Abstract
Rationale
Preference for and reaction to novelty are strongly associated with addiction to cocaine and other drugs. However, the genetic variants and molecular mechanisms underlying these phenomena remain largely unknown. Although the relationship between novelty- and addiction-related traits has been observed in rats, studies in mice have failed to demonstrate this association. New, genetically diverse, high-precision mouse populations including Diversity Outbred (DO) mice provide an opportunity to assess an expanded range of behavioral variation enabling detection of associations of novelty- and addiction-related traits in mice.
Methods
To examine the relationship between novelty- and addiction-related traits, male and female DO mice were tested on open field exploration, hole board exploration, and novelty preference followed by intravenous cocaine self-administration (IVSA; ten 2-hour sessions of fixed-ratio 1 and one 6-hour session of progressive ratio).
Results
We observed high variation of cocaine IVSA in DO mice with 43% reaching and 57% not reaching conventional acquisition criteria. As a group, mice that did not reach these criteria still demonstrated significant lever discrimination. Mice experiencing catheter occlusion or other technical issues (n = 17) were excluded from analysis. Novelty-related behaviors were positively associated with cocaine IVSA. Multivariate analysis of associations among novelty- and addiction-related traits revealed a large degree of shared variance (45%).
Conclusions
Covariation among cocaine IVSA and novelty-related phenotypes in DO mice indicates that this relationship is amenable to genetic dissection. The high genetic precision and phenotypic diversity in the DO may facilitate discovery of previously undetectable mechanisms underlying predisposition to develop addiction disorders.
Keywords: addiction, behavior, cocaine, genetics, novelty, anxiety, self-administration, Diversity Outbred, Diversity Outcross
INTRODUCTION
Personality traits such as impulsivity, reaction to novelty, and preference for novelty predispose individuals to initiate or progress from controlled to compulsive psychostimulant use. Although exposure to psychostimulants may itself affect novelty related traits in humans (Ersche et al. 2010), rats exhibiting elevated impulsivity (Belin et al. 2008; Molander et al. 2011), elevated preference for novelty (Belin et al. 2011), elevated reactivity to a novel environment (Piazza et al. 1990), or reduced anxiety (Bush and Vaccarino 2007; cf. Dilleen et al. 2012), are more likely to initiate or develop compulsive patterns of psychostimulant use (reviewed in Bardo et al. 2013; Belin and Deroche-Gamonet 2012; Dalley et al. 2011; Flagel et al. 2014).
The relationship between predisposing and addiction-related traits in experimental animals suggests the existence of common underlying mechanisms. In support of this hypothesis, Cervantes et al. (2013) have recently provided evidence which suggests a genetic relationship between impulsivity and cocaine self-administration in BXD recombinant inbred mice. However, the genetic underpinnings of the relationship between novelty- and addiction-related traits remain largely unexplored. Despite this, these traits are heritable in humans (Goldman et al. 2005) and mice (Kliethermes and Crabbe 2006; Ruiz-Durantez et al. 2006) making them amenable to genetic dissection. This is particularly true for cocaine addiction as it has the highest heritability of all abused drugs (h = 0.72; Goldman et al. 2005).
Past studies examining interrelationships between novelty- and addiction-related traits have been conducted using mouse and rat populations with limited genetic diversity and precision. This limits interpretability of these studies for two reasons. First, the low allelic diversity in these populations contributes to limited behavioral variation, a characteristic that has been suggested as the reason for prior failures to observe relationships between novelty- and addiction-related traits in mice (Kliethermes et al. 2007). Second, due to widespread linkage of loci across the genome in these populations (Payseur and Place 2007; Petkov et al. 2005), it is possible that some previously observed relationships between novelty- and addiction-related traits in rats are not the result of pleiotropic actions of the same polymorphic loci, but instead reflect parallel effects due to genetic linkage.
Recently developed mouse populations including the Collaborative Cross inbred strains (CC) (Aylor et al. 2011; Chesler et al. 2008; Churchill et al. 2004; Collaborative Cross Consortium 2012; Philip et al. 2011) and the Diversity Outbred mouse population (DO) (Churchill et al. 2012; Logan et al. 2013; Svenson et al. 2012) were designed to overcome these limitations through high allelic diversity and recombination precision. These populations were derived from an intercross of eight mouse strains consisting of five commonly used strains derived from the earliest laboratory strains (A/J, C57BL/6J, 129S1/SvImJ, NOD/LtJ, NZO/HILtJ) and three wild derived strains (CAST/EiJ, PWK/PhJ, and WSB/EiJ) (Chesler et al. 2008). Both the DO and CC offer advantages over commonly used experimental populations such as (1) substantially increased genetic diversity compared to classical laboratory mouse strains (Yang et al. 2011), (2) high behavioral diversity (Philip et al. 2011, Logan et al. 2013), (3) high precision quantitative trait locus (QTL) mapping of behaviors (Philip et al. 2011, Logan et al. 2013), and (4) reduced linkage disequilibrium enabling dissociation of relationships caused by true pleiotropic effects from those secondary to genetic linkage. Moreover, DO mice are outbred, providing a tremendous source of novel allelic combinations, the potential for high sample size mapping studies, and restoration of the broad and continuous range of behavioral phenotypes which were constrained in the derivation of common mouse resources (for review, Chesler 2014). This expanded range of variation enables detection of variation and covariation not typically observed in laboratory mice.
In the present study, we assessed the relationship between intravenous cocaine self-administration (IVSA) and several novelty-related behaviors (activity and center time in a novel open field, exploration of a hole board, and novelty preference) in male (n = 51) and female (n = 47) DO mice. We chose to examine multiple novelty-related behaviors because they likely represent phenotypically distinct and genetically independent constructs (Kliethermes and Crabbe 2006). The added diversity in the DO, combined with the high construct validity of the cocaine IVSA paradigm, provides an opportunity to discover interrelationships between novelty- and addiction-related traits which have been undetected in previous mouse genetic studies.
MATERIALS AND METHODS
Subjects
The DO population was derived from 144 CC lines obtained from Oak Ridge National Laboratory (Chesler 2008) at generations F4 - F12 of inbreeding (Churchill et al. 2012). DO mice (J:DO, JAX stock number 009376) from G10 - G12 were obtained from the mouse production facility at The Jackson Laboratory (JAX) at 4 weeks of age and transferred to the housing and phenotyping facility via standard JAX shipping container. Mice were housed in same sex groups of 3 – 5 in duplex polycarbonate cages on ventilated racks which provided 99.997% HEPA filtered air to each cage. The lid of each cage was fitted with a filtered top which reduced cross-cage odor exposure when mice were removed from the ventilated racks for testing.
Mice were maintained in a climate-controlled room under a standard 12:12 light-dark cycle (lights on at 0600 h). Bedding was changed weekly and mice were provided free access to food (NIH31 5K52 chow, LabDiet/PMI Nutrition, St. Louis, MO) and acidified water. A Nestlet and Shepherd Shack were provided in each cage for enrichment. All procedures and protocols were approved by The Jackson Laboratory Animal Care and Use Committee and were conducted in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Behavioral Testing
DO mice (N = 98) were assessed for exploration in an open field, novelty preference in a divided open field, and exploration in a hole board apparatus (Kliethermes and Crabbe 2006). Increased time spent in the center of an open field is commonly considered to reflect reduced levels of anxiety in mice, but the motivation to explore a novel environment is dependent on neophobia of that environment (Bailey and Crawley 2009; Crawley 1985). Therefore, time spent in the center of the open field during novel open field testing was used as an additional novelty-related measure.
Following assessment of novelty-related behaviors, mice were implanted with a jugular catheter and tested on a cocaine IVSA task (1 mg/kg/infusion) using a fixed-ratio 1 (FR1) schedule. Following 10 sessions of testing using these criteria, mice were tested for a single session on a progressive ratio (PR) schedule to assess motivation to take cocaine. Following behavioral testing, interrelationships between IVSA and novelty-related measures were assessed.
Novelty-related behavioral testing
Open field testing occurred on day 1, novelty preference testing occurred on day 2, and hole board exploration testing occurred on day 3. Mice were between 12 – 16 weeks of age on the first day of testing and were habituated to the testing room for at least 48 hours prior to testing. All pieces of apparatus were cleaned using 70% ethanol between each mouse. Novelty testing was conducted between 10 AM and 2 PM.
