Abstract
Background
Aspiration pneumonia represents an under-reported complication of chemoradiotherapy in head-and-neck cancer. This study evaluated the incidence, risk factors, and mortality of aspiration pneumonia in a large cohort of head-and-neck cancer patients treated with concurrent chemoradiotherapy.
Methods
Patients with head-and-neck cancer diagnosed between 2000 and 2009 were identified from the SEER-Medicare database. Aspiration pneumonia was identified from Medicare billing claims. The cumulative incidence, risk factors, and survival after aspiration pneumonia were estimated and compared to a non-cancer population.
Results
Of 3,513 head-and-neck cancer patients, 801 patients developed aspiration pneumonia at a median time of 5 months after initiating treatment. The 1- and 5-year cumulative incidence of aspiration pneumonia was 15.8% and 23.8% for head-and-neck cancer patients and 3.6% and 8.7% for non-cancer controls, respectively. Among cancer patients multivariate analysis identified independent risk factors (p<0.05) for aspiration pneumonia including hypopharyngeal and nasopharyngeal tumors, male gender, older age, increased comorbidity, no surgery prior to radiation, and care received at a teaching hospital. Among cancer patients who experienced aspiration pneumonia, 674 (84%) were hospitalized of which 301 (45%) were admitted to an intensive care unit. Thirty-day mortality after hospitalization for aspiration pneumonia was 32.5%. Aspiration pneumonia was associated with a 42% increased risk of death (HR=1.42, p<0.001) after controlling for confounders.
Conclusions
This study found that nearly one-quarter of elderly patients will develop aspiration pneumonia within 5 years of chemoradiotherapy for head-and-neck cancer. A better understanding of mitigating factors will help identify patients at risk for this potentially lethal complication.
Keywords: Aspiration pneumonia; chemoradiotherapy; head and neck neoplasms; Surveillance, Epidemiology and End Results Program; Medicare
Introduction
Chemotherapy combined with radiation represents a standard treatment approach for locally advanced head-and-neck cancer. While this joint treatment modality has a proven survival benefit, it also poses the risk of significant acute and late toxicities that can have a profound impact on survivorship and quality of life of among head-and-neck cancer survivors.
Aspiration pneumonia―defined as pneumonia secondary to inhalation of food particles, saliva, or other foreign substances―represents an under-reported side effect of head-and-neck cancer after treatment with chemoradiotherapy1, 2. Aspiration pneumonia after radiation is likely due to a combination of contributing factors including acute and chronic radiation induced mucosal changes, muscle fibrosis, and xerostomia 1, 3–6. These factors lead to swallowing dysfunction which in turn increases the risk of both aspiration, and aspiration pneumonia.
Previous studies characterizing the risk of swallowing dysfunction after radiation have identified dysphagia or aspiration rates ranging from 33 to 81% 4, 6–8. Additional research has shown that aspiration pneumonia is a major source of post-treatment morbidity as well as a potential cause of death among head-and-neck cancer patients 1, 9. The current body of literature on aspiration pneumonia includes single institution analyses 7–12 often with small sample sizes 7–11 or limited follow-up 13, 14. To date, population-based studies that characterize the risk of aspiration pneumonia after chemoradiation do not exist. The purpose of this study was to use SEER-Medicare linked data to evaluate the incidence, risk factors, morbidity, and mortality of aspiration pneumonia in a large cohort of head-and-neck cancer patients treated with concurrent chemoradiotherapy.
Methods
Data source
This study evaluated head-and-neck cancer patients within the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. The SEER program consists of a collection of cancer registries geographically spread across the US which collect demographic, clinical, treatment, and survival information for individuals with cancer 15. The Medicare program provides federally funded health insurance for people over the age of 65. The SEER-Medicare linkage combines longitudinal Medicare claims data for patients within the SEER database providing a valuable resource to understand patterns of care and health outcomes for cancer patients from before diagnosis to throughout treatment with complete follow-up extending through death.
Aspiration pneumonia represents a known complication among older adults without cancer; therefore this study compared aspiration pneumonia rates among head-and-neck cancer patients to that of non-cancer controls. The non-cancer patients were drawn from a 5% random sample of Medicare beneficiaries residing in the same geographic regions covered by SEER. The contents of Medicare files for the non-cancer control group were identical to those available for patients with cancer 15. This study was found to be exempt from approval by the Institutional Review Board at the University of California San Diego.
