Abstract
Objective
To evaluate long term changes in cholesterol levels in early rheumatoid arthritis (RA) patients randomized to initiate methotrexate (MTX) monotherapy, methotrexate + etanercept (MTX+ETA), or triple therapy (TT) [MTX + sulfasalazine (SSZ) + hydroxychloroquine (HCQ)] in the Treatment of Early Aggressive Rheumatoid Arthritis (TEAR) trial.
Methods
Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels were analyzed in 416 patients participating in the TEAR trial over 102 weeks of follow-up. Associations of cholesterol changes with disease activity and drug treatment were evaluated using repeated measures analysis with mixed effect linear models to model the within-subject covariance over time.
Results
Mixed effect models controlling for traditional CV risk factors, TEAR treatment, and baseline prednisone and statin use demonstrated significant inverse associations of RA disease activity with changes in cholesterol over time. Decreases in DAS28, CRP, or ESR were associated with increases in HDL-C, LDL-C, and TC in all treatment groups (p values <0.001–0.035). TT was strongly associated with higher HDL-C and lower LDL-C and TC/HDL-C ratios (p values <0.001) compared to MTX monotherapy and MTX + ETA over two year follow-up.
Conclusion
Decreases in RA disease activity over long term follow-up was associated with increases in cholesterol in early RA patients treated with either biologic or non-biologic therapies. The use of TT over two year follow-up was associated with higher HDL-C and lower LDL-C and TC/HDL-C ratios compared to MTX monotherapy or MTX + ETA combination therapy.
Keywords: rheumatoid arthritis, etanercept, methotrexate, hydroxychloroquine, cholesterol, lipoprotein, cardiovascular
Patients with rheumatoid arthritis (RA) suffer significantly increased cardiovascular (CV) morbidity and mortality when compared to the general population (1–3). Both traditional CV risk factors such as dyslipidemia as well as RA disease activity contribute to this increased CV risk (4;5).
High levels of systemic inflammation have been associated with the suppression of circulating cholesterol levels in disease states such as endotoxemia as well as RA (6). Previous studies have suggested that cholesterol levels may increase after RA treatment; however, a wide range of cholesterol changes has been reported by different studies with different drug treatments, study designs, durations, and patient populations (7–10). Few studies have been able to analyze long-term follow up of a large group of early RA patients randomized to biologic and non-biologic disease modifying therapies. It remains unclear whether serum cholesterol increases over time are related solely to specific drug mechanisms, suppression of inflammation, or a combination of these and other patient factors.
The Treatment of Early Aggressive Rheumatoid Arthritis (TEAR) trial enrolled 755 patients with very early, active RA in which 90% of patients had disease duration less than one year and 75% of patients had received no disease modifying anti-rheumatic drug (DMARD) therapy prior to study entry. These patients were randomized to methotrexate (MTX) monotherapy versus MTX combination therapy with etanercept (MTX+ETA) or triple therapy (TT) including MTX, sulfasalazine (SSZ), and hydroxychloroquine (HCQ) and followed for two years (11).
We previously reported that marked increases in total, low-density lipoprotein (LDL), and high density lipoprotein (HDL) cholesterol occurred after 6 months of treatment in the TEAR trial (12). We now describe the cholesterol changes over two years of follow-up with specific evaluation of relationships to disease activity, systemic inflammation, and the treatments received.
Patients and Methods
Study Design
The TEAR trial was a two year randomized clinical trial of 755 early RA patients with minimal prior DMARD use who were initially randomized to MTX monotherapy, MTX combination therapy with ETA, or TT(11). After 6 months, participants receiving MTX monotherapy who did not achieve low disease activity (DAS28(ESR) < 3.2) were “stepped up” to either MTX + ETA combination therapy or TT as determined by a baseline randomization algorithm. All treatment arms included matching placebos. No further changes in treatment assignment were allowed after 24 weeks of the study.
All patients met the 1987 American College of Rheumatology (ACR) criteria for RA and were autoantibody (rheumatoid factor or anti-citrullinated protein antibody) positive or had evidence of erosive disease on baseline radiographs of the hands and feet. Low dose prednisone (≤ 10 mg/day) was allowed, but had to be stable for at least 2 weeks prior to screening. Full results of the clinical trial have been previously published (11). Patients provided consent for participation in the TEAR trial and separately, for the biorepository study. Characteristics of the participants electing to participate in the TEAR study biorepository were similar to those in the main TEAR trial(12)(11) (data not shown).
Laboratory Testing
Non-fasting serum samples from a total of 416 patients participating in the TEAR biorepository study who had long term biorepository samples and data available through 48 weeks were used to measure total cholesterol (TC), HDL-C, LDL-C, and triglycerides (TG) at 0, 24, 48, and 102 weeks using a Beckman-Coulter with AU400 automated chemistry and Beckman-Coulter reagents (Brea, CA, USA). The coefficients of variation for TC and HDL-C were 2.5% and 1% within run and between run (day-to-day) 3.5% and 2.9% as previously reported (12). LDL-C was calculated according to the Friedwald formula(13) and samples in which TG were > 400 mg/dL were excluded. The Clinical and Epidemiological Research Laboratory at Children’s Hospital in Boston measured C-reactive protein (CRP) levels in mg/L using a high-sensitivity immunoturbidimetric assay on a Hitachi 917 autoanalyzer (Roche Diagnostics, Indianapolis, IN), with the use of reagents and calibrators from Denka Seiken (Tokyo, Japan). Erythrocyte sedimentation rates (ESR), 28 tender and swollen joint counts, and patient/physician global assessments were assessed locally at each site and the disease activity scores using a 28 joint count (DAS28(ESR)) calculated.
