Abstract
Large demyelinating inflammatory central nervous system (CNS) lesions may present with contrast enhancement on magnetic resonance imaging and may mimic CNS tumors such as glioma. In ambiguous cases, new diagnostic tools that may be helpful for distinguishing between demyelinating inflammatory and neoplastic CNS lesions are required. The current study presents the case of a patient with a large contrast-enhanced frontal brain lesion, who was initially diagnosed with tumefactive multiple sclerosis. Following the progression of the brain lesion, an 18F-fluoroethyl-L-tyrosine positron emission tomography (18F-FET PET) was performed, revealing markedly elevated static 18F-FET uptake parameters along with time activity-curves consistent with glioma. Subsequently, a biopsy was undertaken, which confirmed the presence of anaplastic oligoastrocytoma. This case illustrates that 18F-FET PET may provide useful diagnostic information in cases where distinction between neoplastic and demyelinating inflammatory CNS lesions is challenging. However, further systematic and prospective analyses are warranted to explore the value of this method in this setting.
Keywords: 18F-fluoroethyl-L-tyrosine positron emission tomography, tumefactive multiple sclerosis, dynamic 18F-FET PET, static 18F-FET PET, glioma
Introduction
Tumefactive multiple sclerosis (MS) is an uncommon tumor-like variant of MS that is characterized as a demyelinating inflammatory CNS disease with large acute lesions of ≥2 cm in diameter (1). In a large proportion of cases, these lesions are accompanied by edema, ring enhancement on imaging studies, and mass effects (2). As other space occupying lesions, including primary brain tumors, abscesses, metastases and stroke, may present similarly on magnetic resonance imaging (MRI), it is often challenging to determine the correct diagnosis (1). Diagnosis is further hampered when the patient has not yet been diagnosed with MS. In addition, although even more uncommon, the coincidence of brain tumors and tumefactive MS is possible, even within the same lesion (3,4). Therefore, correct diagnosis of tumefactive MS is essential and frequently requires biopsy (5).
However, biopsy may be reluctantly undertaken due to its inherent small but non-negligible risks (6). As recently suggested, factors advocating the diagnosis of tumefactive MS and supporting deferral of biopsy include the additional presence of oligoclonal cerebrospinal fluid (CSF) banding, and/or white matter lesions suggestive of MS, and/or a sustained response to corticosteroids. In the presence of these conditions and the absence of clinical deterioration, a ‘wait and see’ strategy without biopsy and with frequent follow-up MRI may be justifiable (7).
In cases of suspected MS with tumefactive lesions, it has been suggested that further imaging modalities be utilized to guide clinical decision-making (8). In particular, positron emission tomography (PET) using radioactively labelled, metabolically active tracers, such as 18F-fluorodeoxyglucose (18F-FDG) and 11C-methionine, is considered potentially useful. As 18F-FDG uptake has a high background activity in the brain, there is a high false-negative rate for the detection of an underlying glioma. This is not the case with 11C-methionine (9). However, 11C-methionine has a short half-life. Therefore, its use is restricted to centers with an on-site cyclotron (9).
By contrast, 18F-fluoroethyl-L-tyrosine (18F-FET), another commonly used radiolabelled amino acid, is characterized by a longer half-life and is thus more suitable for widespread clinical usage (10). 18F-FET uptake in glioma is high even in the presence of an intact blood-brain barrier (11). Contrast-enhancing non-tumoral lesions usually exhibit a normal 18F-FET uptake (12). Despite these properties, 18F-FET PET has so far not been systematically used with regard to distinction between inflammatory and tumorous lesions.
To the best of our knowledge, the current study presents the first documented case of a patient with a large space-occupying lesion initially diagnosed as tumefactive MS based on clinical and imaging factors, in whom subsequent 18F-FET PET correctly predicted a diagnosis of a glioma.
