Abstract
The correlation between the expression of DNA methyltransferase-1 (DNMT1) and estrogen receptor α (ERα), as well as the methylation status of ERα, was analyzed to investigate the clinical significance of DNMT1 and ERα in breast cancer. Substance P immunohistochemistry and reverse transcription-polymerase chain reaction (RT-PCR) were utilized to detect the protein and mRNA expression levels of DNMT1 and ERα in 112 breast cancer and 20 normal breast specimens. Methylation specific PCR was utilized to detect the methylation status of ERα in ERα-positive and -negative breast cancer specimens and 20 normal breast specimens. The results of the present study revealed that DNMT1 protein and mRNA levels were low in normal breast specimens (10.00 and 46.05%, respectively) and ERα-positive breast cancer specimens (15.00 and 48.68%, respectively), compared with increased levels in ERα-negative breast cancer specimens (81.11 and 88.89%, respectively; P<0.05). The methylation rate of ERα was highest in ERα-negative breast cancer specimens (86.11%) compared with normal breast specimens and ERα-positive breast cancer specimens (10.00 and 36.84%, respectively; P<0.05). Positive expression of ERα protein was observed to be associated with progesterone receptor expression (P<0.05), however, no such association was observed for age, menopause state, tumor size, number of positive nodes, Tumor-Node-Metastasis stage or tumor type (P>0.05). The protein and mRNA expression levels of DNMT1 were negatively correlated with ERα expression (P<0.05). DNMT1 expression was positively correlated with methylation of ERα (P<0.05), and was positively correlated with the methylation of CpG islands of ERα, indicating that the detection of DNMT1 expression may be significant for the diagnosis and typing of breast cancer.
Keywords: breast cancer, DNA methyltransferase 1, estrogen receptor α, methylation
Introduction
Breast cancer is a significant threat to the health of the majority of women, and is the most frequently observed malignancy in women. In the previous 10 years, breast cancer incidence has increased by 47%, reaching 25/10,000,000 (2). Breast cancer has become one of the leading causes of cancer-associated mortality, particularly in developed countries (3). Clinically, the positive or negative expression of estrogen receptor α (ERα) is a significant prognostic indicator in breast cancer (4). ERα-positive breast cancer is associated with increased rates of disease-free survival and an overall improved prognosis (5). However, a previous study identified that 1/3 breast cancer cases exhibited negative ERα expression, which was associated with poor histological differentiation, more negative clinical outcomes and a lack of response to endocrine therapy (6).
A number of mutations in the ERα gene have been identified and demonstrated to be involved in negative ERα expression and estradiol binding (7). However, mutation of the ERα gene has rarely been observed in breast cancer, suggesting that there may be alternative mechanisms other than genetic changes underlying negative ERα expression (8,9). Increasing evidence has revealed that epigenetic changes occur frequently and may be associated with the development and progression of breast cancer (10). Epigenetic alterations in cancer may result in promoter methylation of certain tumor suppressor genes, leading to gene silencing (11). DNA methylation is catalyzed by DNA methyltransferases (DNMTs), which have a significant role in the maintenance of genomic stability (12,13). The aberrant expression of DNMTs and disruption of DNA methylation patterns has been observed to be associated with breast cancer (14). DNMT1 has been identified as important for maintenance of methylation (15). In ERα-negative breast cancer cell lines, the expression levels and activity of DNMT1 were observed to be increased 2- to 10-fold, in accordance with the methylation level (16). These results indicated a potential association between ERα-negative expression and hypermethylation of the ERα gene (17,18).
Substance P (S-P) immunohistochemistry and reverse transcription-polymerase chain reaction (RT-PCR) were employed to investigate the protein and messenger (m)RNA levels of DNMT1 and ERα in 112 breast cancer and 20 normal breast specimens. The methylation status of ERα was detected using methylation specific (MS)-PCR in ERα-positive or ERα-negative breast cancer specimens and 20 normal breast specimens. The correlation between the expression of DNMT1 and ERα, and the methylation status of ERα in breast cancer, was investigated.