Statistical analysis
The subgroup of 416 patients participating in the biorepository study who had long term biorepository samples and data available through 48 weeks of the study were included in the analyses. Baseline clinical characteristics between treatment groups were compared using one-way ANOVA for continuous variables and Chi-Square test for categorical variables. Paired t test was used to evaluate changes in cholesterol between two different time points within each treatment group. Mean cholesterol changes in tertiles of change in DAS28, CRP, and ESR were compared by linear trend analysis. Specifically, orthogonal polynomial contrasts were created and linear trend was tested when fitting a generalized linear model. To determine the relative contribution of RA treatments, RA disease activity/systemic inflammation, and other patient characteristics to changes in cholesterol over time, repeated measures analysis with linear mixed effect models (14) was used to model the within-subject covariance over time. Measures of disease activity/inflammation and cholesterol at four time points were included in the models as fixed effects and separate models were constructed for each cholesterol outcome (TC, LDL-C, or HDL-C) and each measure of disease activity/inflammation (ESR, CRP, or DAS28). Other fixed-effect patient covariates included treatment assignment at each time point, age, sex, race, RA disease duration, baseline body mass index (BMI), smoking status, statin use, prednisone use, diabetes, and presence of cardiovascular disease. All statistical testing was two-sided with 0.05 alpha level threshold for declaring significance. Statistical analyses were carried out using SAS version 9.3 (SAS Institute Inc. 2012).
RESULTS
Demographic, Laboratory, and Clinical Characteristics
The baseline clinical characteristics of the TEAR patients participating in the bio-repository subsstudy included in the analyses are shown in Table 1. The population studied was very similar in demographics to the main TEAR trial population (n=755) and to the total substudy population with data and serum samples available for the 24 week cholesterol analysis (n= 459) (12)(11). Patients were categorized by their final treatment assignment at 48 weeks, after any change in therapy at 24 weeks (Table 1). Patients receiving MTX monotherapy were slightly younger than patients receiving MTX + ETA, however, no significant differences were observed in important demographic and clinical variables including sex, race, body mass index (BMI), and smoking status. Over 86% of patients in each group were rheumatoid factor positive with the mean disease duration less than six months in all groups. Patients had very active arthritis at baseline with mean DAS28 scores in all treatment groups above 5.4. The baseline presence of co-morbidities including diabetes and known cardiovascular disease was similar across groups as was the use of prednisone and statins. No differences in baseline TC, LDL-C, HDL-C, and TC/HDL-C ratios were observed.
Table 1.
Baseline Characteristics of Patients Receiving MTX + Etanercept, Triple Therapy, or MTX Monotherapy at 48 weeks.
| MTX + ETA N=245* |
Triple Therapy N=119** |
MTX Monotherapy N=52*** |
p-value | |
|---|---|---|---|---|
| Age (years) | 50.87 ± 12.58 | 48.66 ± 11.98 | 45.98 ± 11.70 | 0.02 |
| BMI | 29.87 ± 7.05 | 30.28 ± 8.83 | 29.55 ± 5.96 | 0.82 |
| Female | 176(71.84%) | 88(73.95%) | 35(67.31%) | 0.67 |
| RF positive | 219(89.39%) | 105(88.24%) | 45(86.54%) | 0.83 |
| Smoking (baseline) | 80(32.65%) | 35(29.41%) | 17(32.69%) | 0.81 |
| Disease Duration (mos) | 3.30 ± 5.70 | 5.13 ± 8.35 | 4.20 ± 7.65 | 0.05 |
| DAS28 (baseline) | 5.82 ± 1.07 | 5.77 ± 1.04 | 5.48 ± 1.03 | 0.11 |
| CRP (baseline) | 16.12 ± 25.43 | 14.18 ± 19.79 | 10.19 ± 12.79 | 0.23 |
| TC (baseline) | 189.26 ± 47.46 | 191.29 ± 48.33 | 188.54 ± 46.13 | 0.91 |
| HDL-C (baseline) | 54.41 ± 14.10 | 58.22 ± 19.14 | 54.83 ± 13.79 | 0.09 |
| LDL-C (baseline) | 106.78 ± 37.37 | 103.89 ± 35.62 | 104.16 ± 36.64 | 0.75 |
| TC/HDL (baseline) | 3.59 ± 0.90 | 3.42 ± 0.81 | 3.56 ± 0.88 | 0.21 |
| Race/Ethnicity | 0.47 | |||
| African American | 30(12.24%) | 9(7.56%) | 3(5.77%) | |
| Caucasian | 191(77.96%) | 99(83.19%) | 45(86.54%) | |
| Other | 24(9.80%) | 11(9.24%) | 4(7.69%) | |
| Medications | ||||
| Prednisone Use | 105(42.86%) | 49(41.18%) | 24(46.15%) | 0.83 |
| Statin Use | 31(12.65%) | 11(9.24%) | 6(11.54%) | 0.63 |
| Comorbidities | ||||
| Diabetes | 25(10.20%) | 13(10.92%) | 3(5.77%) | 0.56 |
| CV Events | 14(5.71%) | 4(3.36%) | 1(1.92%) | 0.37 |
Data shown as mean ± SD, or N (%); p-values are obtained from one-way ANOVA (for continuous variables) or Chi-Square test (for categorical variables). BMI= body mass index, RF= rheumatoid factor, DAS28 = disease activity score using a 28 joint count, CRP = C reactive protein, TC= total cholesterol, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol. Patients were classified into treatment groups according to therapy at week 48.