Case report
A 41-year-old Caucasian woman was admitted to University of Bonn Medical Center (Bonn, Germany) with a generalized seizure in December 2010. MRI revealed a left frontal ring-enhancing lesion with additional non-enhancing periventricular lesions and further lesions in the corpus callosum (Fig. 1A and B). As treatment, dexamethasone (8 mg, intravenously, 3 times per day) was administered for several days, and the patient commenced levetiracetam (1,000 mg, intravenously, 3 times per day) as anti-epileptic medication. Physical examination at that time and on follow-up visits revealed no pathological findings. A serum screen for anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, anti-cardiolipin antibodies, angiotensin converting enzyme, lysozyme and C-reactive protein yielded negative results or normal values. An infection screen, including human immunodeficiency virus, treponemal and borrelia serology tests yielded negative results or results within the normal ranges. There was no elevated cell count in the CSF, glucose and lactate values were within normal range, and fluorescence-activated cell sorting analysis revealed no atypical cells. However, the CSF was positive for oligoclonal bands. Somatosensory and visual evoked potentials were normal.
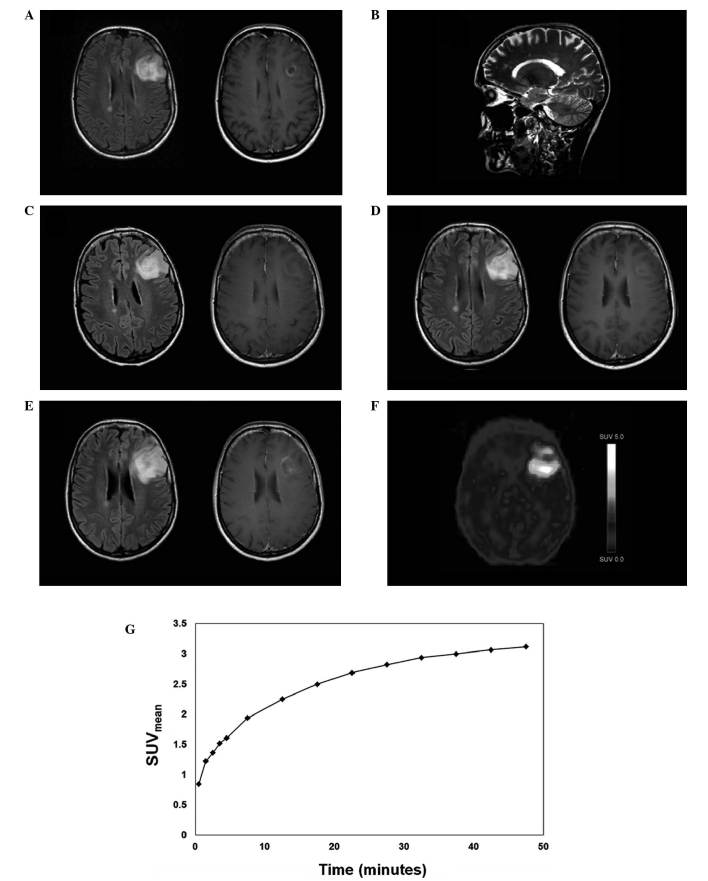
Figure 1.
MRI and 18F-FET PET imaging. (A) MRI scans at patient's initial presentation with several small periventricular hyperintense lesions and a large left frontal lesion on axial FLAIR imaging (left) with ring-shaped enhancement on T1-weighted (right) spin echo sequences following intravenous administration of gadolinium. (B) Persisting linear and ovoid lesions in the periventricular white matter on a sagittal T2-weighted turbo spin-echo sequence. (C) Sustained response and (D) size decrease of the hyperintense lesion on FLAIR (left) and T1-weighted (right) imaging following contrast, observed over a course of 3 years in response to corticosteroid pulse treatment. (E) 3 years later, another increase in size observed on FLAIR (left) and T1-weighted (right) pulse sequences following contrast administration. (F) 18F-FET PET imaging showing high 18F-FET uptake in the left frontal lesion with (G) initial rapid increase in SUVmean followed by slower further tracer accumulation. MRI, magnetic resonance imaging; 18F-FET, 18F-fluoroethyl-L-tyrosine; PET, positron emission tomography; FLAIR, fluid attenuated inversion recovery; SUVmean, mean standardized uptake value.