Study population
The initial query of the SEER-Medicare database identified 42,250 patients with head-and-neck cancer over the age of 66 diagnosed between 2000 through 2009. Even though Medicare coverage starts at 65, only patients over 66 were included to ensure a complete year of Medicare claims data to enable calculation of Charlson comorbidity scores (described below). Patients with invalid dates of diagnosis (n=297) and those with non-squamous cell carcinoma histology (n=9,326) were excluded. The study cohort was further refined by removing patients diagnosed with multiple primary tumors (n=8,529) and those diagnosed at autopsy or death (n = 202). Patients with missing data were excluded (n=8,642) including those with non-continuous part A and part B coverage or any part C enrollment (enrollment in an HMO) from 12 months prior to diagnosis to death or last follow-up, leaving 15,254 remaining patients. Finally, only patients who received radiation with concurrent chemotherapy within 6 months of diagnosis were included leaving 3,513 cancer patients in the final study cohort.
Study covariates
Demographic covariates obtained from SEER included patient age at diagnosis, gender, race, and marital status. Geographic location was based on the location of SEER cancer registries, which were categorized into East (Connecticut, New Jersey), Midwest (Detroit, Iowa), South (Atlanta, Rural Georgia, Kentucky, Louisiana), and West (San Francisco, Hawaii, New Mexico, Seattle, Utah, San Jose, Los Angeles, Greater California). Median regional household income was determined from year 2000 Census tract data and divided into quartiles. Patients without household income data (1%) were grouped into the bottom quartile. Tumor site was categorized into six groups: oral cavity, nasopharynx, oropharynx and tonsil, larynx, hypopharynx, and other (salivary gland, other oral cavity and pharynx, and nose, nasal cavity, and middle ear). The SEER Historic Stage A staging system16 was used to classify cancer stage as TNM staging data was limited for the years studied and defined differently across head-and-neck cancer sites. Patients staged as “distant” in the historic SEER system for head-and-neck cancer have locally advanced disease equivalent to T4a and T4b in the American Joint Committee on Cancer (AJCC) staging system17.
Comorbidity score was calculated during the year prior to diagnosis using inpatient and outpatient Medicare claims with the Deyo modification of the Charlson comorbidity index 18. Patient care within a teaching hospital was defined as any indirect medical education charge on a Medicare claim during a hospitalization after the patient’s diagnosis of cancer.
Treatment covariates included surgery, radiation, and chemotherapy. Information on whether the patient had surgery directed at the primary tumor was obtained from SEER and Medicare billing claims (Table 1). The delivery of radiation and chemotherapy were determined from Medicare billing claims (Table 1) using methods as described previously 19, 20. Concurrent chemotherapy was defined as a chemotherapy claim anytime from 2 weeks before the start of radiation through 2 weeks after completing radiation. Additionally, we assessed for the use of additional chemotherapy before (neoadjuvant), or after (adjuvant) the patient received radiotherapy. Use of positron emission tomography (PET) was defined from Medicare claims from 3 months prior to diagnosis extending through the start of radiation. Intensity-modulated radiation therapy (IMRT) was defined as the existence of any IMRT billing claim during the course of radiation. Table 2 summarizes the baseline characteristics of the 3,513 head-and-neck cancer patients included in this study.
Table 1.
Medicare and SEER codes used in study
| Variable | Codes |
|---|---|
| Radiotherapy | HCPCS codes: 61796–61800, 63620–63621, 77371–77373, 77401–77416, 77418, 77421–77423, 77470, 77520, 77522, 77523, 77525, 0197T, G0173-G0174, G0243, G0251, and G0339-G0340. |
| Surgery |
ICD-9 procedure codes: 21.5–21.6, 22.31, 22.42, 22.60–22.66, 24.31, 25.1–25.4, 26.2, 26.29–26.32, 27.3, 27.32, 27.4, 27.42–27.43, 27.49, 27.72, 28.92, 29.33, 29.39, 30.0, 30.09, 30.1, 30.21–30.22, 30.29, 30.3–30.5, 31.5, 40.40–40.42, 76.2, 76.31, 76.39–76.42, or 76.44–76.45. HCPCS codes: 21044–21045, 21555–21557, 30117–30118, 30130, 30140, 30150, 31200–31201, 31205, 31225, 31230, 31299, 31365, 31367–31368, 31370, 31375, 31380, 31382, 31390, 31395, 31420, 38700, 38720, 38724, 40810, 40812, 40814, 40816, 40819, 41110, 41112–41114, 41116, 41120, 41130, 41135, 41140, 41145, 41150, 41153, 41155, 41825–41827, 42104, 42106–42107, 42120, 42140, 42410, 42415, 42420, 42425–42456, 42440, 42450, 42842, 42844–42845, or 42890. |
| Chemotherapy |
ICD-9 procedure code: 99.25. ICD-9 diagnosis codes: V58.1, V58.11, V58.12, V66.2, or V67.2. HCPCS codes: 96400 to 96599, J8999 to J9999, J8520, J8521, or Q0083 to Q0085. Revenue Center codes: 0331, 0332, or 0335. NDC: 00004110013, 00004110020, 00004110051, 00004110113, 00004110116, 00004110150, 00004110151. BETOS codes: 01D. |
| Aspiration pneumonia | ICD-9 diagnosis code: 507.0. |
| IMRT | HCPCS codes: 77301, 77338, 77418, G0174, G0178. |
| PET | HCPCS codes: 78810–78816, G0223-G0225. |
Abbreviations: HCPCS, Healthcare Common Procedure Coding System codes; CPT, Current Procedural Terminology; NDC, National Drugs Codes; BETOS, Berenson-Eggers Type of Service; IMRT, Intensity-Modulated Radiation Therapy; PET, Positron Emission Tomography.
