Abstract
Perioperative and postoperative blood transfusions (BT), anemia and inflammation are associated with poor survivals in patients with non-small cell lung cancer (NSCLC). This study investigated the impact of perioperative BT on the survival of patients with NSCLC taking into account their preoperative inflammatory status and the presence of anemia. Demographic, perioperative, and survival data for 861 patients with stage I NSCLC was collected retrospectively. The primary endpoints of interest were recurrence-free (RFS) and overall survival (OS). Before and after propensity score matching, univariate and multivariable Cox proportional hazards models were used to evaluate the association between covariates and survival. A neutrophil-to-lymphocyte ratio (NLR) < 5 (hazard ratio [HR]: 0.58, 95% CI: 0.38-0.87; p = 0.009) and normal Hb concentration (HR: 0.72, 95% CI: 0.72; p = 0.022) were independently associated with longer RFS. The administration of blood perioperatively was associated with a trend towards worse RFS (HR: 0.69, 95% CI: 0.47-1.02; p = 0.066). The multivariate analysis also revealed that an NLR < 5 (HR: 0.48, 95% CI: 0.3-0.76; p = 0.001) and the absence of BT (HR: 0.63, 95% CI: 0.4-0.98; p = 0.04) were significantly associated with lower mortality risk. The propensity score matching analysis did not confirm the association between BT and poor RFS (HR: 0.63, 95% CI: 0.35-1.1; p = 0.108) and OS (HR: 0.52, 95% CI: 0.26-1.04; p = 0.06). Inflammation and anemia are common finding in patients with stage 1 NSCLC. After adjusting for these two important confounders, this study confirms that previous reports demonstrating an association between BT and poor survival after NSCLC surgery.
Keywords: Non-small cell, blood transfusions, anemia, cancer recurrence, survival
Introduction
Approximately 2.8 million units of packed red blood cells (pRBCs) are transfused into patients undergoing surgery every year in the United States [1, 2]. Blood transfusions can expose patients to serious complications, including hemolytic reactions and transfusion-related immune suppression [3, 4], which may be one of the mechanisms underlying the association between blood transfusions and poor clinical outcomes [4]. Clinical studies have shown that in patients with gastrointestinal malignancies, perioperative allogeneic blood transfusion (ABT) is an independent risk factor for poor survival [5-7], and in-addition a recent meta-analysis demonstrated an association between ABT and shorter recurrence-free survival (RFS) and overall survival (OS) in lung cancer patients [8].
Other factors may influence the effect that ABT has on survival outcomes. In patients with non–small cell lung cancer, preoperative anemia is common and is an independent prognostic factor [9, 10]. In these patients, preoperative anemia can be multifactorial; however, low hemoglobin (Hb) concentrations, particularly in patients with more aggressive tumors, may also be related to complex interactions among the immune system, tumor microenvironment, and cancer cells [11, 12]. Moreover, the presence of both anemia and leukocytosis may be associated with worse prognosis than the presence of either condition alone is [10]. Preoperative inflammatory status is another important predictor of survival in patients with stage I NSCLC. In this patient population, those with a neutrophil-to-lymphocyte ratio (NLR) ≥ 5 have recurrence and mortality risks that are 2 times higher than those of patients with an NLR < 5 [13, 14].
To date no study has studied the clinical relevance of ABT, preoperative anemia and inflammatory status in patients with stage I NSCLC. We hypothesized that perioperative ABT, after adjustment for both preoperative anemia and inflammatory status, is an independent prognostic factor for poor survival in patients with stage I NSCLC. To test this hypothesis, we retrospectively assessed the effect that the combination of high NLR, preoperative anemia, and perioperative ABT has on the RFS and OS of these patients.
Materials and methods
Patients
This retrospective study was approved by The University of Texas MD Anderson Cancer Center's Institutional Review Board. Study data were collected and managed using REDCap electronic data capture tools hosted at MD Anderson Cancer Center. We retrieved demographic, perioperative, and survival data for 861 patients with stage I NSCLC who underwent surgery with curative intent between January 1, 2004, and July 31, 2014, at The University of Texas MD Anderson Cancer Center. We excluded patients who had undergone palliative surgery or had secondary malignancies.
Preoperative Hb concentrations were obtained from routine blood tests. Preoperative anemia was defined according to World Health Organization (WHO) criteria (Hb concentration < 13 g/dL for men and < 12 g/dL for women) [15]. Perioperative ABT, given at the discretion of the treating physician, was defined as any pRBC unit given during surgery or within 30 days after surgery. The preoperative NLR was calculated by dividing the neutrophil count by the lymphocyte count. An NLR ≥ 5 and an NLR < 5 were used to define high preoperative inflammatory status and low preoperative inflammatory status, respectively [13].
Statistical analysis
The primary endpoint of interest was RFS, defined as the time from surgery to recurrence or death, whichever occurred first. OS was defined as the time from surgery to death from any cause. Descriptive statistics, including means, standard deviations, medians, and ranges, were used to describe continuous variables such as age, body mass index (BMI), and NLR. Frequency counts and percentages were used to describe categorical variables such as gender, American Society of Anesthesiologists (ASA) physical status, and perioperative ABT rate. The Fisher exact test or chi-square test was used to assess associations between 2 categorical variables. The Wilcoxon rank-sum test or Kruskal-Wallis test was used to assess differences in continuous variables between or among patient groups. The Kaplan-Meier method was used to estimate RFS and OS. The median RFS and OS durations and/or rates and 95% confidence intervals (CIs) for different patient groups were calculated. The log-rank test was used to evaluate the difference in RFS and OS durations and/or rates between patient groups. Univariate Cox proportional hazards models were fitted to evaluate the effects of continuous variables on RFS and OS durations and/or rates. Univariate analysis revealed to be significantly associated with RFS and OS durations were included in the multivariate analysis. Multivariable Cox proportional hazards models were used for the multivariate analysis.