Apparatus
All behavioral tests were performed using eight VersaMax Animal Activity Monitors (AccuScan Instruments, Inc; Columbus, OH). Each square arena (39 × 39 × 39 cm) was constructed of clear acrylic. Infrared detectors enabling automated data collection were positioned around the bottom edges of the arena walls. Arenas were housed in a rectangular room (3 × 4.5 meters) and illumination in each apparatus was maintained at 150 – 200 lux. Open field activity was collected in an empty arena. An insert which separated each arena into two equally-sized rectangular-halves (one with clear walls, the other with black walls) was used for novelty preference testing. The two sides were connected by a small opening in the central partition. A separate insert consisting of 16 holes positioned in a 4 × 4 grid was used for hole board exploration testing. The open field apparatus was in the same location when assessing open field activity, hole board exploration, and novelty preference.
Open field activity
Mice were placed into the center of the empty arena and allowed to explore for 20 minutes. Total distance traveled, time in center, and additional measures were recorded. We chose to use a relatively short 20 minute session because the environment becomes less novel over time and our goal was to assess novelty reactivity.
Novelty preference
A partition was placed into the arena to create two equally spaced halves. Mice were placed into one side of the two-sided arena (the familiar side) and allowed to explore for 10 minutes. The wall color of the familiar side (black or clear) was randomized for each mouse. During this initial phase, access to the other side of the chamber (the novel side) was blocked. Immediately following this initial 10-minute familiarization phase, mice were allowed access to both the familiar and novel sides of the arena for 10 minutes. The time spent in each side of the arena during this 10-minute testing phase was recorded.
Hole board exploration
The hole board insert was placed into the arena. Mice were placed into the center of the arena and allowed to explore for 10 minutes. The total number of exploratory hole pokes was recorded.
Intravenous cocaine self-administration
Catheterization surgery
Following testing on novelty-related paradigms, an indwelling catheter was implanted into the right external jugular vein under oxygen/isoflurane anesthesia using procedures described in detail by Thomsen and Caine (2007) and Kmiotek et al. (2012). Briefly, the catheter was inserted 12 mm into the jugular vein and anchored with sutures. The catheter traveled subcutaneously to an incision at the mid scapular region and attached to an external vascular access harness (Instech Laboratories, Inc., Plymouth Meeting, PA).
Apparatus
IVSA data were collected using 16 Med Associates operant conditioning chambers (307W) enclosed in sound attenuating cubicles (ENV-022MD). Two retractable response levers (ENV-310W) were mounted on the front wall. A stimulus light (ENV-321W) was mounted above each lever. A house light (ENV-315W) was centrally mounted on the rear wall. A 25 gauge single-channel stainless steel swivel was mounted to a counterbalanced lever arm attached to the outside of the chamber. Tubing was used to connect a syringe mounted on the infusion pump to the swivel, and to connect the swivel to a vascular access harness. Operant conditioning chambers were controlled by a Med Associates control unit using MED-PC IV software.
Acquisition of intravenous cocaine self-administration
Following three days of post-surgical recovery, mice were tested for 5 consecutive days on a cocaine IVSA paradigm using an FR1 schedule at a dose of 1.0 mg/kg/infusion. Infusion rate was 11.76 μl per second and infusion duration was 2 seconds. Each day mice were tested for two 2-hour sessions separated by a 2-hour timeout resulting in a total of 10 testing sessions. IVSA testing began at 9 AM each day. During the entire IVSA study, mice were continuously housed in the operant conditioning chambers with free access to food and water. To verify and maintain patency, mice were briefly removed from the chamber once daily at which point blood was aspirated and catheters were flushed with 25 μl of a heparin lock solution (100 U/ml heparin/saline). Mice were dropped from the study if catheters became non-patent.
Each 2-hour session began with the extension of the two response levers. A left (active) lever press resulted in a cocaine infusion and the illumination of both stimulus lights for two seconds with house light off. This was followed by a twenty-second time-out during which the house light was off and lever presses were recorded but had no consequences. The following dependent variables were collected during each 120 min cocaine IVSA session: (1) number of infusions, (2) number of responses on the active lever, and (3) number of responses on the inactive lever. All mice were tested for 10 sessions.
Progressive ratio
Following the acquisition stage of cocaine IVSA testing, all mice were tested on a PR schedule for a single session to assess motivation to take cocaine. The schedule was identical to the FR1 schedule used during the acquisition stage with the exception that, following an earned cocaine infusion, the number of active lever presses required to receive a subsequent cocaine infusion was increased within the session as follows: 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, etc. The number of responses required for each infusion was determined using the following equation: [5e(infusion number × 0.2)] − 5 (Richardson and Roberts 1996). Breakpoint was reached when 60 min had elapsed without earning a cocaine infusion and corresponded to the last completed step (i.e., the total number of infusions earned during the session). If breakpoint was not reached within 6 hours the session was terminated.
Drugs
Cocaine hydrochloride was obtained from the NIDA Drug Supply Program. Non-sterile cocaine solution was filtered through 0.22 μm syringe filters. Heparinized saline was purchased from Fisher Scientific (Pittsburgh, PA).
Mortality and attrition of experimental subjects
Although 98 mice were tested on the three novelty-related behavioural tasks, hole board exploration data were unavailable for 6 mice and novel place-preference data were unavailable for 3 mice because of equipment failure. Open field data were unavailable for 1 mouse and novel place-preference data were unavailable for 1 mouse because these mice escaped from the apparatus during testing.
Following testing on novelty-related paradigms, 87 mice were successfully catheterized. Out of these mice, 17 mice did not complete cocaine IVSA testing for the following reasons: non-patent catheter (n = 7), systemic infection (n = 5), local infection at the catheter port (n = 3), cocaine overdose (n = 1), air embolism (n = 1). These mice were not included in analyses involving cocaine IVSA measures. Mice from cohort 1 were tested on a different version of the PR schedule than those from subsequent cohorts, and were therefore excluded from the PR analysis. Performance distributions of mice that completed novelty testing but did not complete cocaine IVSA testing are provided in Supplementary Figure 1. Performance of these two groups did not differ significantly on any of the novelty-related assays (p > .21 for all tests).
Statistical analysis
Prior to performing inferential statistics, normality and linearity of all measures were assessed by inspecting normal probability plots and scatterplots. Z-scores and Mahalanobis distance scores were examined to identify univariate and multivariate outliers, respectively. Z-scores in excess of the absolute value of 3.29 (outlier at p < .001, two-tailed test) and extreme Mahalanobis distance scores (outlier at p < .001, χ2 value) were considered significant outliers. Data which violated statistical assumptions were, depending on the severity of the violation, square root or log base 10 transformed using the methods recommended by Tabachnick and Fidell (2007). One-way and factorial mixed-design analysis of variance (ANOVA) was used to assess group differences on novelty-related and cocaine IVSA measures. Pearson product-moment correlations and canonical correlation (Sherry and Henson 2005; Tabachnick and Fidell 2007) were used to assess interrelationships between novelty-related and cocaine IVSA measures. When repeated-measures ANOVA was used, the assumption of homogeneity of variance across groups was assessed using Mauchly’s test of sphericity. When this assumption was violated, the Huynh–Feldt correction was used.
RESULTS
Outlier detection
Univariate outliers were identified for several measures including active lever presses (z = 3.67), inactive lever presses (z = 5.23), time in the center of the open field (z = 3.42), and exploratory hole pokes (z = 4.27). Notably, the elevated z-scores in these four mice reflect statistically significant abnormal behaviour on these measures as opposed to equipment failure or some other artifact related to testing. In isogenic populations, these aberrant values typically reflect environmentally driven events or other experimental noise. In a high diversity population such as the DO, outliers are to be expected due to the presence of rare combinations of alleles. With high sample sizes, the genetic basis of systematic outlier behaviour can be uncovered, highlighting a key advantage of utilizing an outbred population with high genetic and phenotypic diversity such as the DO.
Novelty preference
Prior to examining the relationships between novelty- and addiction-related behaviors, we examined performance on the novelty preference task to determine if mice exhibited a preference for the novel side during the choice stage and if this preference was affected by side color. We performed a 2 × 2 × 2 mixed-design ANOVA with percentage of time on a side as the dependent variable, color of the novel side (black or clear) and sex as between-subjects factors, and side-novelty (novel or familiar) as a within-subjects factors. Repeated measures ANOVA revealed a statistically significant two-way interaction of side-novelty and color of the novel side [F (1, 91) = 16.80, p = 9.00 × 10−5] as well as a significant main effect of color of the novel side [F (1, 91) = 22.75, p = 6.98 × 10−6]. Post hoc tests indicated that mice spent significantly more time on the novel side when walls were black (M = 58.87%, SD = 15.03) than when walls were clear (M = 44.89, SD = 17.50). When collapsing across color of the novel side, mice as a group demonstrated a small but non-significant preference for the novel side (M = 51.96, SD = 17.67) over the familiar side (M = 48.04, SD = 17.67) [F (1, 91) = 1.25, p = .26].