Materials and methods
Human tissue samples
Patient clinicopathological data was obtained from the Breast Surgery Department of The First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) between January and June 2013, including 112 specimens from sporadic breast cancer cases and 20 normal breast specimens, with patient ages ranging from 32–78 years (median age, 58.6 years). Written informed consent was obtained from all patients and the study was approved by the ethics committee of Zhengzhou University. The histological grades of the specimens were as follows: grade I, 30 cases; grade II, 50 cases; and grade III, 32 cases. Each specimen was isolated and immediately stored on ice. One half of each section was embedded in paraffin and underwent S-P immunohistochemistry, and the remaining half was frozen in liquid nitrogen for RNA or DNA isolation. Pathological diagnoses of all specimens were clear, and patients had received no treatment prior to their surgery.
S-P immunohistochemistry. Tissue samples from breast cancer and normal breast specimens were studied using S-P immunohistochemistry. Slides were deparaffinized using xylol, rehydrated in a graded alcohol series and subsequently stained with hematoxylin and eosin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). For immunostaining, endogenous biotin activity was blocked using the SP-9000 kit (ZSGB-BIO, Beijing, China). An immunostaining assay was performed using two primary antibodies: anti-DNMT1 mouse monoclonal antibody (cat. no. ab134148; 1:500; Abcam, Cambridge, MA, USA) and anti-ER mouse monoclonal antibody (cat. no. ab32063; 1:500; Abcam). Samples were incubated with primary antibody at 37°C for 1 h. Polyclonal goat anti-rabbit immunoglobulin G (cat. no. A0277; 1:1,000; Beyotime Biotech, Jiangsu, China) was used as a secondary antibody, and was incubated with samples for 30 min at room temperature. Slides were washed three times between steps using Tris-buffered saline. Immunoreactions were visualized by a streptavidin-biotin complex using the 3,3′-Diaminobenzidine Chromogenic kit (ZSGB-BIO). The specimens were counterstained using hematoxylin.
RNA isolation and analysis of semi-quantitative RT-PCR
Total cell RNA was extracted from breast cancer and normal breast tissue specimens using TRIzol® reagent (Gibco; Thermo Fisher Scientific, Waltham, MA, USA). The A260/A280 absorption of isolated RNA was analyzed using a UV–VIS spectrophotometer (UV-2450; Shimadzu Corporation, Kyoto, Japan) to evaluate the purity and concentration of RNA. A Thermoscript™ RT-PCR System (Fermentas; Thermo Fisher Scientific) was employed to synthesize complementary DNA using 1 µg RNA. The ERα gene was amplified using the following primers: Forward, 5′-TGATGAAAGGTGGGATACGAAA-3′ and reverse, 5′-GGCTGTTCTTCTTAGAGCGTTTG-3′, to create a 168 bp product. The DNMT1 gene was amplified using the following primers: Forward, 5′-CTACCAGGGAGAAGGACAGG-3′ and reverse, 5′-CTCACAGACGCCACATCG-3′, to create a 152 bp product. The β-actin gene was amplified using the following primers: forward, 5′-AGGCATTGTGATGGACTCCG-3′) and reverse, 5′-AGTGATGACCTGGCCGTCAG-3′, to create a 301 bp product, which was utilized as an internal control. The program was monitored and processed using the GeneAmp® PCR System 9700 (Applied Biosystems; Thermo Fisher Scientific), and the thermal cycling conditions used were as follows: an initial heating cycle of 95°C for 5 min, 35 cycles of 94°C for 30 sec, annealing at 65°C (β-actin) for 45 sec or 54°C (ERα, DNMT1) for 30 sec, 72°C for 90 sec and a final 5 min extension step at 94°C. The targeted PCR products were loaded onto 1.5% agarose gels and confirmed using electrophoresis and sequencing. The relative gene expression data was analyzed using the 2−∆∆Cq method.
MS-PCR
Genomic DNA from breast cancer and normal breast tissues was isolated using a DNA Extraction kit (Axygen; Corning Life Sciences, Corning, NY, USA), followed by treatment with sodium bisulfite using the CpGenome™ DNA Modification kit (Epigentek Group, Inc., Farmingdale, NY, USA) according to the manufacturer's protocol. MS-PCR was conducted using the GeneAmp® PCR System 9700.