To adjust for selection bias, we conducted a propensity score matching (PSM) analysis. The propensity score was the conditional probability of receiving a blood transfusion on a set of observed covariates. Age, BMI, ASA (1/2 vs. 3/4), tumor histology (no adenocarcinoma vs. adenocarcinoma), preoperative NLR, preoperative Hb, preoperative chemoradiation, and surgery type (thoracotomy vs. thoracoscopy) the covariates included in the multicovariate logistic model to estimate the propensity scores. Some of these prognostic covariates were significantly imbalanced between the groups of patients who received or not BT (BMI (<=25 vs. >25; p-value=0.0029), tumor histology (no adenocarcinoma vs. adenocarcinoma; p-value=0.0368), preop NLR (<5 vs. >=5; p-value= 0.0024), preoperative Hb (abnormal vs. normal; p-value<0.0001)).
Among the 861 patients, the propensity score was calculated. The Greedy 5→1 digit match algorithm was used to match the baseline covariates, so that the two groups (with perioperative BT or without perioperative BT) would have similar propensity scores. Sixty-two patients who received BT and with non-missing values for the covariates were matched with a 1:1 ratio to the non-transfused patients BT and with non-missing values for the covariates. Univariate and multivariate Cox proportional hazards models were fitted on the data after PSM to assess the association between BT and RFS or OS. P values < 0.05 were considered statistically significant.
All statistical analyses were performed using the statistical software programs SAS 9.3 (SAS, Cary, NC) and S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA).
Results
Patient characteristics
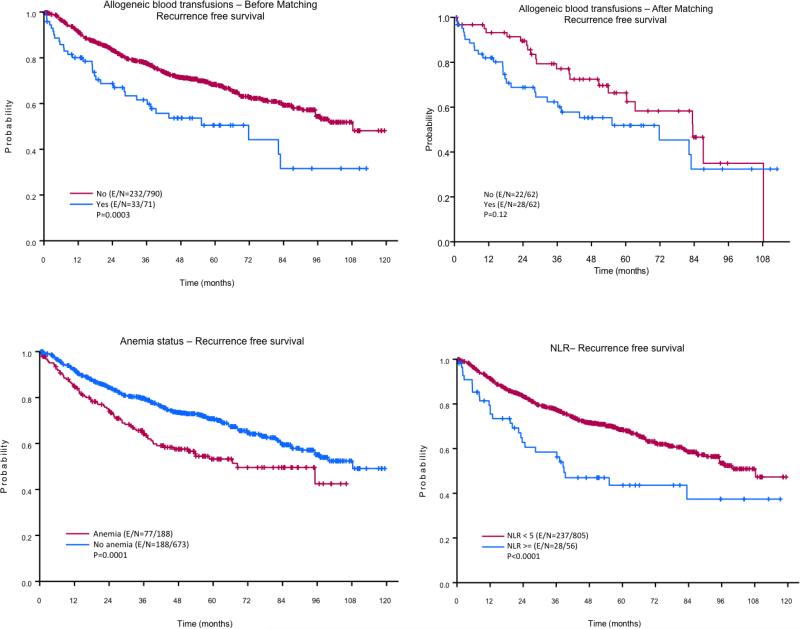
The 861 patients’ clinical and tumor characteristics are given in Table 1. Overall, 56 patients (6.5%) had an NLR ≥ 5, 188 patients (21.84%) had preoperative anemia, and 71 patients (8.25%) received perioperative ABT. Of the patients who received ABT, more than three-fourths (78.87%; 56 patients) received 1–3 units of pRBCs. Compared with patients who did not receive perioperative ABT, those who did receive perioperative ABT were significantly more likely to have a BMI < 25 (p = 0.002), preoperative anemia (p = 0.0001), an NLR ≥ 5 (p < 0.0001), a histology other than adenocarcinoma (p = 0.036), and adjuvant radiation (p = 0.028). We found no statistically significant differences between the patients who did and those who did not receive ABT in terms of age, gender, ASA physical status, neoadjuvant chemotherapy, neoadjuvant radiation, or adjuvant chemotherapy.
Table 1.