Interrelationships between novelty-related measures
To assess relationships among the four novelty-related measures, we performed Pearson product-moment correlations. As shown in Table 1, all dependent measures were positively correlated. With the exception of novelty preference, all of these relationships reached statistical significance. Additionally, total distance traveled in the three novelty-related tasks was positively associated. Specifically, total distance traveled in the open field was positively correlated with total distance traveled in the novelty preference task (r = .45, p < .001) and the hole board exploration task (r = .53, p < .001). In order to statistically correct for the significant effect of novel side color on novelty preference scores, the novelty preference measure was regressed on color of the novel side to create a residual. This residual was used as the novelty preference measure in this and all subsequent analyses.
Table 1.
Pearson product-moment correlations of novelty-related and addiction-related dependent measures
| Dependent measures | Novelty-related
|
Addiction-related
|
||||||
|---|---|---|---|---|---|---|---|---|
| Distance traveled OF (cm) | Hole pokes | Novelty preference (%) | Time in center OF (s) | Infusions FR1 | Active presses FR1 | Inactive presses FR1 | Breakpoint PR | |
| Novelty | ||||||||
| Distance traveled OF (cm) | - | - | - | - | - | - | - | - |
| Hole pokes | .273** | - | - | - | - | - | - | - |
| Novelty preference (%) | .156 | .200 | - | - | - | - | - | - |
| Time in center OF (s) | .547** | .316** | .157 | - | - | - | - | - |
| Addiction | ||||||||
| Infusions FR1 | .037 | .257* | −.227 | .192 | - | - | - | - |
| Active presses FR1 | .090 | .220 | −.225 | .298* | .916** | - | - | - |
| Inactive presses FR1 | .144 | .181 | −.103 | .146 | .260* | .394** | - | - |
| Breakpoint PR | .117 | .203 | −.002 | .190 | .231 | .262* | .086 | - |
Notes. Degrees of freedom for correlations between two novelty-related variables were 88 – 95. Degrees of freedom for correlations between novelty- and addiction-related variabes were 54 – 67. Degrees of freedom for correlations between two addiction-related variables were 68 if both variables were from the FR1 schedule and 59 if the correlation contained PR breakpoint.
p < .01
p < .05
Sex differences on novelty-related measures
To assess the effect of sex on performance during novelty-related tasks, we performed one-way ANOVAs with sex as the independent factor and each of the novelty-related measures as dependent variables. As shown in Table 2, mean values for all novelty-related measures were higher in female mice. These differences reached statistical significance on the hole pokes measure [F (1, 90) = 11.94, p = 8.37 × 10−4] and approached statistical significance on the total distance traveled in the open field measure [F (1, 95) = 3.42, p = 6.71 × 10−2].
Table 2.
Means and standard deviations of novelty-related behaviors in male and female Diversity Outbred mice.
| Dependent Measures | Males | Females |
|---|---|---|
| Distance traveled OF (cm) | 2125.18 (1037.97) | 2516.28 (1041.53) |
| Hole pokes** | 26.13 (11.17) | 36.91 (18.20) |
| Novelty preference (%) | 48.95 (17.50) | 55.16 (17.47) |
| Time in center OF (s) | 78.02 (71.82) | 92.01 (73.08) |
p < .01
Intravenous cocaine self-administration in DO mice
Acquisition
Out of the 70 DO mice that completed the 1 cocaine IVSA testing sessions, 18 males and 12 females met and 20 males and 20 females did not meet conventional acquisition criteria (i.e., >= 10 infusions and >= 75% on the active lever for 3 consecutive sessions). As would be expected in a phenotypically diverse population such as the DO, we observed substantial variability in both active and inactive lever pressing across all mice. All 70 mice earned cocaine infusions during the course of testing. The range of infusions summed across the 10 sessions was 4 to 236, respectively (M = 113.21, SD = 63.96).
To assess performance in mice meeting acquisition criteria, we performed a 10 × 2 × 2 mixed-design ANOVA with number of lever presses as the dependent variable, session (1 – 10) and side (active or inactive) as within-subjects factors, and sex as a between-subjects factor. ANOVA revealed significant main effects of session [F (9, 252) = 3.65, p = 1.35 × 10−3] and lever [F (1, 28) = 160.75, p = 4.02 × 10−13] as well as a significant session x lever interaction [F (9, 252) = 2.50, p = 2.79 × 10−2]. Sex differences did not reach statistical significance. Post hoc tests were examined to determine the nature of the significant session x lever interaction. As shown in Figure 2A, mice that met acquisition criteria began to discriminate between the active and inactive levers on the first session (p = 1.45 × 10−4) and continued to do so for all subsequent sessions. The percentage of responses on the active lever increased across the first five sessions and remained stable at 80 – 90% for all subsequent sessions.
Figure 2.
Intravenous cocaine self-administration in Diversity Outbred mice. (A) As a group, male (n = 18) and female (n = 12) mice that reached conventional acquisition criteria began to discriminate between the active and inactive levers on the first session (p = 1.45 × 10−4) and continued to do so for all subsequent sessions. (B) As a group, male (n = 20) and female (n = 20) mice that did not reach conventional acquisition criteria also showed significant lever discrimination on most days.
Although it is common to categorize mice as having acquired or not acquired cocaine IVSA based on a set of criteria including number of infusions and percentage of responses on the active relative to the inactive lever (Thomsen and Caine 2007), these acquisition criteria are somewhat arbitrary and may obscure the detection of genetic effects on variation in acquisition related traits. It is likely that some, though not all mice which fail to reach these criteria are responding for cocaine, albeit to a somewhat lesser degree. To examine this possibility, we assessed IVSA performance in the group of 40 DO mice that did not to reach acquisition criteria. Repeated measures ANOVA revealed significant main effects of session [F (9, 342) = 5.63, p = 1.98 × 10−5] and lever [F (1, 38) = 33.89, p = 1.00 × 10−6]. As shown in Figure 2B, post hoc tests indicated that, as a group, mice that did not reach formal acquisition criteria individually nevertheless showed a statistically significant preference for the active lever (p < .05) on all sessions with the exception of session 8.
Progressive ratio
Following 10 sessions of testing on the FR1 schedule, all mice were tested for a single session on the PR schedule. Breakpoints ranged between 0 and 19 in male mice (M = 5.28, SD = 4.93) and 0 and 16 in female mice (M = 5.68, SD = 5.27). There was no significant effect of sex on breakpoint. Mice that met acquisition criteria reached a higher breakpoint (M = 6.85, SD = 5.43) than mice that did not meet acquisition criteria (M = 5.51, SD = 5.16), although this difference did not reach statistical significance. As shown in Figure 3D and 3H, the range of breakpoints was the same for both groups. As shown in Table 1, breakpoint was significantly positively correlated with active lever presses on the FR1 schedule. However, the strength of this relationship was modest (r = .26), supporting the hypothesis that progressive and fixed ratio schedules measure distinct constructs (Richardson and Roberts 1996).
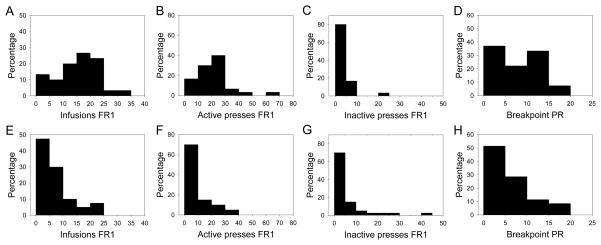
Figure 3.
Performance distributions of mice that reached (A – D) and did not reach (E – F) conventional intravenous cocaine self-administration acquisition criteria. Relative frequency (percentage) is plotted against mean performance on the last session of the final two days of testing for each of the measures.
Relationships between novelty- and addiction-related behaviors
Bivariate relationships
Although we tested mice in two separate sessions each day to maximize training during the five day acquisition period, we used the mean of the last two days of cocaine IVSA testing when assessing the relationship between novelty-related measures and IVSA FR1 measures. We specifically considered the second 2-hour session on each of these days in order to minimize inclusion of (1) variability due to the initial loading phase which occurs during cocaine IVSA (Ahmed and Koob 2005; Lynch and Carroll 2001; Panlilio et al. 2003; Tornatzky and Miczek 2000), and (2) responding for non-drug stimuli such as stimulus lights which occurs most robustly early during exposure (Olsen and Winder 2009; compare first hour responses of 6-hour group presented in Fig 4B with total session responses presented in Fig 4A of this paper). In the present study, these effects are evidenced by significantly higher active lever responding on the first relative to the second testing session on all testing days with the exception of the first, F (1, 69) = 22.12, p = 1.30 × 10−5.