Of the 245 in the group on MTX + ETA at week 48, 141 were assigned that treatment initially and 104 were stepped up from MTX monotherapy to MTX + ETA at 24 weeks.
Of the 119 included in the group on TT at week 48, 70 were assigned that treatment initially and 49 were stepped up from MTX monotherapy to TT at 24 weeks.
205 patients were assigned MTX monotherapy initially, but 104 were stepped up from MTX monotherapy to MTX + ETA at 24 weeks and included in that group for this analysis, and 49 were stepped up from MTX monotherapy to TT at 24 weeks and included in that group for this analysis, leaving 52 patients in the MTX monotherapy group who did not step up to combination therapy at 24 weeks.
Changes in Cholesterol Levels over Two Years of Follow-up
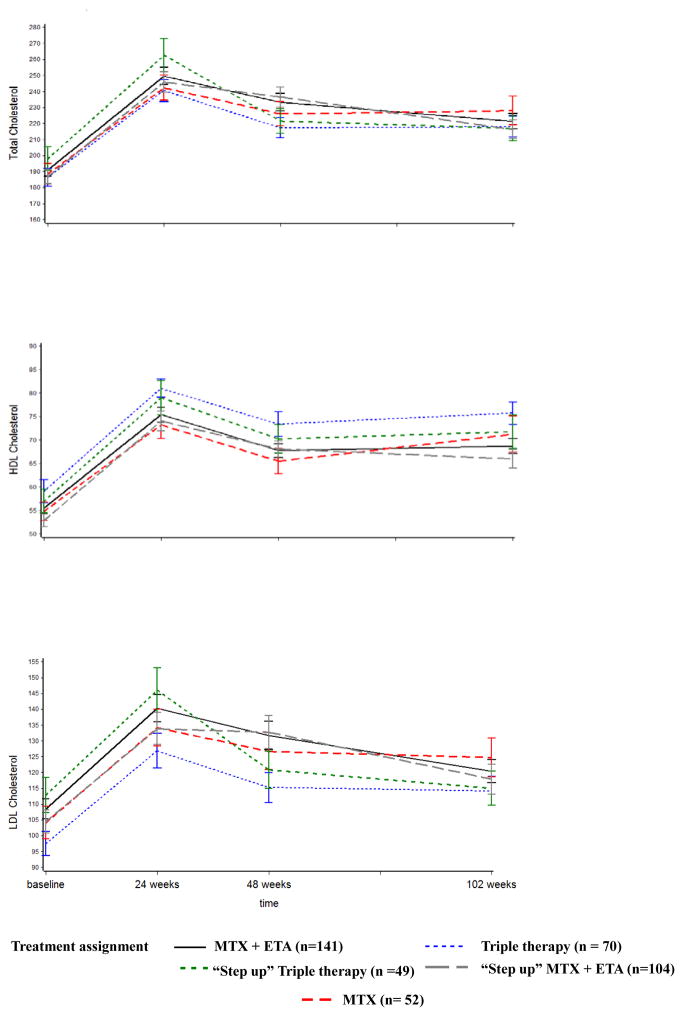
Marked increases in TC, LDL-C, and HDL-C occurred after 24 weeks of the study treatment as reported in initial work (Figure 1) (12). Compared to baseline, cholesterol levels were elevated at 24 weeks, but decreased significantly thereafter compared to the 24 week values in almost all treatment groups (Figure 1). Specifically, by the end of the 2 year follow-up (102 week time point), statistically significant decreases in TC, LDL-C, and HDL-C from 24 weeks (p<0.05 for 24 vs. 102 week comparison) were noted in all treatment groups except for the MTX monotherapy group which showed a similar, but non-significant trend.
Figure 1.
Mean ± standard error for LDL, HDL, and total cholesterol levels (mg/dL) in each treatment group over two year follow-up in the TEAR trial.
Association of Long Term Cholesterol Changes with RA Disease Activity and Systemic Inflammation
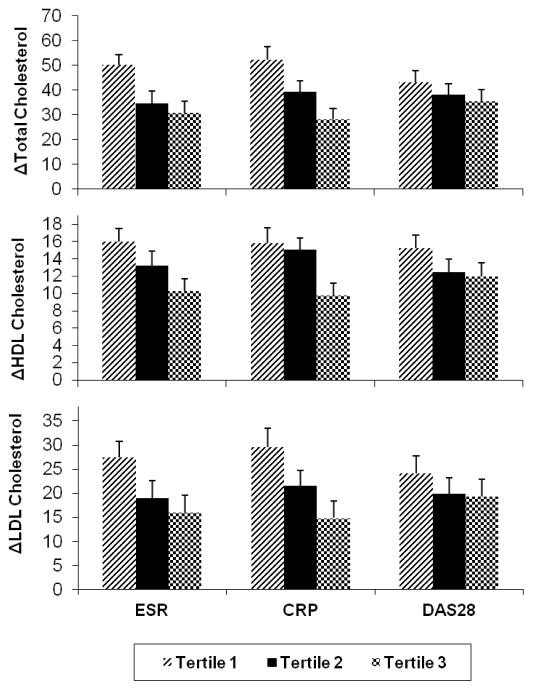
Our previous work showed an association of changes in CRP with short term cholesterol changes after 6 months of therapy (12). In the current work, similar associations were observed with all three measures of RA disease activity/inflammation over two year follow-up. Patients with the largest long term decreases in DAS28, CRP, or ESR (i.e. the greatest negative change) also had the largest increases, (i.e. greatest positive change), in TC, LDL-C, or HDL-C. Figure 2 shows mean changes in cholesterol in the three tertiles of change in DAS28/CRP/ESR at 48 weeks of follow-up. Increases in TC, LDL-C, and HDL-C were highest in tertile 1 which represents the largest mean decrease (negative change) in inflammation over the time period. Sequentially smaller cholesterol changes were noted in tertiles 2 and 3 which reflect sequentially smaller changes in inflammation/RA disease activity (Figure 2). Linear trend analysis confirmed statistically significant trends for all analyses using CRP and ESR at both 48 as well as 102 week time points (exception of LDL-C analyses at 102 weeks (p value =0.17 for ESR, p= 0.28 for CRP)). Similar trends were also noted with DAS28 (Figure 2) but did not reach statistical significance.