Patient and Tumor Characteristics of All Patients and According to Transfusion Status
| Non-matched | Non-matched | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | All patients, n = 861 | No transfusion, n = 790 | Transfusion, n = 71 | p Value | No transfusion, n = 62 | Transfusion, n = 62 | Standardized Difference in %* |
| Mean age, years (SD) | 65.29 (11.02) | 65.08 (11.14) | 67.64 (9.26) | 0.103 | 66.97 (10.10) | 67.33 (8.83) | 3.79 |
| Mean BMI (SD) | 27.41 (5.55) | 26.58 (5.48) | 24.50 (5.95) | 0.002 | 25.86 (4.03) | 25.93 (6.14) | 1.24 |
| Gender | |||||||
| Male | 394 (45.76) | 365 (46.2) | 29 (40.8) | 0.385 | - | - | |
| Female | 467 (54.25) | 425 (53.8) | 42 (59.2) | . | - | - | |
| ASA physical status | |||||||
| 1-2 | 96 (11.15) | 92 (11.6) | 4 (5.6) | 0.166 | 3(42.9%) | 4(57.1%) | 6.94 |
| 3-4 | 765 (88.85) | 698 (88.4) | 67 (94.4) | . | 59(50.4%) | 58(49.6%) | |
| Mean NLR (SD) | 2.67 (1.81) | 2.58 (1.61) | 3.65 (3.13) | 0.002 | 3.07 (2.53) | 3.08 (2.17) | 0.42 |
| NLR | |||||||
| < 5 | 805 (93.5) | 747 (94.6) | 58 (81.7) | <0.0001 | 53(49.5%) | 54(50.5%) | |
| ≥ 5 | 56 (6.5) | 43 (5.4) | 13 (18.3) | . | 9(52.9%) | 8(47.1%) | |
| Mean Hb concentration, g/dL (SD) | 13.43 (1.48) | 13.51 (1.42) | 12.51 (1.82) | <0.0001 | 12.79 (1.47) | 12.84 (1.59) | 3.15 |
| Preoperative anemia | |||||||
| Yes | 188 (21.84) | 159 (20.1) | 29 (40.8) | 0.0001 | - | - | |
| No | 673 (78.13) | 631 (79.9) | 42 (59.2) | . | - | - | |
| Histology adenocarcinoma | |||||||
| No | 360 (41.81) | 322 (40.8) | 38 (53.5) | 0.036 | 35 (52.2%) | 30 (52.6%) | 9.64 |
| Yes | 501 (58.19) | 468 (59.2) | 33 (46.5) | . | 27 (47.4%) | 32 (47.8%) | |
| Neoadjuvant chemotherapy | |||||||
| No | 797 (92.57) | 734 (92.9) | 63 (88.7) | 0.198 | - | - | |
| Yes | 64 (7.43) | 56 (7.1) | 8 (11.3) | . | - | - | |
| Neoadjuvant radiation | |||||||
| No | 857 (99.54) | 787 (99.6) | 70 (98.6) | 0.291 | - | - | |
| Yes | 4 (0.46) | 3 (0.4) | 1 (1.4) | . | - | - | |
| Type of surgery | |||||||
| Thoracotomy | 557 (64.69) | 510 (64.6) | 47 (66.2) | 0.781 | 43 (51.2%) | 41 (48.8%) | 6.85 |
| Thoracoscopy | 304 (35.31) | 280 (35.4) | 24 (33.8) | . | 19 (47.5%) | 21 (52.5%) | |
| Adjuvant chemotherapy | |||||||
| No | 89 (10.34) | 708 (89.6) | 64 (90.1) | 0.890 | - | - | |
| Yes | 772 (89.66) | 82 (10.4) | 7 (9.9) | . | - | - | |
| Adjuvant radiation | |||||||
| No | 22 (2.56) | 773 (97.8) | 66 (93) | 0.028 | - | - | |
| Yes | 839 (97.44) | 17 (2.2) | 5 (7) | . | - | - | |
| pRBC units | |||||||
| 0 | 790 (100) | 0 | 0.0001 | - | - | ||
| 1-3 | 0 | 56 (78.9) | . | - | - | ||
| 4-9 | 0 | 15 (21.1) | - | - | |||
Note: All data are no. of patients (%) unless otherwise specified. SD = standard deviation; BMI = body mass index; ASA = American Society of Anesthesiologists; NLR = neutrophil-to-lymphocyte ratio; Hb = hemoglobin; pRBC = packed red blood cells.
The standardized differences for all covariates were <=10.84% in the post-matching cohort, suggesting substantial reduction of bias between the two groups.
RFS estimates
The median follow-up time after surgery was 108.28 months. The results of the univariate analysis of the effects of different variables on 3- and 5-year RFS are given in Table 2. The 3- and 5-year RFS rates of the patients with an NLR ≥ 5 (58% and 44%, respectively) were significantly lower than those of the patients with an NLR < 5 (77% and 68%, respectively; p = 0.0004). The 3- and 5-year RFS rates of the patients with preoperative anemia (64% and 53%, respectively) were significantly lower than those of the patients without preoperative anemia (80% and 71%, respectively; p = 0.0001). The 3- and 5-year RFS rates of the patients who received ABT (62% and 50%, respectively) were significantly lower than those of the patients who did not receive ABT (78% and 68%, respectively; p = 0.0003). The number of pRBCs administered during and/or after surgery also had a negative impact on RFS rates. As expected patients who received > 4 units had the lowest 3- and 5- year RFS (Table 2). In addition, the 3- and 5- year RFS rates of patients age > 65 years, patients with a BMI < 25, men, patients with an ASA physical status of 3-4, and patients who received adjuvant chemoradiation were significantly lower than those of patients age ≤ 65 years (p < 0.0001), patients with a BMI ≥ 25 (p = 0.012), women (p = 0.001), patients with an ASA physical status of 1-2 (p = 0.003), and patients who did not receive adjuvant chemoradiation (p = 0.0027), respectively.
Table 2.