Figure 4.
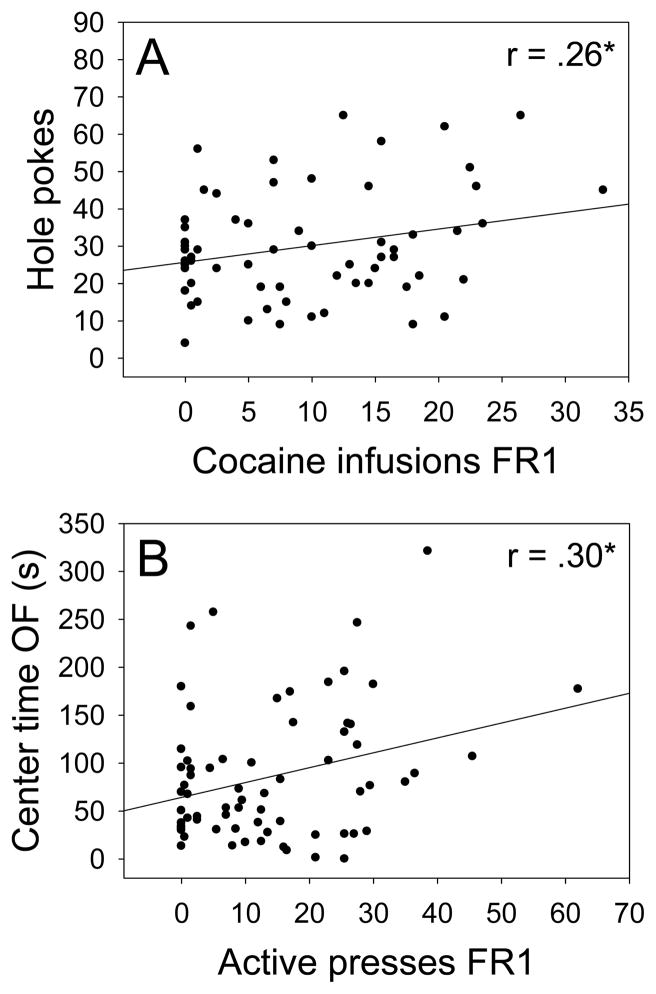
Bivariate relationships between novelty-related variables and cocaine IVSA. (A) Number of exploratory hole pokes and (B) center time in the open field were significantly positively correlated (p < .05) with number of cocaine infusions and active lever presses, respectively. The highest novelty and IVSA correlations are shown. Cocaine infusions and active lever presses were highly positively correlated (Table 1). *p < .05
To estimate trait covariation in a highly diverse population, it is important to avoid range restriction. Therefore, all mice that completed cocaine IVSA testing were included in correlational analyses, regardless of whether they met conventional acquisition criteria. This also served to maintain sufficient power to detect correlations. Distributions of mean performance on four measures obtained during the last session of the final two days of IVSA are shown in Figure 3, separated by attainment of acquisition criteria. Overall, there were significantly fewer self-infusions [F (1, 68) = 26.03, p = 3.00 × 10−6] and active lever presses [F (1, 68) = 17.17, p = 9.60 × 10−5] performed by mice that did not reach acquisition criteria, as expected by definition. The number of inactive lever presses did not differ significantly between the two groups, although six mice that did not reach acquisition criteria responded on the inactive lever more than ten times, while only one mouse that reached acquisition criteria exhibited this behavior.
To assess the bivariate relationships between novelty-related behaviors and cocaine IVSA, we performed Pearson product-moment correlations using each of the novelty- and addiction-related measures. As shown in Fig 4A, the number of exploratory hole pokes was significantly positively correlated with the number of cocaine infusions. Also, as shown in Figure 4B, time in the center of the open field was significantly positively correlated with the number of active lever presses. Bivariate relationships of all novelty- and addiction-related measures are shown in Table 1.
Multivariate relationships
A canonical correlation analysis (Sherry and Henson 2005; Tabachnick and Fidell 2007) was performed to assess the multivariate shared relationship between the two novelty- and addiction-related variable sets. The set of novelty-related variables consisted of distance traveled in the open field, time in the center of the open field, novelty preference, and hole board exploration. The set of addiction-related variables consisted of infusions on the FR1 schedule, breakpoint, and IVSA acquisition (i.e., met or did not meet IVSA acquisition criteria). Both breakpoint and FR1 infusions were included in the analysis because these measures index independent facets of drug use (Richardson and Roberts 1996), evidenced by the modest strength of their association in the present study (r = .23). To avoid high multicollinearity, active lever presses on the FR1 schedule was excluded from the analysis because it was highly correlated with FR1 infusions (r = .925, p = 3.80 × 10−23). All other within-set Pearson product-moment correlation coefficients were ≤ .54. Only mice with scores on all 6 dependent measures were included in the analysis, resulting in an overall sample size of N = 53. To meet assumptions of normality, a square root transformation was applied to center time in the open field. Following this transformation, no univariate or multivariate outliers were identified.
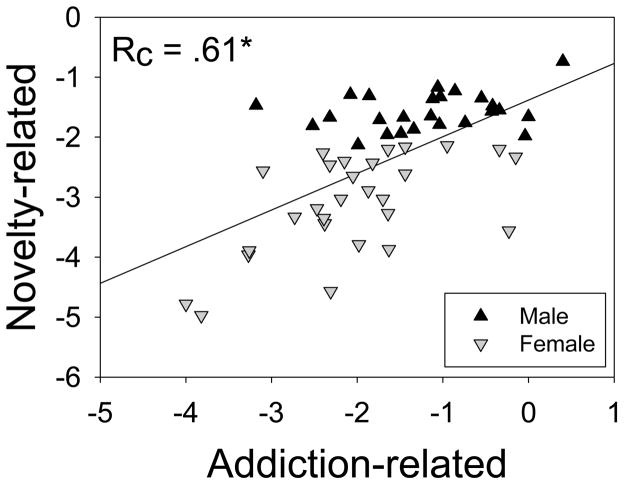
The analysis yielded four canonical correlations which had magnitudes of .61 (37% overlapping variance), .32 (11% overlapping variance), .14 (2% overlapping variance), and < .01 (< 1% overlapping variance). When all four canonical functions were included, the full model was statistically significant [F (16, 138.11) = 1.86, p = 2.80 × 10−2, Wilks λ = .5495] and explained 45% of the shared variance between the variable sets. Canonical functions 2 to 4, 3 to 4, and 4 alone did not explain a statistically significant amount of shared variance between the variable sets (p > .70 on all tests). Therefore, as shown in Figure 5, the first set of canonical variates was sufficient to account for the significant relationships between the novelty- and addiction-related variable sets.
Figure 5.
The multivariate shared relationship between the sets of novelty- and addiction-related variables. Novelty-related variables comprise distance traveled in the open field (cm), novelty preference, hole board exploration, and time in the center of the open field (s). Addiction-related variables comprise infusions on the FR1 schedule, breakpoint, IVSA acquisition, and sex. *p < .05
The standardized canonical function coefficients, structure coefficients, and squared structure coefficients for the first canonical function are presented in Table 1. Using a cutoff correlation of .3, the novelty-related measures that were correlated with the first canonical variate were distance traveled in the open field, exploratory hole pokes in the hole board, and time in the center of the open field. Novelty preference was not correlated with the first canonical variate. The addiction related measures that were correlated with the first canonical variate were infusions on the FR1 schedule and breakpoint. Thus, the first pair of canonical variates indicates that a high propensity to explore novel environments, as assessed using multiple measures, is associated with a high propensity to self-administer cocaine. Notably, the novelty-related measures explained a relatively large degree of variance (45%) in the addiction-related measures.
DISCUSSION
In the present study, we have demonstrated in a high-precision and genetically diverse mouse population that multiple novelty-related measures are predictive of cocaine IVSA. DO mice were assessed on a group of four novelty-related measures consisting of open field exploration, time in the center of the open field, hole board exploration, and novelty preference. Following assessment on the novelty-related tasks, jugular catheters were implanted and mice were tested on a cocaine IVSA task. Multivariate analysis revealed that open field exploration, time in the center of the open field, and hole board exploration were significantly positively related to cocaine IVSA (Table 3 and Figure 5). Collectively, novelty-related measures accounted for a large degree of variance (45%) in the addiction-related measures.
Table 3.
