed a meta-analysis, in which they identified that HER2 amplification and TOP2A amplification and deletion may have certain value in the prediction of responsiveness to anthracycline-containing chemotherapy. However, non-HER2 amplified and non-TOP2A altered tumor types also appear to derive benefits from treatment with anthracyclines. Furthermore, in their meta-analysis, triple-negative breast cancer and moderately hormone-sensitive tumor types appeared to exhibit and improved response to anthracycline treatment compared with treatment with the cyclophosphamide, methotrexate and fluorouracil regimen. Therefore, a differential benefit from anthracyclines may exist within these subtypes. Since all triple-negative tumors, and ~90% of moderately hormone-sensitive tumors, from that previous study revealed no TOP2A gene amplification, other mechanisms of increased anthracycline sensitivity may exist. Du et al (13) suggested that topoisomerase IIα is a predictive factor for breast cancer patients who received anthracycline-containing neoadjuvant chemotherapy using fluorescence in situ hybridization in another meta-analysis. However, the authors could not detect an association between the expression of topoisomerase IIα and sensitivity to anthracycline-containing regimens using IHC, which is similar to the results of the present study.
Notably, the target of anthracycline is the topoisomerase IIα protein as opposed to the gene, and it is known that there is a lack of correlation between gene status and protein expression (28–30). Proliferation signals can lead to overexpression of the topoisomerase IIα protein independently of the TOP2 gene status (29,31). In normal cells, the expression of topoisomerase IIα is regulated according to the cell cycle. In proliferating cells, topoisomerase IIα becomes detectable in the late G1 phase, and the quantity gradually increases, peaking in G2/M. By contrast, increased expression of topoisomerase IIα is commonly observed in malignant tumors, irrespective of the cell cycle stage (10).
There are certain limitations to the present study. Triple-negative, moderately hormone-sensitive and HER2-positive tumors are characterized by high proliferation (15,32,33) and this data further confirmed that topoisomerase IIα overexpression was more frequently observed among the triple-negative and HER2-positive subtypes. However, ideally, quantification of nuclear concentrations of topoisomerase IIα protein may be a more appropriate way to investigate its predictive value as opposed to IHC alone. Furthermore, the present study included breast cancer patients treated with anthracycline combinations, as well as other drugs. Therefore, the use of these other drugs, including taxanes, cyclophosphamide and fluorouracil, may have influenced the activity of topoisomerase IIα and obscured any existing association.
In conclusion, the present findings do not justify the routine use of immunohistochemical staining of topoisomeras IIα as a predictive marker of the response to anthracycline-containing regimens. Women with triple-negative and HER2-positive tumors appear to derive benefits from anthracycline-containing chemotherapy independently of the expression of topoisomerase IIα.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG): Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gianni L, Norton L, Wolmark N, Suter TM, Bonadonna G, Hortobagyi GN. Role of anthracyclines in the treatment of early breast cancer. J Clin Oncol. 2009;27:4798–4808. doi: 10.1200/JCO.2008.21.4791. [DOI] [PubMed] [Google Scholar]
- 3.Ellis P, Smith I, Ashley S, Walsh G, Ebbs S, Baum M, Sacks N, McKinna J. Clinical prognostic and predictive factors for primary chemotherapy in operable breast cancer. J Clin Oncol. 1998;16:107–114. doi: 10.1200/JCO.1998.16.1.107. [DOI] [PubMed] [Google Scholar]
- 4.Bonadonna G, Valagussa P, Brambilla C, Ferrari L. Preoperative chemotherapy in operable breast cancer. Lancet. 1993;341:1485. doi: 10.1016/0140-6736(93)90933-8. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 6.Smith IE, Walsh G, Jones A, Prendiville J, Johnston S, Gusterson B, Ramage F, Robertshaw H, Sacks N, Ebbs S, et al. High complete remission rates with primary neoadjuvant infusional chemotherapy for large early breast cancer. J Clin Oncol. 1995;13:424–429. doi: 10.1200/JCO.1995.13.2.424. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, Howell A, Costa SD, Beuzeboc P, Untch M, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: Review and recommendations. J Clin Oncol. 2003;21:2600–2608. doi: 10.1200/JCO.2003.01.136. [DOI] [PubMed] [Google Scholar]
- 8.Chang J, Powles TJ, Allred DC, Ashley SE, Clark GM, Makris A, Assersohn L, Gregory RK, Osborne CK, Dowsett M. Biologic markers as predictors of clinical outcome from systemic therapy for primary operable breast cancer. J Clin Oncol. 1999;17:3058–3063. doi: 10.1200/JCO.1999.17.10.3058. [DOI] [PubMed] [Google Scholar]
- 9.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 10.Kellner U, Sehested M, Jensen PB, Gieseler F, Rudolph P. Culprit and victim - DNA topoisomerase II. Lancet Oncol. 2002;3:235–243. doi: 10.1016/S1470-2045(02)00715-5. [DOI] [PubMed] [Google Scholar]