Univariate Analysis of the Effects of Different Variables on 3- and 5-Year Recurrence-Free Survival (RFS) Rates
| Variable | HR (95% CI) | 3-Year RFS rate (95% CI) | 5-Year RFS rate (95% CI) | p Value |
|---|---|---|---|---|
| All patients | 0.76 (0.73,0.79) | 0.67 (0.63-0.71) | ||
| Age (continuous) | 1.03 (1.01-1.04) | < 0.0001 | ||
| Age | < 0.0001 | |||
| < 65 | 0.83 (0.79-0.87) | 0.76 (0.71-0.81) | ||
| ≥ 65 | 0.72 (0.67-0.76) | 0.6 (0.55-0.66) | ||
| BMI (continuous) | 0.97 (0.94-0.99) | |||
| BMI | 0.012 | |||
| ≤ 25 | 0.71 (0.66-0.77) | 0.6 (0.54-0.67) | ||
| > 25 | 0.79 (0.76-0.83) | 0.71 (0.66-0.75) | ||
| Gender | 0.001 | |||
| Male | 0.71 (0.67-0.76) | 0.6 (0.54-0.66) | ||
| Female | 0.8 (0.77-0.84) | 0.73 (0.69-0.78) | ||
| ASA physical status | 0.003 | |||
| 1-2 | 0.92 (0.86-0.98) | 0.81 (0.72-0.92) | ||
| 3-4 | 0.74 (0.71-0.78) | 0.65 (0.61-0.69) | ||
| Adenocarcinoma | 0.938 | |||
| No | 0.76 (0.71-0.81) | 0.67 (0.62-0.73) | ||
| Yes | 0.76 (0.73-0.81) | 0.67 (0.62-0.72) | ||
| Type of surgery | 0.396 | |||
| Thoracotomy | 0.76 (0.72-0.8) | 0.65 (0.61-0.7) | ||
| Thoracoscopy | 0.77 (0.72-0.82) | 0.71 (0.65-0.77) | ||
| Neoadjuvant chemotherapy | 0.043 | |||
| No | 0.77 (0.74-0.8) | 0.68 (0.64-0.71) | ||
| Yes | 0.66 (0.55-0.8) | 0.58 (0.46-0.73) | ||
| Neoadjuvant radiation | 0.405 | |||
| No | 0.76 (0.73-0.79) | 0.67 (0.63-0.71) | ||
| Yes | 0.5 (0.19-1) | 0.5 (0.19-1) | ||
| Adjuvant chemotherapy | 0.760 | |||
| No | 0.76 (0.73-0.8) | 0.67 (0.63-0.71) | ||
| Yes | 0.76 (0.68-0.86) | 0.66 (0.57-0.77) | ||
| Adjuvant radiation | 0.012 | |||
| No | 0.77 (0.74-0.8) | 0.68 (0.64-0.71) | ||
| Yes | 0.57 (0.39-0.83) | 0.39 (0.22-0.7) | ||
| NLR (continuous) | 1.13 (1.08-1.18) | <0.0001 | ||
| NLR | 0.0004 | |||
| < 5 | 0.77 (0.74-0.81) | 0.68 (0.65-0.72) | ||
| ≥ 5 | 0.58 (0.46-0.74) | 0.44 (0.31-0.61) | ||
| Preoperative anemia | ||||
| Yes | 0.64 (0.57-0.72) | 0.53 (0.46-0.62) | 0.0001 | |
| No | 0.8 (0.77-0.83) | 0.71 (0.67-0.75) | ||
| Hb (continuous) | 0.89 (0.82-0.96) | 0.006 | ||
| Blood transfusion | ||||
| No | 0.78 (0.74-0.81) | 0.68 (0.65-0.72) | 0.0003 | |
| Yes | 0.62 (0.51-0.75) | 0.5 (0.39-0.66) |
HR = hazard ratio; CI = confidence interval; BMI = body mass index; ASA = American Society of Anesthesiologists; NLR = neutrophil-to-lymphocyte ratio; Hb = hemoglobin.
The multivariate analysis confirmed that age (p < 0.001), BMI (p = 0.015), and gender (p = 0.008) were independent predictors of RFS (Table 4). Moreover, an NLR < 5 (hazard ratio [HR]: 0.58, 95% CI: 0.38-0.87; p = 0.009) and normal Hb concentration (HR: 0.72, 95% CI: 0.72; p = 0.022) were independently associated with longer RFS. Compared with patients who did receive ABT, patients who did not receive ABT showed a trend towards having better RFS (HR: 0.69, 95% CI: 0.47-1.02; p = 0.066). The univariate and multivariate model after PSM demonstrated that non-transfused patients had a lower risk of recurrence (HR: 0.64, 95% CI: 0.36-1.12; p = 0.122 and HR: 0.63, 95% CI: 0.35-1.1; p = 0.108, respectively), although it did not reach statistical significance.
Table 4.
Multivariate Analysis for Recurrence Free Survival (RFS) and Overall Survival (OS) Rates
| Recurrence Free Survival | Overall Survival | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Matching | After Matching | Before Matching | After Matching | |||||||||||||
| Covariate | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | ||||
| Age, years (continuous) | 1.02 | 1.01 | 1.04 | <0.0001 | 1.04 | 1.02 | 1.06 | <0.0001 | ||||||||
| BMI (continuous) | 0.96 | 0.94 | 0.99 | 0.015 | 0.97 | 0.94 | 1.00 | 0.138 | ||||||||
| Gender (female vs. male) | 0.71 | 0.55 | 0.91 | 0.008 | 0.57 | 0.42 | 0.77 | 0.0003 | ||||||||
| ASA physical status (1-2 vs. 3-4) | 0.58 | 0.35 | 0.95 | 0.138 | 0.63 | 0.35 | 1.15 | 0.138 | ||||||||
| Adenocarcinoma (yes vs. no) | 0.78 | 0.58 | 1.06 | 0.117 | ||||||||||||
| Type of surgery (thoracoscopy vs. thoracotomy) | 0.79 | 0.57 | 1.11 | 0.181 | ||||||||||||
| NLR (< 5 vs. ≥ 5) | 0.58 | 0.38 | 0.97 | 0.009 | 0.48 | 0.30 | 0.76 | 0.0017 | ||||||||
| Preoperative anemia (no vs. yes) | 0.72 | 0.54 | 0.95 | 0.053 | 0.72 | 0.52 | 1.00 | 0.053 | ||||||||
| Blood transfusion (no vs. yes) | 0.69 | 0.47 | 1.02 | 0.066 | 0.630 | 0.35 | 1.1 | 0.108 | 0.63 | 0.40 | 0.98 | 0.044 | 0.52 | 0.26 | 1.04 | 0.066 |
HR = hazard ratio; CI = confidence interval; BMI = body mass index; ASA = American Society of Anesthesiologists; NLR = neutrophil-to-lymphocyte ratio; Hb: hemoglobin.