Figure 2.
Mean ± standard error for changes in cholesterol levels (mg/dL) in the three tertiles of change in DAS28/CRP/ESR at 48 week follow-up. Tertile 1 represents the largest mean decrease (negative change) in ESR, CRP, or DAS28 from 0 to 48 weeks followed by tertiles 2 and 3.
Association of Long Term Cholesterol Changes with Treatment Group
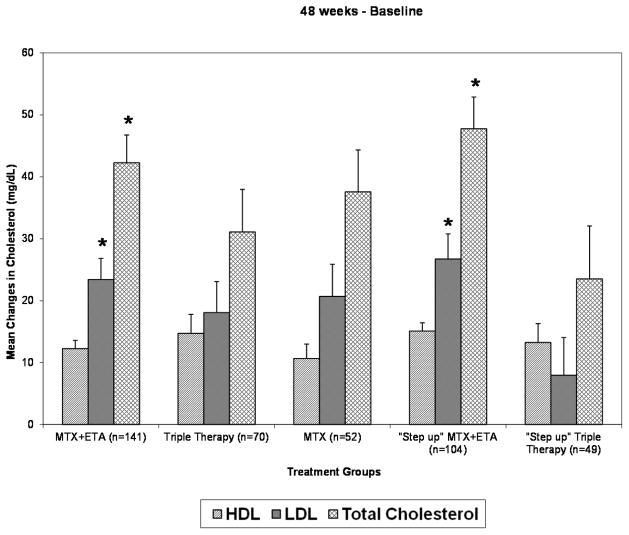
Short term changes in cholesterol at 24 weeks were not significantly different between the treatment groups as previously reported (12). However, after 24 weeks, patients receiving TT had larger decreases in TC and LDL-C compared to non-TT groups. Specifically, patients in the “step-up” TT group had larger decreases in TC and LDL-C from 24 to 48 weeks compared to MTX, MTX+ ETA, and “step-up” MTX +ETA groups (all comparison p values <0.05 except for the MTX/”step up” TT LDL-C comparison (p=0.07)). These larger decreases in TC and LDL-C after 24 weeks resulted in lower overall increases from baseline to 48 weeks in TC and LDL-C in the TT groups, which were statistically significant in the “step-up” TT group (Figure 3). Trends for larger decreases in LDL-C and TC in the TT groups over 102 weeks of follow-up were also noted, although were not as marked as the 48 week changes. Interestingly, at both 48 and 102 weeks, patients in the “step-up” TT group no longer had significant increases in mean LDL-C values from baseline. The mean LDL-C change (±SD) from baseline to 48 wks in the “step-up” TT group was 8 ± 43 mg/dL (p=0.20 for comparison 48 wks to baseline), and 2 ± 35 mg/dL, (p=0.67 for comparison 102 wks to baseline). Significant increases in both TC and HDL-C from baseline to 48 and 102 weeks were still noted in all treatment groups including the TT group.
Figure 3.
Mean ± standard error for changes in cholesterol from baseline to 48 weeks in each treatment group. Asterisks signify greater increases in TC and LDL-C at 48 weeks (p<0.05) compared to the “step-up” triple therapy (TT) group (MTX + SSZ/HCQ).
Determinants of Long Term Cholesterol Changes in the TEAR Cohort
In order to determine the relative contribution of RA disease activity, RA treatments, and other patient characteristics over time to changes in cholesterol in the TEAR cohort, repeated measures analyses with mixed effect linear models were performed. These models included patient characteristics as well as disease activity and treatment data from four time points over two years of follow-up.
In all models tested (Table 2 + supplementary data), measures of RA disease activity were consistently associated with changes in TC, LDL-C, and HDL-C. Specifically, decreases in DAS28, CRP, or ESR over time were significantly associated with increases in TC, LDL-C, and HDL-C after controlling for treatment group, age, sex, race, disease duration, baseline body mass index (BMI), smoking status, statin use, prednisone use, diabetes, and presence of cardiovascular disease. The use of triple therapy was associated with lower LDL-C levels and higher HDL-C levels over time compared to use of MTX or MTX +ETA combination therapy in all models (p values <0.05 for all models with the exception of p= 0.08 and p=0.09 for the comparison of TT with MTX monotherapy in the ESR and DAS28 LDL-C models respectively). Triple therapy was strongly and consistently associated with improvements (decreases) in the TC/HDL-C ratios in all models (all p values <0.001 compared to other therapies).