The unmethylated DNA of ERα was amplified using the following primers: Forward, 5′-GGGGTTGGATGTAGTGGTTTAT-3′ and reverse, 5′-TAAAACTACAAATACCCACCA-3′, to create a 170 bp product. Thermal cycling conditions used were as follows: An initial heating cycle of 94°C for 5 min, 35 cycles of 94°C for 30 sec, 58°C for 45 sec, 72°C for 90 sec and a final 5 min extension step at 72°C. The MS-PCR products were loaded onto 2% agarose gels and resolved by electrophoresis. The band intensities of the reaction products were examined using vision work software LS 6.6a (UVP, Inc., Upland, CA, USA).
Statistical analysis
All experiments were performed in triplicate and data were analyzed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). The χ2 test and Spearman rank correlation coefficient analysis were engaged to assess the univariate association between the correlation of expression of DNMT1 with ERα, as well as the methylation status of ERα and its clinical significance. P<0.05 was considered to indicate a statistically significant difference.
Results
Differential ERα protein expression is observed in normal and cancerous breast tissue
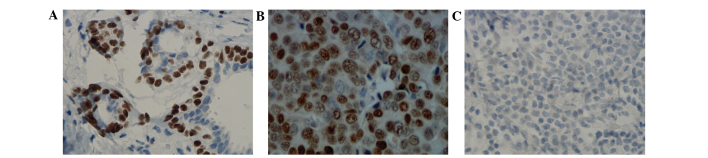
As revealed in Fig. 1, ERα expression is primarily observed in the nucleus, and partially in the cytoplasm; however, ERα was only stained in the cytoplasm in ERα-negative breast cancer samples. The rate of ERα protein positive expression was 95.00% in 20 cases of normal breast tissue, while expression decreased significantly to 67.85% in breast cancer tissue (χ2=6.197; P=0.013).
Figure 1.
Protein expression of ERα (Substrate P, ×400 magnification; hematoxylin and eosin stained). Positive expression of ERα protein in (A) normal breast specimen and (B) breast cancer specimen. (C) Negative expression of ERα protein in breast cancer specimen. ER, estrogen receptor. All images are representative.
Differential DNMT1 protein expression is observed in normal and cancerous breast tissue
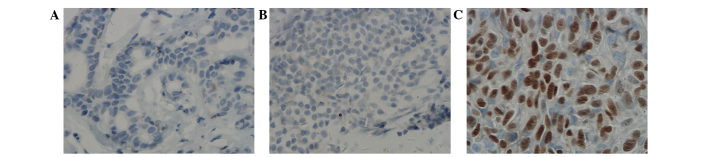
DNMT1 was characterized by yellow or brown staining in the nucleus and cytoplasm in ERα-negative breast cancer tissue, with weaker staining observed in paracarcinoma tissue (Fig. 2). The rate of DNMT1 protein positive expression was 10.00% in normal breast tissue (n=20), 46.05% in ERα-positive breast cancer tissue (n=76) and 81.11% in ERα-negative breast cancer tissue (n=36). The difference between the three groups was statistically significant (χ2=31.960; P<0.0001).
Figure 2.
Protein expression of DNMT1 (Substrate P, ×400 magnification; hematoxylin and eosin stained). Negative expression of DNMT1 protein in (A) normal breast specimen and (B) ERα-positive breast cancer specimen. (C) Positive expression of DNMT1 protein in ERα-negative breast cancer specimen. ER, estrogen receptor; DNMT, DNA methyltransferase. All images are representative.
Expression of DNMT1 is associated with ERα expression and the methylation status of ERα
According to the clinicopathological data of breast cancer patients (Table I), positive expression of ERα protein was associated with progesterone receptor expression (PR; P<0.05), but not with age, menopause state, tumor size, number of positive nodes, Tumor-Node-Metastasis (TNM) stage and tumor type (P>0.05). The protein and mRNA expression levels of DNMT1 were negatively correlated with the expression of ERα (P<0.05), but positively correlated with the methylation of ERα (P<0.05).
Table I.