OS estimates
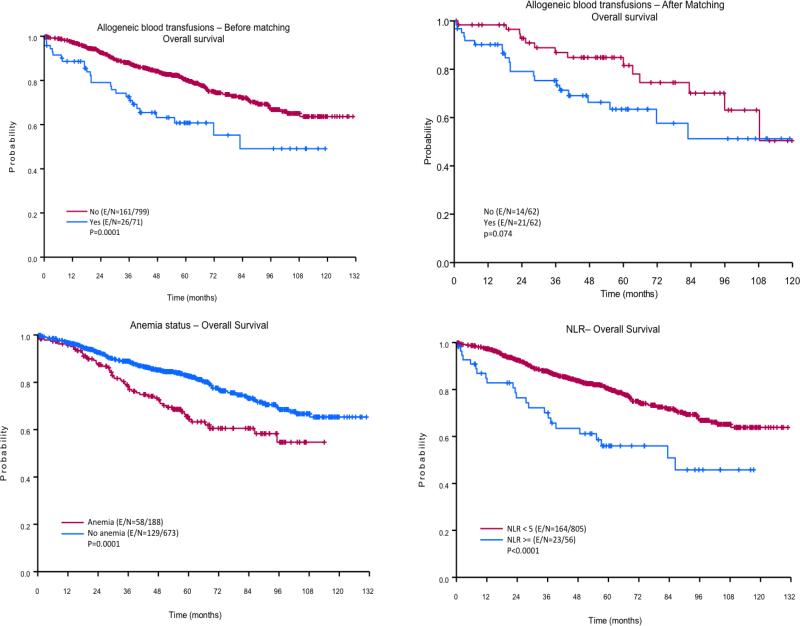
The results of the univariate analysis of the effects of different variables on 3- and 5-year OS are given in Table 3. Patients age < 65 years, women, and patients with an ASA physical status of 1-2 had significantly better 3- and 5-year OS rates compared with patients age ≥ 65 years (p < 0.0001), men (p < 0.0001), and patients with an ASA physical status of 3-4 (p = 0.019), respectively. The 3- and 5-year OS rates of patients with preoperative anemia (78% and 64%, respectively) were significantly lower than those of patients without preoperative anemia (89% and 82%, respectively; p = 0.0001), and the 3- and 5-year OS rates of patients with an NLR ≥ 5 (70% and 56%, respectively) were significantly lower than those of patients with an NLR < 5 (88% and 80%, respectively; p < 0.0001). Patients who received ABT had 3- and 5-year OS rates (73% and 61%, respectively) that were significantly lower than those of patients who did not receive ABT (88% and 80%, respectively; p = 0.0001). The univariate analysis also demonstrated that the number of pRBCs transfused perioperatively also had a negative impact on OS rates. Thus, who received > 4 units had the lowest 3- and 5- year OS (Table 3).
Table 3.
Univariate Analysis of the Effects of Different Variables on 3- and 5-Year Overall Survival (OS) Rates
| Variable | HR (95% CI) | 3-Year OS rate (95% CI) | 5-Year OS rate (95% CI) | p Value |
|---|---|---|---|---|
| All patients | 0.87 (0.84-0.89) | 0.78 (0.75-0.82) | ||
| Age, years (continuous) | 1.05 (1.03-1.06) | < 0.0001 | ||
| Age, years | < 0.0001 | |||
| < 65 | 0.93 (0.9-0.96) | 0.88 (0.84-0.92) | ||
| ≥ 65 | 0.82 (0.78-0.86) | 0.71 (0.67-0.76) | ||
| BMI (continuous) | 0.97 (0.95-1.00) | 0.157 | ||
| BMI | 0.254 | |||
| ≤ 25 | 0.86 (0.82-0.9) | 0.76 (0.71-0.82) | ||
| > 25 | 0.87 (0.84-0.9) | 0.8 (0.76-0.84) | ||
| Gender | < 0.0001 | |||
| Male | 0.81 (0.77-0.85) | 0.7 (0.65-0.75) | ||
| Female | 0.92 (0.89-0.94) | 0.86 (0.82-0.9) | ||
| ASA physical status | 0.011 | |||
| 1-2 | 0.98 (0.95-1) | 0.93 (0.86-0.99) | ||
| 3-4 | 0.85 (0.83-0.88) | 0.77 (0.73-0.8) | ||
| Adenocarcinoma | 0.088 | |||
| No | 0.83 (0.78-0.87) | 0.75 (0.7-0.81) | ||
| Yes | 0.89 (0.87-0.92) | 0.81 (0.77-0.85) | ||
| Type of surgery | 0.180 | |||
| Thoracotomy | 0.86 (0.83-0.89) | 0.77 (0.73-0.81) | ||
| Thoracoscopy | 0.89 (0.85-0.93) | 0.83 (0.78-0.88) | ||
| Neoadjuvant chemotherapy | 0.112 | |||
| No | 0.87 (0.85-0.9) | 0.79 (0.76-0.83) | ||
| Yes | 0.78 (0.69-0.9) | 0.69 (0.58-0.83) | ||
| Neoadjuvant radiation | 0.824 | |||
| No | 0.87 (0.84-0.89) | 0.78 (0.75-0.82) | ||
| Yes | 0.75 (0.43-1) | 0.75 (0.43-1) | ||
| Adjuvant chemotherapy | 0.486 | |||
| No | 0.87 (0.84-0.89) | 0.78 (0.75-0.82) | ||
| Yes | 0.87 (0.81-0.95) | 0.81 (0.73-0.9) | ||
| Adjuvant radiation | 0.147 | |||
| No | 0.87 (0.84-0.89) | 0.79 (0.76-0.82) | ||
| Yes | 0.77 (0.62-0.97) | 0.58 (0.38-0.86) | ||
| NLR (continuous) | 1.16 (1.1-1.22) | < 0.0001 | ||
| NLR | < 0.0001 | |||
| < 5 | 0.88 (0.85-0.9) | 0.8 (0.77-0.83) | ||
| ≥ 5 | 0.7 (0.58-0.84) | 0.56 (0.43-0.72) | ||
| Hb (continuous) | 0.87 (0.79-0.96) | 0.005 | ||
| Preoperative anemia | 0.0001 | |||
| Yes | 0.78 (0.72-0.85) | 0.64 (0.57-0.73) | ||
| No | 0.89 (0.86-0.92) | 0.82 (0.79-0.86) | ||
| Blood transfusion | 0.0001 | |||
| No | 0.88 (0.85-0.9) | 0.8 (0.77-0.83) | ||
| Yes | 0.73 (0.63-0.84) | 0.61 (0.49-0.75) |
HR = hazard ratio; CI = confidence interval; BMI = body mass index; ASA = American Society of Anesthesiologists; NLR = neutrophil-to-lymphocyte ratio; Hb: hemoglobin.