- 11.Fry AM, Chresta CM, Davies SM, Walker MC, Harris AL, Hartley JA, Masters JR, Hickson ID. Relationship between topoisomerase II level and chemosensitivity in human tumor cell lines. Cancer Res. 1991;51:6592–6595. [PubMed] [Google Scholar]
- 12.Di Leo A, Gancberg D, Larsimont D, Tanner M, Jarvinen T, Rouas G, Dolci S, Leroy JY, Paesmans M, Isola J, et al. HER-2 amplification and topoisomerase II alpha gene aberrations as predictive markers in node-positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate, and 5-fluorouracil. Clin Cancer Res. 2002;8:1107–1116. [PubMed] [Google Scholar]
- 13.Du Y, Zhou Q, Yin W, Zhou L, Di G, Shen Z, Shao Z, Lu J. The role of topoisomerase IIα in predicting sensitivity to anthracyclines in breast cancer patients: A meta-analysis of published literatures. Breast Cancer Res Treat. 2011;129:839–848. doi: 10.1007/s10549-011-1694-9. [DOI] [PubMed] [Google Scholar]
- 14.Di Leo A, Desmedt C, Bartlett JM, Piette F, Ejlertsen B, Pritchard KI, Larsimont D, Poole C, Isola J, Earl H, et al. HER2/TOP2A Meta-analysis Study Group: HER2 and TOP2A as predictive markers for anthracycline-containing chemotherapy regimens as adjuvant treatment of breast cancer: A meta-analysis of individual patient data. Lancet Oncol. 2011;12:1134–1142. doi: 10.1016/S1470-2045(11)70231-5. [DOI] [PubMed] [Google Scholar]
- 15.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 16.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 18.Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 19.Nogi H, Kobayashi T, Suzuki M, Tabei I, Kawase K, Toriumi Y, Fukushima H, Uchida K. EGFR as paradoxical predictor of chemosensitivity and outcome among triple-negative breast cancer. Oncol Rep. 2009;21:413–417. [PubMed] [Google Scholar]
- 20.Sørlie T, Perou CM, Fan C, Geisler S, Aas T, Nobel A, Anker G, Akslen LA, Botstein D, Børresen-Dale AL, et al. Gene expression profiles do not consistently predict the clinical treatment response in locally advanced breast cancer. Mol Cancer Ther. 2006;5:2914–2918. doi: 10.1158/1535-7163.MCT-06-0126. [DOI] [PubMed] [Google Scholar]
- 21.Martin M, Romero A, Cheang MC, López García-Asenjo JA, García-Saenz JA, Oliva B, Román JM, He X, Casado A, de la Torre J, et al. Genomic predictors of response to doxorubicin versus docetaxel in primary breast cancer. Breast Cancer Res Treat. 2011;128:127–136. doi: 10.1007/s10549-011-1461-y. [DOI] [PubMed] [Google Scholar]
- 22.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology: College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 23.Berger JM, Gamblin SJ, Harrison SC, Wang JC. Structure and mechanism of DNA topoisomerase II. Nature. 1996;379:225–232. doi: 10.1038/379225a0. [DOI] [PubMed] [Google Scholar]
- 24.Smith K, Houlbrook S, Greenall M, Carmichael J, Harris AL. Topoisomerase II alpha co-amplification with erbB2 in human primary breast cancer and breast cancer cell lines: Relationship to m-AMSA and mitoxantrone sensitivity. Oncogene. 1993;8:933–938. [PubMed] [Google Scholar]
- 25.Järvinen TA, Tanner M, Rantanen V, Bärlund M, Borg A, Grénman S, Isola J. Amplification and deletion of topoisomerase IIalpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol. 2000;156:839–847. doi: 10.1016/S0002-9440(10)64952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arriola E, Moreno A, Varela M, Serra JM, Falo C, Benito E, Escobedo AP. Predictive value of HER-2 and Topoisomerase II alpha in response to primary doxorubicin in breast cancer. Eur J Cancer. 2006;42:2954–2960. doi: 10.1016/j.ejca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Arriola E, Rodriguez-Pinilla SM, Lambros MB, Jones RL, James M, Savage K, Smith IE, Dowsett M, Reis-Filho JS. Topoisomerase II alpha amplification may predict benefit from adjuvant anthracyclines in HER2 positive early breast cancer. Breast Cancer Res Treat. 2007;106:181–189. doi: 10.1007/s10549-006-9492-5. [DOI] [PubMed] [Google Scholar]
- 28.Coon JS, Marcus E, Gupta-Burt S, Seelig S, Jacobson K, Chen S, Renta V, Fronda G, Preisler HD. Amplification and overexpression of topoisomerase IIalpha predict response to anthracycline-based therapy in locally advanced breast cancer. Clin Cancer Res. 2002;8:1061–1067. [PubMed] [Google Scholar]
- 29.Durbecq V, Desmed C, Paesmans M, Cardoso F, Di Leo A, Mano M, Rouas G, Leroy JY, Sotiriou C, Piccart M, et al. Correlation between topoisomerase-IIalpha gene amplification and protein expression in HER-2 amplified breast cancer. Int J Oncol. 2004;25:1473–1479. doi: 10.3892/ijo.25.5.1473. [DOI] [PubMed] [Google Scholar]
- 30.Mueller RE, Parkes RK, Andrulis I, O'Malley FP. Amplification of the TOP2A gene does not predict high levels of topoisomerase II alpha protein in human breast tumor samples. Genes Chromosomes Cancer. 2004;39:288–297. doi: 10.1002/gcc.20008. [DOI] [PubMed] [Google Scholar]
- 31.Campiglio M, Somenzi G, Olgiati C, Beretta G, Balsari A, Zaffaroni N, Valagussa P, Ménard S. Role of proliferation in HER2 status predicted response to doxorubicin. Int J Cancer. 2003;105:568–573. doi: 10.1002/ijc.11113. [DOI] [PubMed] [Google Scholar]
- 32.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al. Ki67 index, HER2 status and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]