Table 2.
Descriptive characteristics of the study patients
| Variables | N (%) |
|---|---|
| Total | 3,513 (100) |
| Age at diagnosis, y | |
| 66–74 | 2,255 (64) |
| 75–79 | 691 (20) |
| ≥80 | 567 (16) |
| Metropolitan area | |
| Yes | 2,920 (83) |
| No | 593 (17) |
| Teaching hospital | |
| Yes | 2,013 (57) |
| No | 1,500 (43) |
| Region | |
| East | 776 (22) |
| Midwest | 406 (12) |
| South | 959 (27) |
| West | 1372 (39) |
| Sex | |
| Male | 2,471 (70) |
| Female | 1,042 (30) |
| Race | |
| White | 2,961 (84) |
| Black | 329 (9) |
| Other | 223 (6) |
| Marital status | |
| Married | 1,894 (54) |
| Other | 1,619 (46) |
| Charlson comorbidity score | |
| 0 | 1,861 (53) |
| 1 | 947(27) |
| 2 | 395 (11) |
| ≥3 | 310 (9) |
| Year of diagnosis | |
| 2000–2003 | 988 (28) |
| 2004–2006 | 1,109 (32) |
| 2007–2009 | 1,416 (43) |
| Tumor site | |
| Hypopharynx | 391 (11) |
| Larynx | 834 (24) |
| Nasopharynx | 148 (4) |
| Oral cavity | 583 (17) |
| Oropharynx | 1,298 (37) |
| Other | 259 (7) |
| Historic stage | |
| Localized | 478 (14) |
| Regional | 2,219 (63) |
| Distant | 695 (20) |
| Unknown | 121 (3) |
| PET | |
| Yes | 1,800 (51) |
| No | 1,713 (49) |
| IMRT | |
| Yes | 1,821 (52) |
| No | 1,692 (48) |
| Surgery prior to radiation | |
| Yes | 753 (21) |
| No | 2,760 (79) |
| Sequence of chemotherapy and radiotherapy | |
| During only | 1,982 (56) |
| During, and before | 458 (13) |
| During, and after | 894 (25) |
| During, before, and after | 179 (5) |
| Types of chemotherapy | |
| Cisplatin | 768 (22) |
| Cetuximab | 588 (17) |
| Other | 2,157 (61) |
Abbreviations: PET, Positron Emission Tomography; IMRT, Intensity-Modulated Radiation Therapy.
Aspiration pneumonia
Aspiration pneumonia, the primary endpoint of this study, was identified from ICD-9 diagnosis code of 507.0 (Table 1) which represents pneumonitis or pneumonia due to inhalation of food or vomitus 21. The ICD-9 code for aspiration pneumonia is distinct from codes that describe bacterial pneumonia. A study attempting to determine the validity of this ICD-9 code found that 91% of patients with the ICD-9 code for aspiration pneumonia had objective clinical evidence or a physician note documenting aspiration pneumonia 22. In this study, aspiration pneumonia was defined as the presence of this ICD-9 diagnosis code within inpatient or outpatient Medicare billing claims any time after the start of radiation until the end of the study period, December 31, 2009, or the patient’s date of death. Aspiration pneumonia associated with hospitalization was defined as any aspiration pneumonia diagnosis code during a hospitalization. Similarly, admission to an intensive care unit (ICU) was defined as any aspiration pneumonia diagnosis code during admission to an ICU.
Statistical analysis
The rate of aspiration pneumonia was estimated with a competing risk analysis to account for the competing event of death. Time to an aspiration pneumonia event was defined from the start of radiation through the first aspiration pneumonia claim, censoring at last follow-up and treating death of any cause as a competing event. Unadjusted estimates of the rate of aspiration pneumonia were determined with nonparametric cumulative incidence functions using Gray’s test to compare differences between head-and-neck cancer patients and non-cancer controls as well as between subgroups among head-and-neck cancer patients 23. Multivariate predictors of aspiration pneumonia were assessed with Fine-Gray regression models to estimate the sub-distribution hazards 24, 25. All covariates were defined a priori based on factors that could impact survival or radiation therapy treatment. All variables included in the multivariate model are noted in Table 3.
Table 3.