The multivariate analysis revealed that a preoperative NLR < 5 (HR: 0.48, 95% CI: 0.3-0.76; p = 0.001) and avoidance of ABT (HR: 0.63, 95% CI: 0.4-0.98; p = 0.04) were associated with longer overall survival than an NLR ≥ 5 (Table 4). Compared with patients without preoperative anemia, Patients with preoperative anemia showed a trend towards having worse OS (HR: 0.72, 95% CI: 0.52-1.00; p = 0.053). Gender and age were also independent predictors of OS. The univariate and multivariate model after PSM demonstrated that non-transfused patients had an important trend to have a lower risk of overall mortality (HR: 0.53, 95% CI: 0.27-1.06; p = 0.07 and HR: 0.52, 95% CI: 0.26-1.04; p = 0.06, respectively) compared to patients who received pRBCs
Discussion
The ways in which ABT promotes tumor growth and cancer recurrence remain largely unclear; however, this association appears to be related to 2 mechanisms: One associated with the proposed immunosuppressive effect of blood transfusion (i.e., transfusion-related immune suppression) and the other associated with the direct action growth factors have on cancer cells [4, 16]. Our study confirmed that, compared with NSCLC patients who do not receive perioperative ABT, those who do have an increased mortality risk and tend to have shorter RFS, although significant selection bias is a concern since the PSM analysis did not show such association. These results are in line with those of a recent meta-analysis demonstrating that patients with stage I NSCLC who receive ABT are 1.5 times as likely as patients who do not receive ABT to have cancer recurrence and 1.39 times as likely to die of their cancer [8]. However, the present study and the meta-analysis differ in several ways [17-22]. The meta-analysis included fewer patients, defined perioperative ABT as occurring within 7 days (rather than 30 days) following surgery, and did not report preoperative Hb concentrations. The patient population in the study by Peñalver et al., which included more than 800 patients with stage I NSCLC, was much more similar to that of the present study. Peñalver et al. found no association between blood transfusion and OS; however, the authors did not consider preoperative anemia as a possible confounding variable. More importantly, the 5-year survival rate of the ABT patients in the present study (80%) was higher than that of those Peñalver et al. reported on (63%) [19].
Other groups have investigated the impact preoperative anemia has on lung cancer patients’ postoperative oncological outcomes. In agreement with our study, Nosotti et al. and Ng et al. found that transfused patients had lower Hb concentrations than those not transfused [17, 18]. The preoperative Hb concentrations of the patients who did or did not receive transfusions in those studies were nearly identical to those we report here; however, Nosotti et al. did not include anemia as a variable in their analysis [17, 18]. Ng et al. also reported that anemia was associated with increased risks of cancer progression and cancer-related mortality, although the latter was not statistically significant [17]. In agreement with Tomita et al., we found that the 5-year survival rate of patients with preoperative anemia was significantly lower than that of patients without preoperative anemia [9]. Several groups have offered hypotheses to explain how preoperative anemia reduces the survival of cancer patients; it has been suggested that the reduced partial pressure of oxygen at the tumor level triggers an exaggerated expression of hypoxia-inducible factor-1, which in turn triggers angiogenesis, epithelial-mesenchymal transformation, genetic mutations, and apoptosis resistance [23-26]. Although, our study did not address mechanisms by which anemia can be linked to cancer progression we accounted for an important mediator of preoperative anemia that is inflammation and for ABT that is a treatment of perioperative anemia.
The preoperative NLR is an independent predictor of survival in patients with a wide variety of cancers[14]. Previous studies from our group and others have highlighted the importance of the NLR as an independent predictor of survival in the overall NSCLC patient population [13, 27-29]. To date, only Sarraf et al. have assessed the impact of preoperative NLR on the survival of patients with stage I NSCLC [27]. The study, which included 83 patients and used an NLR cut-off value different from that used in the present study, revealed that patients with an NRL > 3.83 had poor survival, which is in agreement with our findings [27]. In our study, the multivariate analysis showed that the preoperative NLR, compared with anemia and postoperative NLR, had the strongest predictive value for RFS and OS, indicating the importance of stratifying patients with stage I NSCLC according to their preoperative inflammatory status.