Table 2 shows results from the mixed effect linear models for TC, LDL-C, and HDL-C using CRP as the disease activity covariate. Similar results were found with substitution of either ESR or DAS28 for CRP in this model (supplementary tables 1 and 2). Decreases in CRP over time were associated with significant increases in TC, LDL-C, and HDL-C. Use of triple therapy over time was associated with lower LDL-C levels by 8.1 mg/dL compared to use of MTX + ETA therapy and lower LDL-C levels by 7.3 mg/dL compared to MTX monotherapy. TT was also associated with significantly higher HDL-C levels over time by 4.6 mg/dL compared to MTX + ETA therapy and by 4.2 mg/dL compared to MTX monotherapy. Strong associations for lower TC/HDL-C ratios with TT were noted compared to other therapies (p values <0.0001).
Table 2.
Repeated measures analysis, linear mixed effect model of variables associated with cholesterol changes over 2 year follow-up in the TEAR trial.
| TC | HDL-C | LDL-C | TC/HDL-C | |||||
|---|---|---|---|---|---|---|---|---|
| Effect | p-value | Effect | p-value | Effect | p-value | Effect | p-value | |
| CRP- per unit decrease | 0.36 | <0.001 | 0.08 | 0.001 | 0.20 | <0.001 | −0.00 | 0.626 |
| Age- per year increase | 1.02 | <0.001 | 0.23 | <0.001 | 0.64 | <0.001 | 0.00 | 0.296 |
| BMI -per unit increase | 1.00 | <0.001 | −0.33 | <0.001 | 0.84 | <0.001 | 0.03 | <0.001 |
| Treatment(Overall) | 0.382 | 0.004 | 0.049 | <0.001 | ||||
| MTX + ETA vs TT | 6.06 | 0.165 | −4.59 | 0.001 | 8.13 | 0.018 | 0.24 | <0.001 |
| MTX vs TT | 4.12 | 0.394 | −4.16 | 0.009 | 7.33 | 0.048 | 0.27 | <0.001 |
| MTX + ETA vs MTX | 1.94 | 0.639 | −0.43 | 0.752 | 0.79 | 0.800 | −0.02 | 0.710 |
| Female vs Male | 14.68 | 0.002 | 12.24 | <0.001 | 4.90 | 0.196 | −0.42 | <0.001 |
| Race(overall) | 0.005 | 0.007 | 0.289 | 0.100 | ||||
| AA vs Caucasian | −15.42 | 0.029 | −0.28 | 0.904 | −5.49 | 0.337 | −0.22 | 0.063 |
| Others vs Caucasian | −18.62 | 0.009 | −7.29 | 0.002 | −7.67 | 0.183 | 0.11 | 0.363 |
| AA vs Others | 3.20 | 0.738 | 7.01 | 0.024 | 2.18 | 0.778 | −0.32 | 0.041 |
| Baseline Smoking Yes vs No | 8.66 | 0.051 | −3.34 | 0.020 | 11.27 | 0.002 | 0.28 | <0.001 |
| Baseline Predisone Use Yes vs No | 9.71 | 0.018 | 5.55 | <0.001 | 3.89 | 0.244 | −0.13 | 0.062 |
| Baseline Statin User: CVD vs no CVD | −34.59 | 0.024 | −3.67 | 0.458 | −31.46 | 0.013 | −0.47 | 0.064 |
| Baseline No Statin: CVD vs no CVD | −14.43 | 0.285 | −5.36 | 0.220 | −10.92 | 0.319 | 0.08 | 0.720 |
| Baseline CVD : statin vs no statin | −47.14 | 0.015 | −1.56 | 0.803 | −48.41 | 0.002 | −0.70 | 0.028 |
| Baseline No CVD : statin vs no statin | −26.98 | 0.001 | −3.25 | 0.228 | −27.88 | <0.001 | −0.15 | 0.263 |
| Baseline Statin User: diabetes vs no diabetes | −16.88 | 0.180 | −1.92 | 0.638 | −12.03 | 0.246 | −0.18 | 0.383 |
| Baseline No Statin: diabetes vs no diabetes | −13.41 | 0.117 | −3.00 | 0.278 | −15.93 | 0.023 | 0.09 | 0.534 |
| Baseline diabetes : statin vs no statin | −30.45 | 0.024 | −2.16 | 0.620 | −23.97 | 0.030 | −0.42 | 0.058 |
| Baseline No diabetes : statin vs no statin | −26.98 | 0.001 | −3.25 | 0.228 | −27.88 | <0.001 | −0.15 | 0.263 |
Change in TC, HDL-C, LDL-C, and TC/HDL-C are shown per categorical variable or per unit change in continuous variables as specified. Only significant associations besides time are shown in the table. CRP= C reactive protein, BMI= body mass index, MTX = methotrexate, ETA= etanercept, TT= triple therapy, AA= African American, CVD = cardiovascular disease.