Risk factors for aspiration pneumonia among patients with head-and-neck cancer treated with chemoradiotherapy.
| Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|
| Variables | 5-yr rate (%) | SDHR (95% CI) | SDHR (95% CI) |
| Age at diagnosis, y | |||
| 66–74 | 21.4 | Ref | Ref |
| 75–79 | 27.9 | 1.40 (1.17–1.67) | 1.37 (1.14–1.64) |
| ≥80 | 28.9 | 1.45 (1.20–1.75) | 1.49 (1.22–1.82) |
| Metropolitan area | |||
| Yes | 25.4 | 1.64 (1.31–2.06) | 1.22 (0.95–1.57) |
| No | 16.7 | Ref | Ref |
| Teaching hospital | |||
| Yes | 28.3 | 1.79 (1.53–2.09) | 1.87 (1.58–2.20) |
| No | 17.5 | Ref | Ref |
| Region | |||
| West | 25.3 | Ref | Ref |
| East | 26.6 | 1.06 (0.89–1.27) | 0.89 (0.73–1.08) |
| Midwest | 26.6 | 1.04 (0.82–1.30) | 0.86 (0.67–1.09) |
| South | 18.7 | 0.67 (0.55–0.81) | 0.75 (0.60–0.92) |
| Median Income | |||
| Bottom quartile | 19.9 | Ref | Ref |
| 2nd quartile | 23 | 1.17 (0.94–1.46) | 1.24 (0.98–1.55) |
| 3rd quartile | 25.2 | 1.31 (1.06–1.62) | 1.25 (1.00–1.57) |
| Top quartile | 27.9 | 1.54 (1.25–1.89) | 1.37 (1.09–1.73) |
| Sex | |||
| Male | 25.4 | 1.27 (1.08–1.50) | 1.31 (1.09–1.56) |
| Female | 20.2 | Ref | Ref |
| Race | |||
| White | 23.5 | Ref | Ref |
| Black | 24.8 | 1.01 (0.79–1.29) | 1.07 (0.82–1.41) |
| Other | 28.8 | 1.31 (1.01–1.71) | 1.06 (0.80–1.41) |
| Marital status | |||
| Married | 24 | 1.01 (0.88–1.17) | 0.94 (0.81–1.10) |
| Other | 23.8 | Ref | Ref |
| Charlson comorbidity score | |||
| 0 | 22.1 | Ref | Ref |
| 1 | 23.9 | 1.09 (0.92–1.29) | 1.07 (0.90–1.27) |
| 2 | 29.1 | 1.27 (1.02–1.59) | 1.26 (1.01–1.58) |
| ≥3 | NA | 1.15 (0.89–1.49) | 1.13 (0.87–1.48) |
| Year of diagnosis | |||
| 2000–2003 | 22.9 | Ref | Ref () |
| 2004–2006 | 25 | 1.10 (0.92–1.31) | 1.06 (0.87–1.30) |
| 2007–2009 | NA | 0.89 (0.74–1.06) | 0.86 (0.67–1.11) |
| Tumor site | |||
| Oral cavity | 19.9 | Ref | Ref |
| Hypopharynx | 30.5 | 1.68 (1.28–2.20) | 1.58 (1.20–2.10) |
| Larynx | 24.4 | 1.20 (0.94–1.54) | 1.16 (0.90–1.49) |
| Nasopharynx | 34.1 | 1.76 (1.23–2.50) | 1.77 (1.23–2.55) |
| Oropharynx | 23.4 | 1.16 (0.92–1.46) | 1.16 (0.91–1.47) |
| Other | 19.9 | 0.96 (0.68–1.36) | 0.94 (0.67–1.34) |
| Historic stage | |||
| Localized | 23.8 | Ref | Ref |
| Regional | 23.5 | 1.03 (0.83–1.28) | 0.99 (0.79–1.25) |
| Distant | 25.2 | 1.07 (0.83–1.38) | 1.01 (0.77–1.31) |
| Unknown | 29.4 | 1.29 (0.86–1.94) | 1.27 (0.84–1.92) |
| PET | |||
| Yes | 24.5 | 1.00 (0.86–1.15) | 0.99 (0.84–1.17) |
| No | 23.6 | Ref | Ref |
| IMRT | |||
| Yes | 24.1 | 1.00 (0.87–1.16) | 1.06 (0.88–1.26) |
| No | 23.3 | Ref | Ref |
| Surgery prior to raditaion | |||
| Yes | 20.7 | 0.83 (0.69–1.00) | 0.81 (0.67–0.99) |
| No | 24.6 | Ref | Ref |
| Sequence of chemotherapy and radiotherapy | |||
| During | 24.8 | Ref | Ref |
| During and before | 21.6 | 0.85 (0.67–1.08) | 0.86 (0.67–1.11) |
| During and after | 23.9 | 0.96 (0.81–1.14) | 0.94 (0.79–1.11) |
| During, before and after | 21.4 | 0.89 (0.63–1.26) | 0.86 (0.61–1.22) |
| Types of chemotherapy | |||
| Cetuximab | 27.7 | Ref | Ref |
| Cisplatin | 23.9 | 0.86 (0.69–1.09) | 0.89 (0.69–1.14) |
| Other | 23.3 | 0.91 (0.75–1.11) | 0.92 (0.73–1.15) |
Abbreviations: SDHR, Subdistribution Hazard Ratio; PET, Positron Emission Tomography; IMRT, Intensity-Modulated Radiation Therapy.