Canonical correlation analysis of the relationship between novelty-related behaviors and addiction-related behaviors
| Dependent Measures | Coef | rs | rs2 (%) |
|---|---|---|---|
| Novelty | |||
| Distance traveled OF (cm) | 0.2905 | 0.6381 | 40.71 |
| Hole pokes | 0.8282 | 0.9435 | 89.01 |
| Novelty preference (%) | −0.1445 | −0.0541 | 0.29 |
| Time in center OF (s) | 0.0482 | 0.5290 | 27.98 |
| Addiction | |||
| Infusions FR1 | 0.7249 | 0.6092 | 37.11 |
| Breakpoint PR | 0.2550 | 0.4181 | 17.48 |
| Acquired IVSA | −0.2250 | 0.2183 | 4.77 |
| Sex of mouse | 0.7237 | 0.6922 | 47.91 |
Notes. Structure coefficients greater than |.30| are underlined. Coef = standardized canonical function coefficient, rs = structure coefficient, rs2 = squared structure coefficient;
Although phenotypic relationships between novelty-related behaviors and cocaine IVSA have previously been reported in rats, we are unaware of any previous studies showing that novelty-related behaviors are predictive of intravenous cocaine IVSA in mice. Thus, results from the present study indicate that these relationships can be modeled in the mouse species and offer further support for the hypothesis that similar biological mechanisms underlie novelty- and addiction-related traits. Most importantly, the present study indicates that the genetically and phenotypically diverse DO population can be used to discover the underlying genes and mechanisms driving these relationships. Indeed, as discussed below, results from prior studies suggest that the additional phenotypic diversity in the DO may be required to detect relationships between novelty- and addiction-related traits in mice.
Novelty-related behaviors in Diversity Outbred mice
With the exception of novelty preference, all novelty related variables were significantly, though modestly, positively intercorrelated (Table 1). One reason for the modest strength of these relationships may be the genetic independence of these novelty-related traits (Kliethermes and Crabbe 2006). In the case of novelty preference, which was least related to other tests, it is possible that mice experienced less anxiety during this task relative to other tasks due to a habituation phase. Specifically, in order to create a situation in which one side of the apparatus was relatively less familiar (i.e., more novel), mice were introduced to the apparatus and allowed to explore one side prior to the testing phase. In the other two tasks, testing began immediately when mice were placed into the apparatus. Previous studies using different testing methods and apparatus have observed a more robust preference for novelty in mice (Adriani et al. 1998; Palanza et al. 2001). Thus, it is possible that refinements to the task, such as the addition of tactile cues or extending habituation to the familiar side, may result in a more robust side preference
Intravenous cocaine self-administration in Diversity Outbred mice
Mice that met acquisition criteria (43%) exhibited variation in active lever preference, with some mice responding almost exclusively on the active lever following acquisition, while others continued to sample the inactive lever. DO mice that did not meet acquisition criteria individually (57%) nevertheless showed significant lever discrimination as a group. What is most striking about these mice is the range of response patterns. For example, some mice sampled the active lever but never consistently responded, others consistently responded on both levers but did not discriminate between them, and others appeared to acquire but then did not maintain that pattern of responding across sessions. That some mice did not meet conventional acquisition criteria may be viewed as a limitation. However, several factors suggest that high variability in performance observed in the present study was due to the expanded genetic diversity in the DO population, not experimental artifacts. First, mice experiencing catheter failure, systemic infection, or other technical issues (n = 17) were excluded from analysis, indicating that these factors do not explain response patterns in mice that did not reach conventional acquisition criteria. Second, all mice included in the analysis received cocaine infusions during the study (range 4 – 236). Thus, all mice experienced pairings of active lever pressing and cocaine infusions. Third, in contrast to an inbred strain such as C57BL/6J, mice from the genetically diverse DO population would be expected to express high phenotypic diversity on all traits which control responding in the cocaine IVSA paradigm. Therefore, it seems likely that mice which did not meet conventional acquisition criteria did so as a result of the combined effects of broad phenotypic diversity on the spectrum of processes affecting cocaine acquisition and use in IVSA paradigms such as reaction to novelty (present study), impulsivity (Cervantes et al. 2013), rewarding and aversive effects of cocaine (Rademacher et al. 2000), regulation of drug intake (Lynch and Carroll 2001), rewarding effects of non-drug stimuli (Olsen and Winder 2009), and instrumental and Pavlovian learning and memory processes (Beckmann et al. 2011; Schindler et al. 2002). Furthermore, this suggest that the common practice of excluding from statistical analysis mice that do not reach predetermined acquisition criteria may be ill-advised when using genetically diverse populations as this technique serves to reduce the phenotypic diversity of the sample and, as a consequence, the power to detect relationships between behavior and underlying biological processes.
Association of novelty-related behaviors and intravenous cocaine self-administration in Diversity Outbred mice
Statistically significant bivariate relationships were observed (Figure 4A and 4B) between cocaine IVSA and two of the four novelty-related traits (hole board exploration and center time in the open field). These findings in mice are consistent with previous findings in humans indicating that high novelty seeking and low anxiety are positively related to cocaine and other drug use (Gunnarsdottir et al. 2000; Wills et al. 1994). However, these bivariate relationships were fairly modest with respect to overall variance explained. In contrast, when we considered the multivariate relationship between the four novelty-related traits and the addiction related traits in the present study, it was evident that a much larger degree of variance was shared between these two variable sets than was suggested by the bivariate relationships alone (Figure 5 and Table 3). This observation is consistent with that from a previous study in which Cain et al. (2005) observed that the use of multiple novelty-related traits (open field activity, novel object exploration, and novelty preference), in comparison to a single trait, significantly improved prediction of amphetamine IVSA in rats using a regression model. One reason for this may be that bivariate analysis does not adequately account for the complex relationships within and between the varied psychological constructs which underlie novelty-related and addiction-related behaviors. (Arnett 1994; Belin et al. 2011; Bush and Vaccarino 2007; Castellani and Rugle 1995; Deroche-Gamonet et al. 2004; Dilleen et al. 2012; Molander et al. 2011; Patkar et al. 2002; Patkar et al. 2004; Piazza et al. 1990).
Although there were no main effects of sex on addiction related measures, effects on novelty-related measures were observed; some of these have been previously reported by others (Adriani and Laviola 2000; Beatty 1979; Goodrich and Lange 1986; Gray 1971; Hughes et al. 2004; Ray and Hansen 2004; Siuciak et al. 2007). The high correlation of sex to the first canonical covariate in the multidimensional analysis reflects that variation in novelty-related traits are in part influenced by sex of the mouse, and this must be accounted for when assessing the correlation among novelty- and addiction-related measures. We included this measure on the addiction side of the canonical correlation because it is a predictor of novelty related traits. It is independent of IVSA measures, but enables detection of the canonical correlation of novelty and addiction related behaviors.
In contrast to a previous study in which Kliethermes et al. (2007) found that hole board exploration and oral methamphetamine self-administration were unrelated in mice selectively bred for high and low exploration of a hole board apparatus, we were able to detect such a relationship in the DO. High and low responders in Kliethermes et al. were derived from a B6D2 cross. The increased genetic diversity in DO mice (Chesler 2014) may have enabled detection of relationships which were driven by polymorphisms not present in the B6 and D2 inbred strains from which selected lines were derived in Kliethermes et al. Indeed, several behavioral traits exhibit low between-strain variation in the B6 and D2 strains (Philip et al. 2010), and Kliethermes et al. suggested that the low levels of methamphetamine intake observed in their study were likely due to limited genetic diversity in these strains. A second consideration is that the route of administration differed between the two studies, with Kliethermes et al. using an oral self-administration paradigm while an intravenous self-administration paradigm was employed in the present study. This may be relevant because Kliethermes et al. observed marked aversion to the methamphetamine solution which resulted in low levels of intake across groups, likely creating a floor effect which limited the ability to detect relationships between methamphetamine oral self-administration and hole board exploration. Finally, it should be noted that Kliethermes et al. examined genetic correlations between methamphetamine preference and hole board exploration using selected lines, while in the present study we examined phenotypic correlations between novelty-related traits and cocaine IVSA in individual DO mice.