Several patient characteristics were also associated with cholesterol changes over time in the mixed linear effect models (Table 2/supplementary data). Older age was associated with higher TC, HDL-C, and LDL-C over time in all models. Age was not associated with significant changes in TC/HDL-C ratio. Higher BMI and smoking were both associated with higher TC, LDL-C, and TC/HDL-C ratios and lower HDL-C in all models (all p values <0.05; p =0.05 for associations of smoking with TC in CRP and DAS28 models). Female sex was associated with both higher TC and HDL-C levels over time but lower TC/HDL-C ratios in all models. Caucasian race/ethnicity was associated with higher TC compared to African American (AA) race/ethnicity or “other” race/ethnicity in all models (all p values <0.05 except for comparison of Caucasian with AA in ESR model (p=0.08)). Caucasian and AA races were also associated with higher HDL-C levels compared to the “other” (non-Caucasian/AA) race category although the numbers of patients in the AA and “other” category were relatively small. No effect of disease duration was noted on cholesterol levels in any of the models.
Baseline prednisone use was associated with significantly higher TC and HDL-C levels over time. Statin use was examined in detail with specific evaluation of potential interactions with CVD and diabetes given the known guidelines for use of statins in these high risk populations. As expected, patients with or without CVD on statins had significantly lower TC and LDL-C levels over time compared to patients not on statin therapy (Table 2). Interestingly, patients with known CVD on statins had significantly lower TC and LDL-C compared to patients without CVD on statins. These data suggest more intensive statin therapy in the CVD group as recommended by standard guidelines. No differences in cholesterol levels over time were noted between patients not on statins with or without CVD. Patients with diabetes on statins also had significantly lower TC and LDL-C levels over time compared to diabetic patients not on statin therapy (Table 2).
DISCUSSION
The current study is the first randomized, long term placebo-controlled trial to describe changes in cholesterol in a large, early RA population randomized to both biologic therapy with etanercept and non-biologic therapies including triple therapy. Decreases in RA disease activity were consistently associated with increases in cholesterol (TC, LDL-C, and HDL-C) over time in all analyses using DAS28, ESR, or CRP as the measure of RA disease activity. Triple therapy was also strongly associated with decreases in the TC/HDL-C ratio over two year follow-up compared to other therapies after controlling for RA disease activity/inflammation. A main strength of the current study is the robust repeated measures analyses which incorporated data from 4 different time points over 2 years of follow-up including both RA treatments as well as measures of RA disease activity.
Smaller RA studies have previously suggested a potential association between changes in RA disease activity/systemic inflammation and changes in serum cholesterol (15;16). Boers et al. reported in the COBRA trial that changes in TC were best predicted by changes in ESR with treatment (15). Most of the larger studies to date on lipid changes in RA patients have been observational in nature and none of the large randomized controlled trials other than TEAR have included both a biologic DMARD therapy as well as non-biologic therapies including hydroxychloroquine. It has been hypothesized that agents which are more effective in suppressing inflammation may have greater effects on cholesterol levels, particularly during the early phases of therapy when the suppression of inflammation is most marked (17). The current work supports this hypothesis, showing marked increases in cholesterol in the short term, (6 months of treatment), in patients with very active, previously untreated RA, which then decreased with time.
The marked increases in cholesterol at 6 months were not maintained for the duration of the two year trial. Decreases in lipids occurred after 6 months, particularly by 48 weeks, with lesser change thereafter to 102 weeks. This data is interesting in its potential implications for the timing of lipid-lowering therapies in these patients, however, it should be noted that this was a very early RA population (mean disease duration < 6 months), with no significant previous DMARD therapy, predominantly seropositive, and these results may not be generalized to all RA patients. A much smaller study of early RA patients treated with infliximab by Dahlqvist et al. also reported initial increases in HDL-C and TC which decreased over long term follow-up (8). Mean cholesterol levels remained significantly elevated at the end of the TEAR study compared to baseline values for all groups with the exception of LDL-C levels in the “step-up” TT group. Of note, the current report does not describe the function of lipoproteins which may be of significant relevance to CV risk in RA patients beyond cholesterol levels (18;19).
In a recent systemic review and meta-analysis, Souto et al. reported that moderate changes in lipids were observed in patients treated with tocilizumab or tofacitinib but not with TNF antagonists (20). This work included 25 randomized controlled trials, however, did not specifically select patients with early RA. One might hypothesize that the marked cholesterol increases with TNF inhibition, (as well as non-biologic DMARD therapy), in the very early TEAR population, (less than 6 months of disease/no prior DMARD therapy), represents the course of treating a more “acute” RA inflammatory response rather than a “chronic” RA inflammatory response in patients of longer disease duration and DMARD exposure. Further increases in lipids were not observed in the “step-up” TEAR treatment groups who changed therapy due to persistent disease activity, (DAS28 ≥3.2), after 24 weeks of DMARD therapy, perhaps in part related to the same mechanisms. Hudgins et al. described marked changes in cholesterol and phospholipid levels in healthy volunteers injected with endotoxin to induce an acute inflammatory reaction which then normalized with resolution of the inflammation (6).
A favorable effect of triple therapy on lipid profiles over 2 years of follow-up was noted in the work which was very consistent in all repeated measures models, (CRP, ESR, DAS28). Over time, triple therapy was associated with lower LDL-C and TC/HDL-C ratios as well as higher HDL-C compared to MTX monotherapy and MTX combination therapy with etanercept. This data supports previous observational studies which have suggested potentially favorable effects of hydroxychloroquine (HCQ) on lipid profiles in RA patients (21;22). Morris et al reported that HCQ use (compared to non-use) was associated with an average LDL-C decrease of 7.55 mg/dl (p<0.001) in a 706 RA patient cohort extracted from electronic medical records. The magnitude of this decrease in LDL-C is similar to the magnitude of the difference in LDL-C noted with TT compared to MTX monotherapy or MTX + ETA combination therapy (7.3 mg/dL and 8.1 mg/dL respectively) in the current work. Trends for increases in HDL-C with HCQ have also been noted in previous observational studies but did not reach statistical significance (21;23). Less data is available regarding the effects of sulfasalazine therapy on lipid levels (15) although it is possible that this therapy may also have influenced the changes noted with TT. In the current work, TT was consistently associated with increases in HDL-C over time as well as decreases in the TC/HDL-C ratio. The TC/HDL-C ratio has previously been described as potentially the most useful predictor of CVD in patients with RA (9).