Three weeks later, a follow-up MRI revealed a slight reduction of contrast-enhancement (Fig. 1C). Barkhof criteria (13) for the MRI supported a diagnosis of MS. Owing to a decrease of contrast-enhancement on follow-up MRI, a planned biopsy was withdrawn, and the patient was further observed by clinical examination and MRI.
This reduction of contrast-enhancement sustained for >3 years (Fig. 1D), until it reappeared along with an increase in lesion size (Fig. 1E). However, this finding was not accompanied by any clinical deterioration of the patient. Subsequently, methlyprednisolone pulse therapy (1,000 mg, intravenously, 3 times per day) was administered for 5 days, following which no effect on the contrast-enhancing lesion was observed on a follow-up MRI. To explore the possibility of the coexistence of a primary brain tumor, 18F-FET PET was performed (213 MBq 18F-FET, intravenous; dynamic acquisition over 50 min; reconstruction of a static frame from 20–40 min). Static 18F-FET PET revealed high tumor-to-brain ratio (TNR) values, indicating a neoplastic lesion (TNRmax, 3.8; Fig. 1F) (14). Kinetic analysis revealed a clear uptake pattern in the mean standardized uptake value, with a rapid initial increase followed by a slow further tracer accumulation (Fig. 1G), a pattern which has been described in low-grade glioma (15). A stereotactic biopsy revealed a World Health Organization (WHO) grade II glioma (16). Subsequent complete resection of the tumor provided material that allowed the diagnosis of a WHO grade III oligoastrocytoma. Following diagnosis, the patient was irradiated and consequently treated with a combination of procarbazine (100 mg daily, days 8–22) and lomustine (110 mg/kg, day 1) for 6 eight-week cycles. Brain MRI examination performed at the most recent follow-up in November 2015, revealed that the patient exhibited stable disease.
This report was approved by the local ethics committee, and the patient provided written informed consent for its publication.
Discussion
The present case report outlines the clinical course of a patient initially diagnosed with tumefactive MS, with white matter lesions reminiscent of MS, positive unmatched oligoclonal bands in the CSF, sustained response to corticosteroids and clinical stability following corticosteroid therapy. However, as proven by histology, the diagnosis was eventually determined to be an anaplastic oligoastrocytoma, likely coexisting with MS, and was established >3 years after initial clinical presentation.
The decision in favor of biopsy and subsequent resection in this particularly eloquent brain area was supported by 18F-FET PET results. Static and dynamic analyses of 18F-FET uptake yielded results typical for glioma. Several larger case series have analyzed 18F-FET uptake in cerebral lesions of unknown significance, among which few cases of demyelinating lesions have been reported (16,17).
In line with these reports, the presented case highlights the potential value of 18F-FET PET as a tool to distinguish between MS and primary brain tumors. Both static and dynamic parameters (as demonstrated for the first time in the present case) are important to make this distinction. In particular, the present case demonstrates that these parameters allow the detection of a glioma on a background of an unequivocal diagnosis of MS, where larger lesions may be highly suggestive of tumefactive MS. In such cases, 18F-FET PET should be added early to the portfolio of diagnostic procedures. Overall, further systematic evaluation is warranted to explore the value of 18F-FET PET imaging in the workup of unclear, putatively inflammatory cerebral lesions.