Although we assessed relatively short-term cocaine use in the present study, some studies in rats have revealed that certain addiction-related traits only emerge following longer-term cocaine use (Belin et al. 2011; Deroche-Gamonet et al. 2004). In the present study, mice were tested daily in two independent two-hour sessions separated by a two-hour timeout and were housed in the testing chambers 24 hours a day during the six day IVSA testing period. In similar studies, mice have been tested in a single daily session and, with the exception of those testing sessions, were housed in the home cage (e.g., Cervantes et al. 2013; Dickson et al. 2014; Griffin and Middaugh 2003; Ruiz-Durantez et al. 2006). Housing in the testing chamber could reduce anxiety during the testing session or reduce the drive to explore the relatively less-novel environment. Prior to IVSA testing, mice were group housed and provided with enrichment materials consisting of a Nestlet and Shepard Shack. Environmental enrichment (Chauvet et al. 2009; Gipson et al. 2011; Green et al. 2010) and group housing (Smith 2012; van der Veen et al. 2007) have been shown to affect cocaine self-administration. In the case of group housing, gene by environment interactions have been observed (van der Veen et al. 2007). In the present study, assessment of IVSA and novelty behaviors occurred during the light phase of the light:dark cycle. Strain-dependent effects of light-phase on some high-throughput behavioral tasks such as the elevated plus maze and open field have been observed (Hossain et al. 2004; Post et al. 2011). Many (Caine et al. 2012; Soria et al. 2008; Thomsen and Caine 2006; 2011; Zhang et al. 2009) though not all (Fuchs et al. 2003; Trigo et al. 2009; Ward et al. 2009) mouse IVSA studies have been conducted during the light phase.
The Diversity Outbred population as a resource for the discovery of genes and mechanisms underlying addiction and predisposing traits
The ability to use mice to examine the genetic underpinnings of the novelty-addiction relationship carries distinct advantages, and the mouse remains the most deeply characterized and cost effective resource for genetic analysis. Several mouse genetic resources have been employed for the study of addiction including the BXD recombinant inbred strains (Belknap and Crabbe 1992; Belknap et al. 1993; Crabbe et al. 1994; Gallaher et al. 1996; Grisel et al. 1997; Kest et al. 2004; Philip et al. 2010; Phillips et al. 1998; Roberts et al. 1995; Tarantino et al. 1998), F2 hybrid crosses (Bergeson et al. 2001; Doyle et al. 2008; Ferraro et al. 2005; Kest et al. 2004), the Hybrid Mouse Diversity Panel (Bryant et al. 2012a; Ghazalpour et al. 2012; Park et al. 2011; Parker et al. 2012a), and advanced intercross lines (Bryant et al. 2012b; Parker et al. 2012a; Parker et al. 2012b; Samocha et al. 2010). While these resources have enabled substantial progress in the understanding of mechanisms underlying the effects of addictive drugs, each has some limitations in power, precision, genetic diversity, and genetic architecture. The Collaborative Cross inbred strains (CC), DO mouse population, and related populations (Ghazalpour et al. 2012; Iancu et al. 2010) complement these resources with a wealth of allelic variation, high mapping precision and a greater range of behavioral diversity (reviewed in Chesler 2014). Allelic variants can be identified using a QTL mapping approach in combination with functional genomic data resources (Recla et al. 2014). We have successfully mapped several additive behavioral QTLs in the DO using approximately 300 mice, though more power will enable reliable detection of a larger number of loci and more complex genetic effects (Logan et al. 2013). Because DO mice are generated from the same eight founder strains as the CC and these founders have been sequenced (Keane et al. 2011), CC lines can be intercrossed to assess allelic effects at the QTLs identified using the DO. Once identified, positional candidate genes can be validated with knockout mice from the knockout mouse project (Austin et al. 2004) or derived using CRISPR/Cas systems (Wang et al. 2013; Yang et al. 2014).
Conclusions
Using mice from the genetically and phenotypically diverse DO population, we have shown that performance on multiple novelty-related tasks is predictive of cocaine IVSA. Novelty-related traits as a group explained 45% of the variance in addiction related traits, indicating the high explanatory power of the observed relationships in the DO population. A broad range of behavioral variation in acquisition and discrimination patterns was observed. The high genetic diversity in the DO combined with the high construct validity of the cocaine IVSA paradigm enabled the detection of relationships between novelty- and addiction-related behaviors which have been undetected in past studies. These results indicate for the first time that the relationship between novelty-related traits and traits related to cocaine addiction can be modeled in mice, and that the enhanced genetic diversity in the DO population can be utilized to discover the underlying genetic mechanisms which drive the development of addiction through predisposing personality traits.
Supplementary Material
Figure 1.
Performance distributions of all mice tested on novelty and intravenous cocaine self-administration assays.
Acknowledgments
The authors gratefully acknowledge support from The Jackson Laboratory to EJC, NIDA IRP to DTL; NIDA Drug Supply Program, Cancer Center Core Grant CA034196 and NIGMS P50 GM076468. We would also like to thank Dr. Yavin Shaham of NIDA IRP and Dr. Guy Mittleman at Ball State University for their consultation on the mouse IVSA procedure, and Andree Lapierre of JAX Surgical Services for consultation on mouse surgical techniques for the study.
Footnotes
Conflicts of Interest: None
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–46. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology. 2005;180:473–90. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Arnett J. Sensation seeking: A new conceptualization and a new scale. Personality and Individual Differences. 1994;16:289–296. [Google Scholar]
- Austin CP, Battey JF, Bradley A, Bucan M, Capecchi M, Collins FS, Dove WF, Duyk G, Dymecki S, Eppig JT, Grieder FB, Heintz N, Hicks G, Insel TR, Joyner A, Koller BH, Lloyd KC, Magnuson T, Moore MW, Nagy A, Pollock JD, Roses AD, Sands AT, Seed B, Skarnes WC, Snoddy J, Soriano P, Stewart DJ, Stewart F, Stillman B, Varmus H, Varticovski L, Verma IM, Vogt TF, von Melchner H, Witkowski J, Woychik RP, Wurst W, Yancopoulos GD, Young SG, Zambrowicz B. The knockout mouse project. Nat Genet. 2004;36:921–4. doi: 10.1038/ng0904-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylor DL, Valdar W, Foulds-Mathes W, Buus RJ, Verdugo RA, Baric RS, Ferris MT, Frelinger JA, Heise M, Frieman MB, Gralinski LE, Bell TA, Didion JD, Hua K, Nehrenberg DL, Powell CL, Steigerwalt J, Xie Y, Kelada SN, Collins FS, Yang IV, Schwartz DA, Branstetter LA, Chesler EJ, Miller DR, Spence J, Liu EY, McMillan L, Sarkar A, Wang J, Wang W, Zhang Q, Broman KW, Korstanje R, Durrant C, Mott R, Iraqi FA, Pomp D, Threadgill D, de Villena FP, Churchill GA. Genetic analysis of complex traits in the emerging Collaborative Cross. Genome Res. 2011;21:1213–22. doi: 10.1101/gr.111310.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience (Frontiers in Neuroscience) Boca Raton (FL): 2009. [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–90. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW. Gonadal hormones and sex differences in nonreproductive behaviors in rodents: organizational and activational influences. Hormones and behavior. 1979;12:112–63. doi: 10.1016/0018-506x(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216:159–65. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–79. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med. 2012;2:1–20. doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC. Chromosome mapping of gene loci affecting morphine and amphetamine responses in BXD recombinant inbred mice. Ann N Y Acad Sci. 1992;654:311–23. doi: 10.1111/j.1749-6632.1992.tb25977.x. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Metten P, Helms ML, O’Toole LA, Angeli-Gade S, Crabbe JC, Phillips TJ. Quantitative trait loci (QTL) applications to substances of abuse: physical dependence studies with nitrous oxide and ethanol in BXD mice. Behav Genet. 1993;23:213–22. doi: 10.1007/BF01067426. [DOI] [PubMed] [Google Scholar]