Further mechanistic studies may be warranted to understand the effects of HCQ/TT on cholesterol and lipoprotein metabolism in RA patients. Chloroquine has previously been reported to inhibit hepatic cholesterol synthesis in animal studies of isolated rat hepatocytes (24), however, data regarding HCQ-specific effects on HDL-C as well as LDL-C metabolism is lacking. In addition, whether the long term use of HCQ/TT is associated with lower CV risk in RA patients due to these small but significant effects on cholesterol is unknown and warrants further investigation.
Several patient characteristics were significantly associated with cholesterol changes over long term follow-up in the TEAR cohort. Many of these patient characteristics such as smoking, age, sex and BMI showed very similar effects on cholesterol levels as previously reported in the general population (25–27). For example, both BMI and smoking use were strongly associated with higher TC, LDL-C, and TC/HDL-C ratios, and lower HDL-C levels over time. This data parallels work in the Framingham study in which cigarette smoking was strongly associated with “atherogenic” lipoprotein cholesterol profiles in young adults, particularly women (26). Obesity has also previously been associated with adverse effects on cholesterol profiles in non-RA patients (25). This data reinforces the importance of both RA specific and non-specific patient factors to the cholesterol changes which occur over long term follow-up.
As reported previously, there were few cardiovascular events during the two year TEAR trial, with 3 deaths due to cardiac disorders (general [unattended death], coronary heart failure, and ventricular septal defect) (11). A CV adjudication process was not used during the study. While reliable correlations between changes in lipid levels and CV events could not be made for these reasons, further prospective CV outcome studies are warranted. Two large, phase 4 clinical trials are currently ongoing to evaluate the effects of lipid changes on CV events in RA patients treated with either tocilizumab or tofacitinib as compared to etanercept and adalimumab respectively (Clinical Trials.gov NCT01331837, NCT02092467).
There are some limitations to the current work. As discussed above, the study describes a very early RA patient population with high disease activity at baseline, primarily seropositive, and naïve to prior DMARDs; these results may not be generalized to RA patients with more established disease or with lower levels of disease activity. In addition, data on prednisone and statin use were only available at the baseline visit for the TEAR patients. It is possible that changes in these medications over two year follow-up could have affected cholesterol changes in the study. However, it is unlikely that these effects would be of significant magnitude to alter the strong and consistent relationships between RA disease activity, treatment group, and cholesterol levels described in the multiple models tested. In addition, the use of both medications at baseline was associated with the expected relationships to total cholesterol (statin-decrease, prednisone-increase) (15). Finally, additional medications including non-statin cholesterol-lowering medications and supplements which could have affected cholesterol levels were not available for the current analyses. The serum samples were also collected in a nonfasting state. While the lack of fasting is a potential limitation, there is also data which suggests that fasting time has little association with lipid levels (e.g. variation in LDL of only as high as 10%) (28) and that fasting lipid assessments are not required for optimum CVD risk stratification (29). Lastly, the frequency and intensity of cardiovascular exercise as a potential confounder for lipid changes was not assessed in this study.
In summary, while research regarding the increased CV morbidity and mortality in RA patients has burgeoned in recent years, a need for better understanding of the effects of widely used DMARDs on CV risk factors and CV risk in RA patients remains. The current work describes the long-term effects of disease activity, DMARD use, and patient characteristics on cholesterol levels in a large clinical trial of early RA patients, demonstrating conclusively two major points: 1) suppression of RA-related inflammation is associated with increases in cholesterol, which are highest in the early phase of therapy, regardless of treatment and 2) use of triple therapy is associated with decreases in the TC/HDL-C ratio over long term follow-up. The effect of these cholesterol changes on CV events in RA patients warrants further study.
Supplementary Material
Acknowledgments
Funding: Amgen provided funding for the TEAR trial but was not responsible for data collection or analysis; UAB, UCLA, and co-authors, are solely responsible for all data collection, management, and statistical analysis. The TEAR bio-repository was funded by the NIH R01 AR052658. Dr. Charles-Schoeman received support from the NHLBI (5K23HL094834, R01HL123064) and NIAMS (R21 AR 057913-01A1). Dr. Wang received support from NCRR (1UL1RR033176). Dr. Curtis received support from the NIH (AR053351) and the Agency for Healthcare Research and Quality (R01HS018517). This ancillary study was funded by the Arthritis Foundation through a grant to JRC and by NIAMS (R21 AR 057913-01A1) to CCS.