References
- 1.Nilsson P, Larsson EM, Kahlon B, Nordström CH, Norrving B. Tumefactive demyelinating disease treated with decompressive craniectomy. Eur J Neurol. 2009;16:639–642. doi: 10.1111/j.1468-1331.2009.02547.x. [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131:1759–1775. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green AJ, Bollen AW, Berger MS, et al. Multiple sclerosis and oligodendroglioma. Mult Scler. 2001;7:269–273. doi: 10.1177/135245850100700410. [DOI] [PubMed] [Google Scholar]
- 4.Khan SH, Buwembo JE, Li Q. Concurrence of glioma and multiple sclerosis. Can J Neurol Sci. 2005;32:349–351. doi: 10.1017/S031716710000425X. [DOI] [PubMed] [Google Scholar]
- 5.Given CA, 2nd, Stevens BS, Lee C. The MRI appearance of tumefactive demyelinating lesions. AJR Am J Roentgenol. 2004;182:195–199. doi: 10.2214/ajr.182.1.1820195. [DOI] [PubMed] [Google Scholar]
- 6.Butteriss DJ, Ismail A, Ellison DW, Birchall D. Use of serial proton magnetic resonance spectroscopy to differentiate low grade glioma from tumefactive plaque in a patient with multiple sclerosis. Br J Radiol. 2003;76:662–665. doi: 10.1259/bjr/85069069. [DOI] [PubMed] [Google Scholar]
- 7.Hardy TA, Chataway J. Tumefactive demyelination: An approach to diagnosis and management. J Neurol Neurosurg Psychiatry. 2013;84:1047–1053. doi: 10.1136/jnnp-2012-304498. [DOI] [PubMed] [Google Scholar]
- 8.Takenaka S, Shinoda J, Asano Y, et al. Metabolic assessment of monofocal acute inflammatory demyelination using MR spectroscopy and (11)C-methionine-, (11)C-choline-, and (18)F-fluorodeoxyglucose-PET. Brain Tumor Pathol. 2011;28:229–238. doi: 10.1007/s10014-011-0027-3. [DOI] [PubMed] [Google Scholar]
- 9.Gulyás B, Halldin C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imaging. 2012;56:173–190. [PubMed] [Google Scholar]
- 10.Floeth FW, Sabel M, Stoffels G, Pauleit D, Hamacher K, Steiger HJ, Langen KJ. Prognostic value of 18F-fluoroethyl-L-tyrosine PET and MRI in small nonspecific incidental brain lesions. J Nucl Med. 2008;49:730–737. doi: 10.2967/jnumed.107.050005. [DOI] [PubMed] [Google Scholar]
- 11.Langen KJ, Hamacher K, Weckesser M, et al. O-(2-[18F]Fluoroethyl)-L-tyrosine: Uptake mechanisms and clinical applications. Nucl Med Biol. 2006;33:287–294. doi: 10.1016/j.nucmedbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Piroth MD, Pinkawa M, Holy R, et al. Prognostic value of early [18F]Fluoroethyltyrosine positron emission tomography after radiochemotherapy in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;80:176–184. doi: 10.1016/j.ijrobp.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 13.Rovaris M, Barkhof F, Calabrese M, De Stefano N, Fazekas F, Miller DH, Montalban X, Polman C, Rocca MA, Thompson AJ, et al. MRI features of benign multiple sclerosis: toward a new definition of this disease phenotype. Neurology. 2009;72:1693–1701. doi: 10.1212/WNL.0b013e3181a55feb. [DOI] [PubMed] [Google Scholar]
- 14.Rapp M, Heinzel A, Galldiks N, et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med. 2013;54:229–235. doi: 10.2967/jnumed.112.109603. [DOI] [PubMed] [Google Scholar]
- 15.Pöpperl G, Kreth FW, Mehrkens JH, et al. FET PET for the evaluation of untreated gliomas: Correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging. 2007;34:1933–1942. doi: 10.1007/s00259-007-0534-y. [DOI] [PubMed] [Google Scholar]
- 16.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutterer M, Nowosielski M, Putzer D, Jansen NL, Seiz M, Schocke M, McCoy M, Göbel G, la Fougère C, Virgolini IJ, Trinka E, Jacobs AH, Stockhammer G. [18F]-fluoro-ethyl-L-tyrosine PET: A valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013;15:341–351. doi: 10.1093/neuonc/nos300. [DOI] [PMC free article] [PubMed] [Google Scholar]