- Bergeson SE, Helms ML, O’Toole LA, Jarvis MW, Hain HS, Mogil JS, Belknap JK. Quantitative trait loci influencing morphine antinociception in four mapping populations. Mamm Genome. 2001;12:546–53. doi: 10.1007/s003350020022. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Kole LA, Guido MA, Cheng R, Palmer AA. Methamphetamine-induced conditioned place preference in LG/J and SM/J mouse strains and an F45/F46 advanced intercross line. Front Genet. 2012a;3:126. doi: 10.3389/fgene.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Parker CC, Zhou L, Olker C, Chandrasekaran RY, Wager TT, Bolivar VJ, Loudon AS, Vitaterna MH, Turek FW, Palmer AA. Csnk1e is a genetic regulator of sensitivity to psychostimulants and opioids. Neuropsychopharmacology. 2012b;37:1026–35. doi: 10.1038/npp.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DE, Vaccarino FJ. Individual differences in elevated plus-maze exploration predicted progressive-ratio cocaine self-administration break points in Wistar rats. Psychopharmacology. 2007;194:211–9. doi: 10.1007/s00213-007-0835-7. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–75. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, Newman AH, Butler P, Xu M. Cocaine self-administration in dopamine D(3) receptor knockout mice. Exp Clin Psychopharmacol. 2012;20:352–63. doi: 10.1037/a0029135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani B, Rugle L. A comparison of pathological gamblers to alcoholics and cocaine misusers on impulsivity, sensation seeking, and craving. Int J Addict. 1995;30:275–89. doi: 10.3109/10826089509048726. [DOI] [PubMed] [Google Scholar]
- Cervantes MC, Laughlin RE, Jentsch JD. Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology. 2013;229:515–25. doi: 10.1007/s00213-013-3135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–78. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ. Out of the Bottleneck: The Diversity Outcross and Collaborative Cross Mouse Populations in Behavioral Genetics Research. Mamm Genome. 2014;25:3–11. doi: 10.1007/s00335-013-9492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Miller DR, Branstetter LR, Galloway LD, Jackson BL, Philip VM, Voy BH, Culiat CT, Threadgill DW, Williams RW, Churchill GA, Johnson DK, Manly KF. The Collaborative Cross at Oak Ridge National Laboratory: developing a powerful resource for systems genetics. Mamm Genome. 2008;19:382–9. doi: 10.1007/s00335-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GA, Airey DC, Allayee H, Angel JM, Attie AD, Beatty J, Beavis WD, Belknap JK, Bennett B, Berrettini W, Bleich A, Bogue M, Broman KW, Buck KJ, Buckler E, Burmeister M, Chesler EJ, Cheverud JM, Clapcote S, Cook MN, Cox RD, Crabbe JC, Crusio WE, Darvasi A, Deschepper CF, Doerge RW, Farber CR, Forejt J, Gaile D, Garlow SJ, Geiger H, Gershenfeld H, Gordon T, Gu J, Gu W, de Haan G, Hayes NL, Heller C, Himmelbauer H, Hitzemann R, Hunter K, Hsu HC, Iraqi FA, Ivandic B, Jacob HJ, Jansen RC, Jepsen KJ, Johnson DK, Johnson TE, Kempermann G, Kendziorski C, Kotb M, Kooy RF, Llamas B, Lammert F, Lassalle JM, Lowenstein PR, Lu L, Lusis A, Manly KF, Marcucio R, Matthews D, Medrano JF, Miller DR, Mittleman G, Mock BA, Mogil JS, Montagutelli X, Morahan G, Morris DG, Mott R, Nadeau JH, Nagase H, Nowakowski RS, O’Hara BF, Osadchuk AV, Page GP, Paigen B, Paigen K, Palmer AA, Pan HJ, Peltonen-Palotie L, Peirce J, Pomp D, Pravenec M, Prows DR, Qi Z, Reeves RH, Roder J, Rosen GD, Schadt EE, Schalkwyk LC, Seltzer Z, Shimomura K, Shou S, Sillanpaa MJ, Siracusa LD, Snoeck HW, Spearow JL, Svenson K, Tarantino LM, Threadgill D, Toth LA, Valdar W, de Villena FP, Warden C, Whatley S, Williams RW, Wiltshire T, Yi N, Zhang D, Zhang M, Zou F, Complex Trait C. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet. 2004;36:1133–7. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Gatti DM, Munger SC, Svenson KL. The diversity outbred mouse population. Mamm Genome. 2012;23:713–8. doi: 10.1007/s00335-012-9414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Cross Consortium. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics. 2012;190:389–401. doi: 10.1534/genetics.111.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK, Mitchell SR, Crawshaw LI. Quantitative trait loci mapping of genes that influence the sensitivity and tolerance to ethanol-induced hypothermia in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1994;269:184–92. [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dickson PE, Miller MM, Rogers TD, Blaha CD, Mittleman G. Effects of adolescent nicotine exposure and withdrawal on intravenous cocaine self-administration during adulthood in male C57BL/6J mice. Addict Biol. 2014;19:37–48. doi: 10.1111/j.1369-1600.2012.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ, Dalley JW, Belin D. High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology. 2012;222:89–97. doi: 10.1007/s00213-011-2626-4. [DOI] [PubMed] [Google Scholar]
- Doyle GA, Furlong PJ, Schwebel CL, Smith GG, Lohoff FW, Buono RJ, Berrettini WH, Ferraro TN. Fine mapping of a major QTL influencing morphine preference in C57BL/6 and DBA/2 mice using congenic strains. Neuropsychopharmacology. 2008;33:2801–9. doi: 10.1038/npp.2008.14. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–3. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, Martin JF, Schwebel CL, Doyle GA, Buono RJ, Berrettini WH. Confirmation of a major QTL influencing oral morphine intake in C57 and DBA mice using reciprocal congenic strains. Neuropsychopharmacology. 2005;30:742–6. doi: 10.1038/sj.npp.1300592. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76(Pt B):425–36. doi: 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, See RE, Middaugh LD. Conditioned stimulus-induced reinstatement of extinguished cocaine seeking in C57BL/6 mice: a mouse model of drug relapse. Brain Res. 2003;973:99–106. doi: 10.1016/s0006-8993(03)02560-5. [DOI] [PubMed] [Google Scholar]
- Gallaher EJ, Jones GE, Belknap JK, Crabbe JC. Identification of genetic markers for initial sensitivity and rapid tolerance to ethanol-induced ataxia using quantitative trait locus analysis in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1996;277:604–12. [PubMed] [Google Scholar]
- Ghazalpour A, Rau CD, Farber CR, Bennett BJ, Orozco LD, van Nas A, Pan C, Allayee H, Beaven SW, Civelek M, Davis RC, Drake TA, Friedman RA, Furlotte N, Hui ST, Jentsch JD, Kostem E, Kang HM, Kang EY, Joo JW, Korshunov VA, Laughlin RE, Martin LJ, Ohmen JD, Parks BW, Pellegrini M, Reue K, Smith DJ, Tetradis S, Wang J, Wang Y, Weiss JN, Kirchgessner T, Gargalovic PS, Eskin E, Lusis AJ, LeBoeuf RC. Hybrid mouse diversity panel: a panel of inbred mouse strains suitable for analysis of complex genetic traits. Mamm Genome. 2012;23:680–92. doi: 10.1007/s00335-012-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology. 2011;214:557–66. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–32. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Goodrich C, Lange J. A differential sex effect of amphetamine on exploratory behavior in maturing mice. Physiology & behavior. 1986;38:663–6. doi: 10.1016/0031-9384(86)90261-1. [DOI] [PubMed] [Google Scholar]
- Gray JA. Sex differences in emotional behaviour in mammals including man: endocrine bases. Acta psychologica. 1971;35:29–46. doi: 10.1016/0001-6918(71)90029-1. [DOI] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD. Acquisition of lever pressing for cocaine in C57BL/6J mice: effects of prior Pavlovian conditioning. Pharmacol Biochem Behav. 2003;76:543–9. doi: 10.1016/j.pbb.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Grisel JE, Belknap JK, O’Toole LA, Helms ML, Wenger CD, Crabbe JC. Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. J Neurosci. 1997;17:745–54. doi: 10.1523/JNEUROSCI.17-02-00745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsdottir ED, Pingitore RA, Spring BJ, Konopka LM, Crayton JW, Milo T, Shirazi P. Individual differences among cocaine users. Addict Behav. 2000;25:641–52. doi: 10.1016/s0306-4603(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Hossain SM, Wong BK, Simpson EM. The dark phase improves genetic discrimination for some high throughput mouse behavioral phenotyping. Genes, brain, and behavior. 2004;3:167–77. doi: 10.1111/j.1601-183x.2004.00069.x. [DOI] [PubMed] [Google Scholar]
- Hughes RN, Desmond CS, Fisher LC. Room novelty, sex, scopolamine and their interactions as determinants of general activity and rearing, and light-dark preferences in rats. Behavioural processes. 2004;67:173–81. doi: 10.1016/j.beproc.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Iancu OD, Darakjian P, Walter NA, Malmanger B, Oberbeck D, Belknap J, McWeeney S, Hitzemann R. Genetic diversity and striatal gene networks: focus on the heterogeneous stock-collaborative cross (HS-CC) mouse. BMC Genomics. 2010;11:585. doi: 10.1186/1471-2164-11-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellaker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assuncao JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–94. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Juni A, Chesler EJ, Mogil JS. Mapping of a quantitative trait locus for morphine withdrawal severity. Mamm Genome. 2004;15:610–7. doi: 10.1007/s00335-004-2367-3. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Crabbe JC. Genetic independence of mouse measures of some aspects of novelty seeking. Proc Natl Acad Sci U S A. 2006;103:5018–23. doi: 10.1073/pnas.0509724103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL, Kamens HM, Crabbe JC. Drug reward and intake in lines of mice selectively bred for divergent exploration of a hole board apparatus. Genes Brain Behav. 2007;6:608–18. doi: 10.1111/j.1601-183X.2006.00289.x. [DOI] [PubMed] [Google Scholar]