We identified non-cancer control patients from the Medicare 5% sample using a 1:1 match for non-cancer patients with continuous Medicare parts A and B without part C for the entire observation period of 2000–2009 26. The match was based on patient characteristics (gender, income, and registry location), as well as age, survival status, and date of death. The time at risk for aspiration pneumonia in the head-and-neck cancer patients extended from the start of radiation through death or last follow-up. Non-cancer controls did not receive radiation therefore a pseudo-radiation start date was assigned for controls to ensure an identical at-risk time period to evaluate for aspiration pneumonia. Standardized differences were used to assess the balance between the head-and-neck cancer patients and non-cancer controls. The standardized differences for all covariates were less than 0.02 (data not shown) suggesting an acceptable match.
Kaplan-Meier survival plots and multivariate Cox proportional-hazard models were used to estimate the association of aspiration pneumonia with overall survival. The multivariate Cox-model for overall survival included all the covariates listed in Table 2. All statistical tests were two-sided, and p<0.05 was considered significant. Analyses were conducted with SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Among the 3,513 head-and-neck cancer patients in this study, 801 patients developed aspiration pneumonia after radiation with a 5-month median time from initiating treatment to an aspiration pneumonia event. Among the 3,513 matched non-cancer controls, 272 developed aspiration pneumonia. As depicted in Figure 1, the 1-year and 5-year cumulative incidence of aspiration pneumonia was 15.8% (95% CI, 14.6–17.0%) and 23.8% (95% CI, 22.3–25.3%) for head-and-neck cancer patients and 3.6% (95% CI, 3.0–4.2%) and 8.7% (95% CI, 7.7–9.7%) for the non-cancer population, respectively.
Figure 1.
Cumulative incidence of aspiration pneumonia in patients with head-and-neck cancer (N=3,513) compared to non-cancer matched pairs (N=3,513).
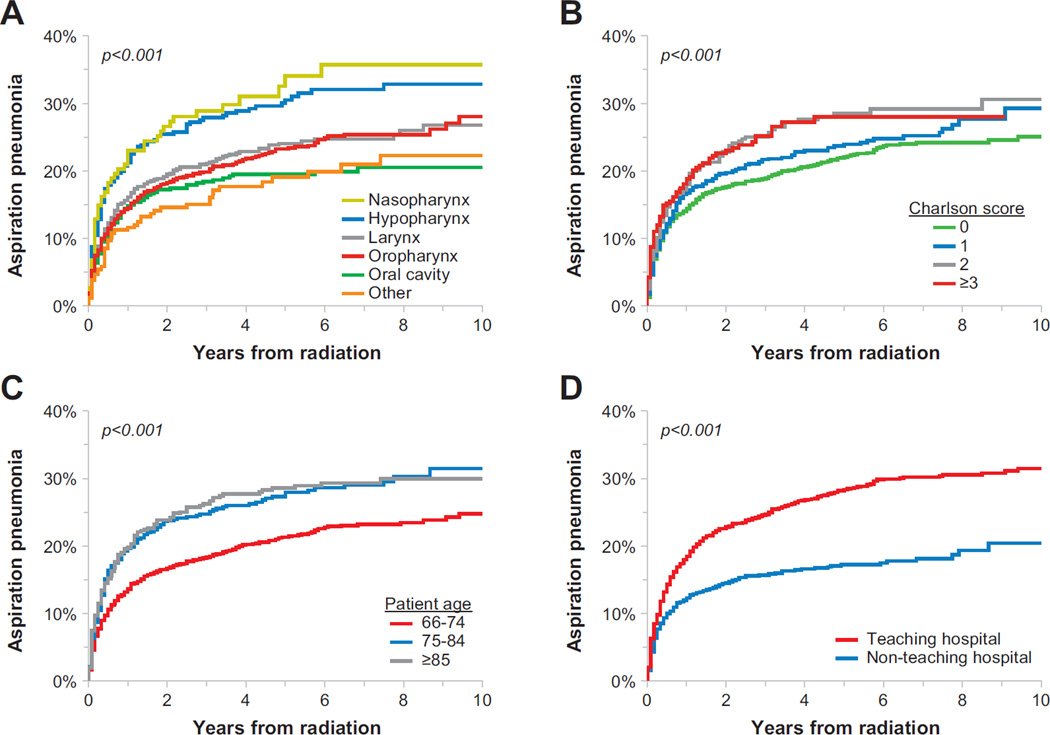
Univariate analysis identified multiple risk factors for aspiration pneumonia of which select predictors are highlighted in cumulative incidence plots shown in Figure 2. Multivariate analysis (Table 3) revealed multiple independent risk factors for aspiration pneumonia including increased age, male gender, delivery of care at a teaching hospital, and tumor location in the nasopharynx or hypopharynx. Lower rates of aspiration pneumonia were identified for patients receiving treatment in the South (versus the West) and for patients with surgery prior to radiation. Of note, disease stage, type of chemotherapy, sequence of chemotherapy with radiation, or the delivery of IMRT did not impact the rate of aspiration pneumonia.