The present study has several potential limitations, including those inherent to all retrospective studies, such as the inability to account for unknown factors that may affect the studied outcomes. For example, our database does not include information on postoperative complications, which are known to negatively impact the mortality of patients who have undergone major thoracic surgery [30]. The present study's statistical analyses also included propensity-score matching that showed a 37% and 48% improvement in RFS and OS. Although this improvement in survivals did not reach statistical significance, it is important to remark that significant number of patients were lost after matching which limits the power of the study detect significances [31].
In addition, because our study included patients who received both leukoreduced and non-leukoreduced pRBCs during the perioperative period, we could not estimate the impact of leukoreduction on survival. Several authors have questioned whether leukoreduced pRBCs affect cancer recurrence; for example, Ng et al. demonstrated that patients who received leukoreduced pRBCs had poorer outcomes than those who did not receive leukoreduced pRBCs [17]. Panagopoulos et al. suggested that pRBC transfusions, regardless of whether they are leukoreduced, explains the association between ABT and poor outcomes [32]. Another limitation is that we did not define the triggers of ABT, intraoperative blood loss or evaluated the impact of ABT timing (intraoperative or postoperative) on survival. Recent studies have suggested that intraoperative ABT has a more deleterious effect on survival than postoperative ABT does [33, 34]. Finally, including both patients with stage Ia and those with stage Ib disease in our analysis might be considered a confounder, as Ng et al. demonstrated that the survival rates of these 2 groups are different [17].
In conclusion, our study's findings suggest that preoperative anemia and an NLR ≥ 5 independent predictors of RFS and OS in patients with stage I NSCLC. Preoperative anemia as defined by the WHO criteria and an NLR ≥ 5 are potential biomarkers for identifying patients who have a high risk of recurrence. In the non-matched population of patients, allogeneic blood transfusion was an independent risk factor of reduced RFS and OS, but this association disappeared after propensity score matching.
Figure 1. Kaplan-Meier curves for recurrence-free survival (RFS).
Patients who did not receive allogeneic blood transfusion (ABT) had significantly better RFS than patients who did receive ABT (top panels). Patients who had preoperative anemia had significantly better RFS than patients who did have preoperative anemia (left bottom panel). The right bottom panel depicts the impact of preoperative neutrophil-to-lymphocyte ratio (NLR) on RFS.
Figure 2. Kaplan-Meier curves for overall survival (OS).
The top panels illustrate the effect of allogeneic blood transfusion (ABT) on OS. The left bottom panel shows the survival curves for patients who did and who did not have preoperative anemia. The right bottom panel depicts the impact of preoperative neutrophil-to-lymphocyte ratio (NLR) on OS.
Acknowledgments
The authors acknowledge Mr. Joseph A. Munch for his contribution in the writing and editing of the manuscript. This work was partially supported by National Cancer Institute - Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Author contributions
JPC was involved in design, or acquisition of data, or analysis and interpretation of data; writing of the manuscript and final approval. CG participate in acquisition of data, or analysis and interpretation of data; writing of the manuscript and final approval. RJM was involved in analysis and interpretation of data; writing of the manuscript and final approval, DR carried out analysis and interpretation of data; writing of the manuscript and final approval, JN was involved in design, analysis and interpretation of data; writing of the manuscript and final approval. Lei Feng contributed with the analysis and interpretation of data; writing of the manuscript and final approval. Andrea Rodriguez-Restrepo participated in acquisition of data, or analysis and interpretation of data; writing of the manuscript and final approval. Fernando Martinez was involved in analysis and interpretation of data; writing of the manuscript and final approval. Gabriel Mena participated in analysis and interpretation of data; writing of the manuscript and final approval. Vijaya Gottumukkala contributed in analysis and interpretation of data; writing of the manuscript and final approval.
To cite this article: Juan P. Cata, et al. Preoperative Anemia, Blood Transfusion, and Neutrophil-to-Lymphocyte Ratio in Patients with Stage I Non–Small Cell Lung Cancer. Can Cell Microenviron 2016; 3: e1116. doi: 10.14800/ccm.1116.
Conflict of interests
The authors have declared that no conflict of interests exist.