- Kmiotek EK, Baimel C, Gill KJ. Methods for intravenous self administration in a mouse model. J Vis Exp. 2012;70:e3739. doi: 10.3791/3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Robledo RF, Recla JM, Philip VM, Bubier JA, Jay JJ, Harwood C, Wilcox T, Gatti DM, Bult CJ, Churchill GA, Chesler EJ. High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes Brain Behav. 2013;12:424–37. doi: 10.1111/gbb.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–43. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Molander AC, Mar A, Norbury A, Steventon S, Moreno M, Caprioli D, Theobald DE, Belin D, Everitt BJ, Robbins TW, Dalley JW. High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology. 2011;215:721–31. doi: 10.1007/s00213-011-2167-x. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–94. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Morley-Fletcher S, Laviola G. Novelty seeking in periadolescent mice: sex differences and influence of intrauterine position. Physiol Behav. 2001;72:255–62. doi: 10.1016/s0031-9384(00)00406-6. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacology. 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Park CC, Gale GD, de Jong S, Ghazalpour A, Bennett BJ, Farber CR, Langfelder P, Lin A, Khan AH, Eskin E, Horvath S, Lusis AJ, Ophoff RA, Smith DJ. Gene networks associated with conditional fear in mice identified using a systems genetics approach. BMC Syst Biol. 2011;5:43. doi: 10.1186/1752-0509-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CC, Cheng R, Sokoloff G, Palmer AA. Genome-wide association for methamphetamine sensitivity in an advanced intercross mouse line. Genes Brain Behav. 2012a;11:52–61. doi: 10.1111/j.1601-183X.2011.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CC, Sokoloff G, Cheng R, Palmer AA. Genome-wide association for fear conditioning in an advanced intercross mouse line. Behav Genet. 2012b;42:437–48. doi: 10.1007/s10519-011-9524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Berrettini WH, Hoehe M, Thornton CC, Gottheil E, Hill K, Weinstein SP. Serotonin transporter polymorphisms and measures of impulsivity, aggression, and sensation seeking among African-American cocaine-dependent individuals. Psychiatry Res. 2002;110:103–15. doi: 10.1016/s0165-1781(02)00098-7. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre-treatment measures of impulsivity, aggression and sensation seeking are associated with treatment outcome for African-American cocaine-dependent patients. J Addict Dis. 2004;23:109–22. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Payseur BA, Place M. Prospects for association mapping in classical inbred mouse strains. Genetics. 2007;175:1999–2008. doi: 10.1534/genetics.106.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov PM, Graber JH, Churchill GA, DiPetrillo K, King BL, Paigen K. Evidence of a large-scale functional organization of mammalian chromosomes. PLoS Genet. 2005;1:e33. doi: 10.1371/journal.pgen.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip VM, Duvvuru S, Gomero B, Ansah TA, Blaha CD, Cook MN, Hamre KM, Lariviere WR, Matthews DB, Mittleman G, Goldowitz D, Chesler EJ. High-throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes Brain Behav. 2010;9:129–59. doi: 10.1111/j.1601-183X.2009.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip VM, Sokoloff G, Ackert-Bicknell CL, Striz M, Branstetter L, Beckmann MA, Spence JS, Jackson BL, Galloway LD, Barker P, Wymore AM, Hunsicker PR, Durtschi DC, Shaw GS, Shinpock S, Manly KF, Miller DR, Donohue KD, Culiat CT, Churchill GA, Lariviere WR, Palmer AA, O’Hara BF, Voy BH, Chesler EJ. Genetic analysis in the Collaborative Cross breeding population. Genome Res. 2011;21:1223–38. doi: 10.1101/gr.113886.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Huson MG, McKinnon CS. Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J Neurosci. 1998;18:3023–34. doi: 10.1523/JNEUROSCI.18-08-03023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Post AM, Weyers P, Holzer P, Painsipp E, Pauli P, Wultsch T, Reif A, Lesch KP. Gene-environment interaction influences anxiety-like behavior in ethologically based mouse models. Behavioural brain research. 2011;218:99–105. doi: 10.1016/j.bbr.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Anders KA, Thompson KJ, Steinpreis RE. The failure of some rats to acquire intravenous cocaine self-administration is attributable to conditioned place aversion. Behav Brain Res. 2000;117:13–9. doi: 10.1016/s0166-4328(00)00277-1. [DOI] [PubMed] [Google Scholar]
- Ray J, Hansen S. Temperament in the rat: sex differences and hormonal influences on harm avoidance and novelty seeking. Behavioral neuroscience. 2004;118:488–97. doi: 10.1037/0735-7044.118.3.488. [DOI] [PubMed] [Google Scholar]
- Recla JM, Robledo RF, Gatti DM, Bult CJ, Churchill GA, Chesler EJ. Precise genetic mapping and integrative bioinformatics in Diversity Outbred mice reveals Hydin as a novel pain gene. Mamm Genome. 2014;25:211–22. doi: 10.1007/s00335-014-9508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Phillips TJ, Belknap JK, Finn DA, Keith LD. Genetic analysis of the corticosterone response to ethanol in BXD recombinant inbred mice. Behav Neurosci. 1995;109:1199–208. doi: 10.1037//0735-7044.109.6.1199. [DOI] [PubMed] [Google Scholar]
- Ruiz-Durantez E, Hall SK, Steffen C, Self DW. Enhanced acquisition of cocaine self-administration by increasing percentages of C57BL/6J genes in mice with a nonpreferring outbred background. Psychopharmacology. 2006;186:553–60. doi: 10.1007/s00213-006-0379-2. [DOI] [PubMed] [Google Scholar]
- Samocha KE, Lim JE, Cheng R, Sokoloff G, Palmer AA. Fine mapping of QTL for prepulse inhibition in LG/J and SM/J mice using F(2) and advanced intercross lines. Genes Brain Behav. 2010;9:759–67. doi: 10.1111/j.1601-183X.2010.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology. 2002;163:327–44. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- Sherry A, Henson RK. Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess. 2005;84:37–48. doi: 10.1207/s15327752jpa8401_09. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Reed TM, Vorhees CV, Repaske DR. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-1B (PDE1B) enzyme. Neuropharmacology. 2007;53:113–24. doi: 10.1016/j.neuropharm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology. 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Barbano MF, Maldonado R, Valverde O. A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology. 2008;199:593–603. doi: 10.1007/s00213-008-1184-x. [DOI] [PubMed] [Google Scholar]
- Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Palmer AA, McMillan L, Churchill GA. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics. 2012;190:437–47. doi: 10.1534/genetics.111.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Pearson; Boston: 2007. [Google Scholar]
- Tarantino LM, McClearn GE, Rodriguez LA, Plomin R. Confirmation of quantitative trait loci for alcohol preference in mice. Alcohol Clin Exp Res. 1998;22:1099–105. [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology. 2006;184:145–54. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Intravenous drug self-administration in mice: practical considerations. Behav Genet. 2007;37:101–18. doi: 10.1007/s10519-006-9097-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. False positive in the intravenous drug self-administration test in C57BL/6J mice. Behav Pharmacol. 2011;22:239–47. doi: 10.1097/FBP.0b013e328345f8f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology. 2000;148:289–98. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Orejarena MJ, Maldonado R, Robledo P. MDMA reinstates cocaine-seeking behaviour in mice. Eur Neuropsychopharmacol. 2009;19:391–7. doi: 10.1016/j.euroneuro.2008.12.010. [DOI] [PubMed] [Google Scholar]
- van der Veen R, Piazza PV, Deroche-Gamonet V. Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacology. 2007;193:179–86. doi: 10.1007/s00213-007-0777-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–8. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Rosenberg M, Dykstra LA, Walker EA. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend. 2009;105:248–55. doi: 10.1016/j.drugalcdep.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. J Subst Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Jaenisch R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat Protoc. 2014;9:1956–68. doi: 10.1038/nprot.2014.134. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang JR, Didion JP, Buus RJ, Bell TA, Welsh CE, Bonhomme F, Yu AH, Nachman MW, Pialek J, Tucker P, Boursot P, McMillan L, Churchill GA, de Villena FP. Subspecific origin and haplotype diversity in the laboratory mouse. Nat Genet. 2011;43:648–55. doi: 10.1038/ng.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34:912–22. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.