Figure 2.
Cumulative incidence of aspiration pneumonia among head-and-neck cancer patients by tumor site (A), Charlson comorbidity score (B), patient age at diagnosis (C) and care received at a teaching hospital (D).
Subsequent evaluation focused on the morbidity and mortality associated with aspiration pneumonia. Among the head-and-neck cancer patients who experienced aspiration pneumonia, 674 patients (84%) had events associated with hospitalizations with a 10 day median length of stay. Among those cancer patients hospitalized, 301 patients (45%) were admitted to an ICU during their hospitalization. Among cancer patients who developed aspiration pneumonia, the 30-day mortality rate after developing aspiration pneumonia was 32.5% (Figure 3A). Head-and-neck cancer patients who experienced aspiration pneumonia had significantly decreased overall survival compared to those without an aspiration event (Figure 3B). The 5-year survival from diagnosis among those with aspiration pneumonia was 15.2% compared to 32.3% among those without aspiration pneumonia (p < 0.001). Multivariate analysis controlling for potential confounders found that aspiration pneumonia after chemoradiation was associated with a 42% increase in the risk of death (HR=1.42, p < 0.001) compared to patients without aspiration pneumonia.
Figure 3.
Kaplan-Meier curves for overall survival. Figure 3A represents survival from the date of aspiration pneumonia among head-and-neck cancer patients who developed aspiration pneumonia (N=801). Figure 3B represents survival from the date of diagnosis among patients with head-and-neck cancer stratified by whether patients developed aspiration pneumonia (N=801) or not (N=2,712).
Discussion
This present US population-based study demonstrates substantial rates of aspiration pneumonia after concurrent chemotherapy with radiation in older patients with head-and-neck cancer. Previously reported rates of aspiration pneumonia for head-and-neck cancer patients after chemoradiotherapy have varied across study populations. A recent single-center study of 324 consecutively treated head-and-neck cancer patients from 2006 to 2008 reported that 5.3% patients developed aspiration pneumonia within in the first year after radiation with or without concurrent weekly cisplatin 12. Eisbruch et al. reported 23% of their 29 patients treated with radiation and concurrent gemicitabine developed aspiration pneumonia within 14 months of completing their treatment [9] while Hunter et al. reported 15% of their 72 patients with locally advanced oropharyngeal cancer treated with IMRT and carboplatin/paclitaxel developed aspiration pneumonia within 24 months of treatment 11. A larger Taiwanese retrospective study of 15,894 head-and-neck cancer patients based on insurance claims reported only a 5% incidence of pneumonia, most commonly associated with aspiration, within three months of treatment. 13 However, only 15% of the patients were older than 65. This current study found higher rates of aspiration pneumonia than the study from Taiwan, but identified similar risk factors such as age, male gender, and hypopharyngeal tumors. The range of incidence rates reported for aspiration pneumonia may be due to differences in patient characteristics, treatment specifics, follow-up, and definitions of aspiration pneumonia.
However, aspiration pneumonia is a relatively common event even among older adults without cancer. For elderly patients, the etiology of aspiration likely stems from multiple causes though data suggest that the progressive age-related loss of protective swallowing reflexes may be contributory 27. Among patients with head-and-neck cancer, the etiology of aspiration pneumonia is likely multifactorial; however, radiation induced dysphagia presumably plays a central role 28. Radiotherapy can produce fibrosis of the treated pharyngeal tissues resulting in nerve impairment and muscular injury causing generalized weakness and uncoordinated swallowing. Additionally, radiation induced xerostomia reduces salivary flow which could also impair the swallowing mechanism thus increasing the risk of aspiration pneumonia.
Radiation may also represent a unique risk factor for the development of aspiration pneumonia compared to other treatment modalities for head-and-neck cancer. A recent population-based study of 93,663 patients who underwent surgery for head-and-neck cancer found only a 2% rate of aspiration pneumonia 29. Our study also found a lower risk of aspiration pneumonia for those treated with surgery prior to radiation though these findings could represent selection bias in that patients treated with surgery may have better overall health, less comorbidity, superior performance status, or more favorable cancer characteristics compared to those not receiving surgery. We also identified an increased risk of aspiration pneumonia in patients receiving care at a teaching hospital, which may reflect differences in un-measured patient characteristics. One could hypothesize that teaching hospitals may care for sicker or more complex patients which could explain the increased risk of aspiration in this sub-population.