References
- 1.UDoHaH Services. The 2011 Nationwide Blood Collection and Utilization Survery Report. 2011 [Google Scholar]
- 2.Morton J, Anastassopoulos KP, Patel ST, Lerner JH, Ryan KJ, Goss TF, Dodd SL. Frequency and outcomes of blood products transfusion across procedures and clinical conditions warranting inpatient care: an analysis of the 2004 healthcare cost and utilization project nationwide inpatient sample database. Am J Med Qual. 2010;25:289–296. doi: 10.1177/1062860610366159. [DOI] [PubMed] [Google Scholar]
- 3.Beale E, Zhu J, Chan L, Shulman I, Harwood R, Demetriades D. Blood transfusion in critically injured patients: a prospective study. Injury. 2006;37:455–465. doi: 10.1016/j.injury.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–244. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Katayama K, Itamoto T, Yano M, Hino H, Okamoto Y, Nakahara H, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999;23:676–680. doi: 10.1007/pl00012367. [DOI] [PubMed] [Google Scholar]
- 7.Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg. 2015;13:102–110. doi: 10.1016/j.ijsu.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Luan H, Ye F, Wu L, Zhou Y, Jiang J. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg. 2014;14:34. doi: 10.1186/1471-2482-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Impact of preoperative hemoglobin level on survival of non-small cell lung cancer patients. Anticancer Res. 2008;28:1947–1950. [PubMed] [Google Scholar]
- 10.Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer Res. 2009;29:2687–2690. [PubMed] [Google Scholar]
- 11.Mercadante S, Gebbia V, Marrazzo A, Filosto S. Anaemia in cancer: pathophysiology and treatment. Cancer Treat Rev. 2000;26:303–311. doi: 10.1053/ctrv.2000.0181. [DOI] [PubMed] [Google Scholar]
- 12.Birgegard G, Aapro MS, Bokemeyer C, Dicato M, Drings P, Hornedo J, Krzakowski M, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3–11. doi: 10.1159/000083128. [DOI] [PubMed] [Google Scholar]
- 13.Choi JE, Villarreal J, Lasala J, Gottumukkala V, Mehran RJ, Rice D, Yu J, et al. Perioperative neutrophil:lymphocyte ratio and postoperative NSAID use as predictors of survival after lung cancer surgery: a retrospective study. Cancer Med. 2015;4:835–833. doi: 10.1002/cam4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Eilam D, Dayan T, Ben-Eliyahu S, Schulman II, Shefer G, Hendrie CA. Differential behavioural and hormonal responses of voles and spiny mice to owl calls. Anim Behav. 1999;58:1085–1093. doi: 10.1006/anbe.1999.1224. [DOI] [PubMed] [Google Scholar]
- 16.Vamvakas EC, Blajchman MA. Blood still kills: six strategies to further reduce allogeneic blood transfusion-related mortality. Transfus Med Rev. 2010;24:77–124. doi: 10.1016/j.tmrv.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng T, Ryder BA, Chern H, Sellke FW, Machan JT, Harrington DT, Cioffi WG. Leukocyte-depleted blood transfusion is associated with decreased survival in resected early-stage lung cancer. J Thorac Cardiovasc Surg. 2012;143:815–819. doi: 10.1016/j.jtcvs.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Nosotti M, Rebulla P, Riccardi D, Baisi A, Bellaviti N, Rosso L, Santambrogio L. Correlation between perioperative blood transfusion and prognosis of patients subjected to surgery for stage I lung cancer. Chest. 2003;124:102–107. doi: 10.1378/chest.124.1.102. [DOI] [PubMed] [Google Scholar]
- 19.Penalver JC, Padilla J, Jorda C, Escriva J, Ceron J, Calvo V, Garcia A, et al. Use of blood products in patients treated surgically for stage I non-small cell lung cancer. Arch Bronconeumol. 2005;41:484–488. doi: 10.1016/s1579-2129(06)60267-x. [DOI] [PubMed] [Google Scholar]
- 20.Keller SM, Groshen S, Martini N, Kaiser LR. Blood transfusion and lung cancer recurrence. Cancer. 1988;62:606–610. doi: 10.1002/1097-0142(19880801)62:3<606::aid-cncr2820620327>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Pastorino U, Valente M, Cataldo I, Lequaglie C, Ravasi G. Perioperative blood transfusion and prognosis of resected stage Ia lung cancer. Eur J Cancer Clin Oncol. 1986;22:1375–1378. doi: 10.1016/0277-5379(86)90148-3. [DOI] [PubMed] [Google Scholar]
- 22.Tartter PI, Burrows L, Kirschner P. Perioperative blood transfusion adversely affects prognosis after resection of Stage I (subset N0) non-oat cell lung cancer. J Thorac Cardiovasc Surg. 1984;88:659–662. [PubMed] [Google Scholar]
- 23.Teicher BA. Acute and chronic in vivo therapeutic resistance. Biochem Pharmacol. 2009;77:1665–1673. doi: 10.1016/j.bcp.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys. 2005;63:25–36. doi: 10.1016/j.ijrobp.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 25.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Schmid T, Schnitzer S, Brune B. Tumor hypoxia and cancer progression. Cancer Lett. 2006;237:10–21. doi: 10.1016/j.canlet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Tomita M, Shimizu T, Ayabe T, Nakamura K, Onitsuka T. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res. 2012;32:3535–3538. [PubMed] [Google Scholar]
- 29.Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31:2995–2998. [PubMed] [Google Scholar]
- 30.Tabutin M, Couraud S, Guibert B, Mulsant P, Souquet PJ, Tronc F. Completion pneumonectomy in patients with cancer: postoperative survival and mortality factors. J Thorac Oncol. 2012;7:1556–1562. doi: 10.1097/JTO.0b013e31826419d2. [DOI] [PubMed] [Google Scholar]
- 31.Gayat E, Pirracchio R, Resche-Rigon M, Mebazaa A, Mary JY, Porcher R. Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med. 2010;36:1993–2003. doi: 10.1007/s00134-010-1991-5. [DOI] [PubMed] [Google Scholar]
- 32.Panagopoulos ND, Karakantza M, Koletsis E, Apostolakis E, Sakellaropoulos GC, Filos KS, Eleni T, et al. Influence of blood transfusions and preoperative anemia on long-term survival in patients operated for non-small cell lung cancer. Lung Cancer. 2008;62:273–280. doi: 10.1016/j.lungcan.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 33.Abel EJ, Linder BJ, Bauman TM, Bauer RM, Thompson RH, Thapa P, Devon ON, et al. Perioperative blood transfusion and radical cystectomy: does timing of transfusion affect bladder cancer mortality? Eur Urol. 2014;66:1139–1147. doi: 10.1016/j.eururo.2014.08.051. [DOI] [PubMed] [Google Scholar]
- 34.Moschini M, Dell' Oglio P, Capogrosso P, Cucchiara V, Luzzago S, Gandaglia G, Zattoni F, et al. Effect of Allogeneic Intraoperative Blood Transfusion on Survival in Patients Treated With Radical Cystectomy for Nonmetastatic Bladder Cancer: Results From a Single High-Volume Institution. Clin Genitourin Cancer. 2015;13:562–567. doi: 10.1016/j.clgc.2015.04.009. [DOI] [PubMed] [Google Scholar]