References
- 1.Boers M, Dijkmans B, Gabriel S, Maradit-Kremers H, O’Dell J, Pincus T. Making an impact on mortality in rheumatoid arthritis: targeting cardiovascular comorbidity. Arthritis Rheum. 2004;50(6):1734–9. doi: 10.1002/art.20306. [DOI] [PubMed] [Google Scholar]
- 2.Van DS, McColl G, Wicks IP. Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum. 2002;46(4):862–73. doi: 10.1002/art.10089. [DOI] [PubMed] [Google Scholar]
- 3.Roman MJ, Moeller E, Davis A, Paget SA, Crow MK, Lockshin MD, et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med. 2006;144(4):249–56. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- 4.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 5.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52(3):722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 6.Hudgins LC, Parker TS, Levine DM, Gordon BR, Saal SD, Jiang XC, et al. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J Lipid Res. 2003;44(8):1489–98. doi: 10.1194/jlr.M200440-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Pollono EN, Lopez-Olivo MA, Lopez JA, Suarez-Almazor ME. A systematic review of the effect of TNF-alpha antagonists on lipid profiles in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29(9):947–55. doi: 10.1007/s10067-010-1405-7. [DOI] [PubMed] [Google Scholar]
- 8.Dahlqvist SR, Engstrand S, Berglin E, Johnson O. Conversion towards an atherogenic lipid profile in rheumatoid arthritis patients during long-term infliximab therapy. Scand J Rheumatol. 2006;35(2):107–11. doi: 10.1080/03009740500474578. [DOI] [PubMed] [Google Scholar]
- 9.Robertson J, Peters MJ, McInnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol. 2013;9(9):513–23. doi: 10.1038/nrrheum.2013.91. [DOI] [PubMed] [Google Scholar]
- 10.Kirkham BW, Wasko MC, Hsia EC, Fleischmann RM, Genovese MC, Matteson EL, et al. Effects of golimumab, an anti-tumour necrosis factor-alpha human monoclonal antibody, on lipids and markers of inflammation. Ann Rheum Dis. 2014;73(1):161–9. doi: 10.1136/annrheumdis-2012-202089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreland LW, O’Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012;64(9):2824–35. doi: 10.1002/art.34498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro-Millan I, Charles-Schoeman C, Yang S, Bathon JM, Bridges SL, Jr, Chen L, et al. Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum. 2013;65(6):1430–8. doi: 10.1002/art.37916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 14.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–74. [PubMed] [Google Scholar]
- 15.Boers M, Nurmohamed MT, Doelman CJ, Lard LR, Verhoeven AC, Voskuyl AE, et al. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62(9):842–5. doi: 10.1136/ard.62.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamnitski A, Visman IM, Peters MJ, Dijkmans BA, Voskuyl AE, Nurmohamed MT. Beneficial effect of 1-year etanercept treatment on the lipid profile in responding patients with rheumatoid arthritis: the ETRA study. Ann Rheum Dis. 2010;69(11):1929–33. doi: 10.1136/ard.2009.127597. [DOI] [PubMed] [Google Scholar]
- 17.Steiner G, Urowitz MB. Lipid profiles in patients with rheumatoid arthritis: mechanisms and the impact of treatment. Semin Arthritis Rheum. 2009;38(5):372–81. doi: 10.1016/j.semarthrit.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Charles-Schoeman C, Watanabe J, Lee YY, Furst DE, Amjadi S, Elashoff D, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 2009;60(10):2870–9. doi: 10.1002/art.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charles-Schoeman C, Lee YY, Shahbazian A, Gorn A, Fitzgerald J, Ranganath V, et al. Association of Paraoxonase 1 Gene Polymorphisms and Enzyme Activity with Carotid Plaque in Rheumatoid Arthritis. Arthritis Rheum. 2013 Nov;65(11):2765–72. doi: 10.1002/art.38118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souto A, Salgado E, Maneiro JR, Mera A, Carmona L, Gomez-Reino JJ. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol. 2015;67(1):117–27. doi: 10.1002/art.38894. [DOI] [PubMed] [Google Scholar]
- 21.Morris SJ, Wasko MC, Antohe JL, Sartorius JA, Kirchner HL, Dancea S, et al. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2011;63(4):530–4. doi: 10.1002/acr.20393. [DOI] [PubMed] [Google Scholar]
- 22.Kerr G, Aujero M, Richards J, Sayles H, Davis L, Cannon G, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken) 2014;66(11):1619–26. doi: 10.1002/acr.22341. [DOI] [PubMed] [Google Scholar]
- 23.Munro R, Morrison E, McDonald AG, Hunter JA, Madhok R, Capell HA. Effect of disease modifying agents on the lipid profiles of patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56(6):374–7. doi: 10.1136/ard.56.6.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beynen AC, van der Molen AJ, Geelen MJ. Inhibition of hepatic cholesterol biosynthesis by chloroquine. Lipids. 1981;16(6):472–4. doi: 10.1007/BF02535017. [DOI] [PubMed] [Google Scholar]
- 25.Garrison RJ, Wilson PW, Castelli WP, Feinleib M, Kannel WB, McNamara PM. Obesity and lipoprotein cholesterol in the Framingham offspring study. Metabolism. 1980;29(11):1053–60. doi: 10.1016/0026-0495(80)90216-4. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PW, Garrison RJ, Abbott RD, Castelli WP. Factors associated with lipoprotein cholesterol levels. The Framingham study Arteriosclerosis. 1983;3(3):273–81. doi: 10.1161/01.atv.3.3.273. [DOI] [PubMed] [Google Scholar]
- 27.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–6. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 28.Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med. 2012;172(22):1707–10. doi: 10.1001/archinternmed.2012.3708. [DOI] [PubMed] [Google Scholar]
- 29.Eberly LE, Stamler J, Neaton JD. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163(9):1077–83. doi: 10.1001/archinte.163.9.1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.