Beyond the increased risk of aspiration with chemoradiotherapy, this study highlights the potential of aspiration pneumonia to substantially impact survival. This current study found significantly increased mortality after aspiration pneumonia. Other studies evaluating survival after aspiration pneumonia in non-cancer patients have found similar increases in the risk of death. A recent single institution retrospective study of 628 patients with aspiration pneumonia found a 30-day mortality rate of 21% though with a higher rate of hospitalization and ICU admission (99% and 38%, respectively)30. Similarly, a multicenter retrospective cohort study of patients admitted with pneumonia found that aspiration pneumonia was the greatest risk factor for 30-day mortality with an adjusted hazard ratio of 5.69 31.
The morbidity and potential impact on mortality of aspiration pneumonia after chemoradiotherapy raises the question of whether action can be taken to reduce the risk of this adverse event. Radiation techniques that spare the constrictor muscles involved with swallowing show potential to reduce dysphagia, a risk factor for aspiration pneumonia 32, 33. Prospective studies to reduce the radiation dose in subsets of head-and-neck cancer patients are ongoing, and hopefully the decreased radiation dose will reduce toxicity without sacrificing local tumor control. Further research should focus on reducing the volume of tissue electively irradiated in head-and-neck cancer for patients with lower risk of regional tumor spread. Beyond modifying radiation fields, a different approach could concentrate on the preservation of swallowing function with early involvement of speech therapy and rehabilitation among patients at high risk of aspiration. Routine swallowing evaluations to screen for dysphagia among this patient population might also be recommended to select patients who are at risk for this complication. These interventions deserve further study as they stand to potentially improve survivorship in patients with head-and-neck cancer.
While this study characterizes the risk of aspiration pneumonia in a large cohort of head-and-neck cancer patients, there are limitations to the presented results. This study population only reported on patients over 66 years old and therefore, these findings cannot be generalized to a younger patient population. Medicare data is collected for administration purposes, and consequentially, confounding factors such as smoking status, dietary intake, body habitus, and performance status were not collected and could not be analyzed. Similarly, SEER-Medicare data does not include information regarding the doses of chemotherapy or radiotherapy delivered which could impact the rate of aspiration pneumonia. Furthermore, the higher aspiration pneumonia rates in this patient population could arise from local cancer progression as opposed to a radiation-induced toxicity. However, we found no correlation between tumor stage and aspiration rates. Additionally, this study could not directly assess the accuracy of the primary endpoint of aspiration pneumonia. While separate diagnosis codes exist for aspiration pneumonia and bacterial pneumonia, distinguishing these two diagnoses can pose a clinical challenge with misclassification potentially impacting our findings. Finally, SEER-Medicare data do not contain information on causality; therefore, future studies with additional longitudinal data are required to fully understand what drives the increased risk of aspiration in this cohort of patients.
Overall, this study represents the first US-based population study to define the incidence of aspiration pneumonia in patients with head-and-neck cancer treated with concurrent chemoradiotherapy contextualized with a comparison to the rate of aspiration in a matched sample of Medicare beneficiaries without cancer. This work also identified several risk factors that can be used to identify patients at a higher risk of developing aspiration pneumonia and suggested avenues for future mitigation of this clinically significant and potentially fatal complication of chemoradiation in head-and-neck cancer.
Acknowledgments
Support: This study was supported by NIH KL2 RR031978 (JDM) and NIH T32 (IJB).
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Disclosures: There are no financial disclosures from any of the authors.
References
- 1.Francis DO, Weymuller EA, Jr, Parvathaneni U, Merati AL, Yueh B. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol. 2010;119:391–397. doi: 10.1177/000348941011900605. [DOI] [PubMed] [Google Scholar]
- 2.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–671. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 3.Logemann JA, Rademaker AW, Pauloski BR, et al. Site of disease and treatment protocol as correlates of swallowing function in patients with head and neck cancer treated with chemoradiation. Head Neck. 2006;28:64–73. doi: 10.1002/hed.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith RV, Kotz T, Beitler JJ, Wadler S. Long-term swallowing problems after organ preservation therapy with concomitant radiation therapy and intravenous hydroxyurea: initial results. Arch Otolaryngol Head Neck Surg. 2000;126:384–389. doi: 10.1001/archotol.126.3.384. [DOI] [PubMed] [Google Scholar]
- 5.Langerman A, Maccracken E, Kasza K, Haraf DJ, Vokes EE, Stenson KM. Aspiration in chemoradiated patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1289–1295. doi: 10.1001/archotol.133.12.1289. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen NP, Moltz CC, Frank C, et al. Dysphagia following chemoradiation for locally advanced head and neck cancer. Ann Oncol. 2004;15:383–388. doi: 10.1093/annonc/mdh101. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen NP, Smith HJ, Dutta S, et al. Aspiration occurence during chemoradiation for head and neck cancer. Anticancer Res. 2007;27:1669–1672. [PubMed] [Google Scholar]
- 8.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen NP, Frank C, Moltz CC, et al. Aspiration rate following chemoradiation for head and neck cancer: an underreported occurrence. Radiother Oncol. 2006;80:302–306. doi: 10.1016/j.radonc.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Purkey MT, Levine MS, Prendes B, Norman MF, Mirza N. Predictors of aspiration pneumonia following radiotherapy for head and neck cancer. Ann Otol Rhinol Laryngol. 2009;118:811–816. [PubMed] [Google Scholar]
- 11.Hunter KU, Lee OE, Lyden TH, et al. Aspiration pneumonia after chemo-intensity-modulated radiation therapy of oropharyngeal carcinoma and its clinical and dysphagia-related predictors. Head Neck. 2014;36:120–125. doi: 10.1002/hed.23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortensen HR, Jensen K, Grau C. Aspiration pneumonia in patients treated with radiotherapy for head and neck cancer. Acta Oncol. 2013;52:270–276. doi: 10.3109/0284186X.2012.742205. [DOI] [PubMed] [Google Scholar]
- 13.Chu CN, Muo CH, Chen SW, Lyu SY, Morisky DE. Incidence of pneumonia and risk factors among patients with head and neck cancer undergoing radiotherapy. BMC Cancer. 2013;13:370. doi: 10.1186/1471-2407-13-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SW, Yang SN, Liang JA, Lin FJ. The outcome and prognostic factors in patients with aspiration pneumonia during concurrent chemoradiotherapy for head and neck cancer. Eur J Cancer Care (Engl) 2010;19:631–635. doi: 10.1111/j.1365-2354.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. [accessed October 13, 2014];Surveillance, Epidemiology and End Results (SEER) Program. Available from URL: http://www.seer.cancer.gov.
- 17.VanderWalde NA, Meyer AM, Liu H, et al. Patterns of care in older patients with squamous cell carcinoma of the head and neck: a surveillance, epidemiology, and end results-medicare analysis. J Geriatr Oncol. 2013;4:262–270. doi: 10.1016/j.jgo.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 20.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40:49–54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 21.Buck CJ, Netter FH American Medical Association. 2013 ICD-9-CM for hospitals, volumes 1, 2, & 3. Professional ed. St. Louis, Mo.: Elsevier; 2013. [Google Scholar]
- 22.McCarthy EP, Iezzoni LI, Davis RB, et al. Does clinical evidence support ICD-9-CM diagnosis coding of complications? Med Care. 2000;38:868–876. doi: 10.1097/00005650-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Lin G, So Y, Johnston G. Analyzing survival data with competing risks using SAS® software. Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 25.Kohl M, Heinze G. PSHREG: A SAS® macro for proportional and nonproportional substribution hazards regression with competing risk data. Vienna: Medical University of Vienna; 2013. [Google Scholar]
- 26.D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in Medicine. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Pontoppidan H, Beecher HK. Progressive loss of protective reflexes in the airway with the advance of age. JAMA. 1960;174:2209–2213. doi: 10.1001/jama.1960.03030180029007. [DOI] [PubMed] [Google Scholar]
- 28.Russi EG, Corvo R, Merlotti A, et al. Swallowing dysfunction in head and neck cancer patients treated by radiotherapy: review and recommendations of the supportive task group of the Italian Association of Radiation Oncology. Cancer Treat Rev. 2012;38:1033–1049. doi: 10.1016/j.ctrv.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Semenov YR, Starmer HM, Gourin CG. The effect of pneumonia on short-term outcomes and cost of care after head and neck cancer surgery. Laryngoscope. 2012;122:1994–2004. doi: 10.1002/lary.23446. [DOI] [PubMed] [Google Scholar]
- 30.Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. Journal of Hospital Medicine. 2013;8:83–90. doi: 10.1002/jhm.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komiya K, Ishii H, Umeki K, et al. Impact of aspiration pneumonia in patients with community-acquired pneumonia and healthcare-associated pneumonia: A multicenter retrospective cohort study. Respirology. 2013;18:514–521. doi: 10.1111/resp.12029. [DOI] [PubMed] [Google Scholar]
- 32.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 33.van der Laan HP, Gawryszuk A, Christianen ME, et al. Swallowing-sparing intensity-modulated radiotherapy for head and neck cancer patients: treatment planning optimization and clinical introduction. Radiother Oncol. 2013;107:282–287. doi: 10.1016/j.radonc.2013.05.004. [DOI] [PubMed] [Google Scholar]