The expression of DNMT1 and ERα and the methylation status of ERα and its clinical significance in breast cancer.
| ERα | DNMT1 | ||||||
|---|---|---|---|---|---|---|---|
| Item | n | Cases (%) | χ2 | P-value | Cases (%) | χ2 | P-value |
| Age, years | 0.320 | 0.571 | 0.627 | 0.428 | |||
| <60 | 51 | 36 (70.59) | 28 (54.90) | ||||
| ≥60 | 61 | 40 (65.57) | 38 (62.30) | ||||
| Menopause state | 0.021 | 0.885 | 0.490 | 0.484 | |||
| Pre | 54 | 37 (6852) | 30 (55.56) | ||||
| Post | 58 | 39 (67.24) | 36 (66.67) | ||||
| Tumor size, cm | 0.774 | 0.679 | 0.482 | 0.786 | |||
| <2.0 | 21 | 13 (61.90) | 12 (57.14) | ||||
| 2–5 | 53 | 38 (71.70) | 33 (62.26) | ||||
| >5 | 38 | 25 (65.79) | 21 (55.26) | ||||
| Number of positive nodes | 0.109 | 0.947 | 1.295 | 0.523 | |||
| 0–3 | 63 | 42 (66.67) | 40 (63.49) | ||||
| 4–9 | 42 | 29 (69.05) | 22 (52.38) | ||||
| >9 | 7 | 5 (71.43) | 4 (57.14) | ||||
| Tumor-Node-Metastasis stage | 0.383 | 0.944 | 0.722 | 0.868 | |||
| pT1 | 10 | 7 (70.00) | 6 (60.00) | ||||
| pT2 | 46 | 31 (67.39) | 28 (60.87) | ||||
| pT3 | 44 | 29 (65.91) | 24 (54.55) | ||||
| pT4 | 12 | 9 (75.00) | 8 (66.67) | ||||
| Tumor type | 0.030 | 0.985 | 0.102 | 0.950 | |||
| Infiltrative ductal carcinoma | 79 | 54 (68.35) | 47 (59.49) | ||||
| Infiltrative lobular carcinoma | 18 | 12 (66.67) | 10 (55.56) | ||||
| Other | 15 | 10 (66.67) | 9 (60.00) | ||||
| Progesterone receptor | 11.069 | 0.001 | 0.319 | 0.572 | |||
| Positive | 74 | 58 (78.38) | 45 (60.81) | ||||
| Negative | 38 | 18 (47.37) | 21 (55.26) | ||||
| Human epidermal growth factor-2 | 3.210 | 0.073 | 2.842 | 0.092 | |||
| Positive | 34 | 19 (55.88) | 26 (76.47) | ||||
| Negative | 78 | 57 (73.08) | 50 (64.10) | ||||
DNMT1, DNA methyltransferase-1; ERα, estrogen receptor α.
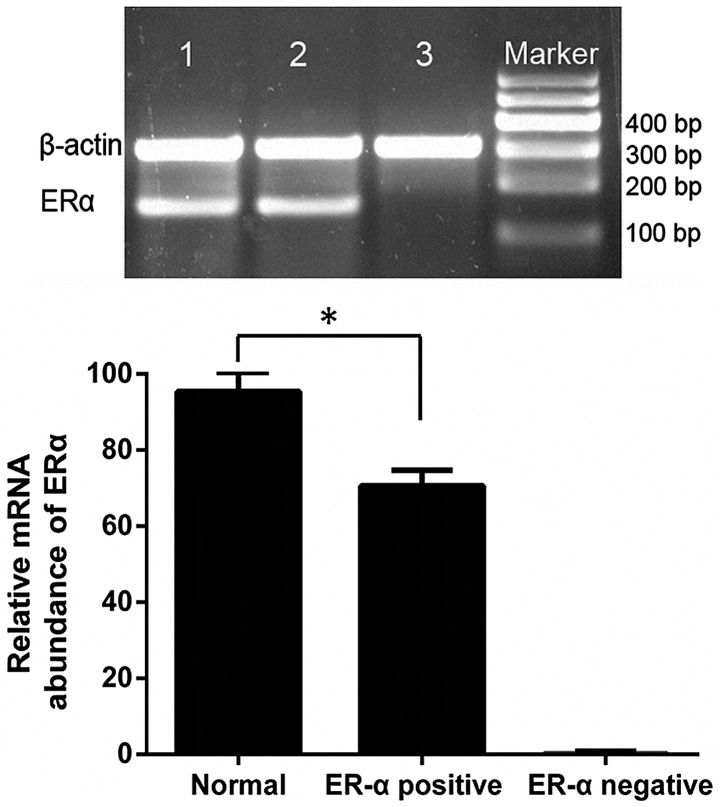
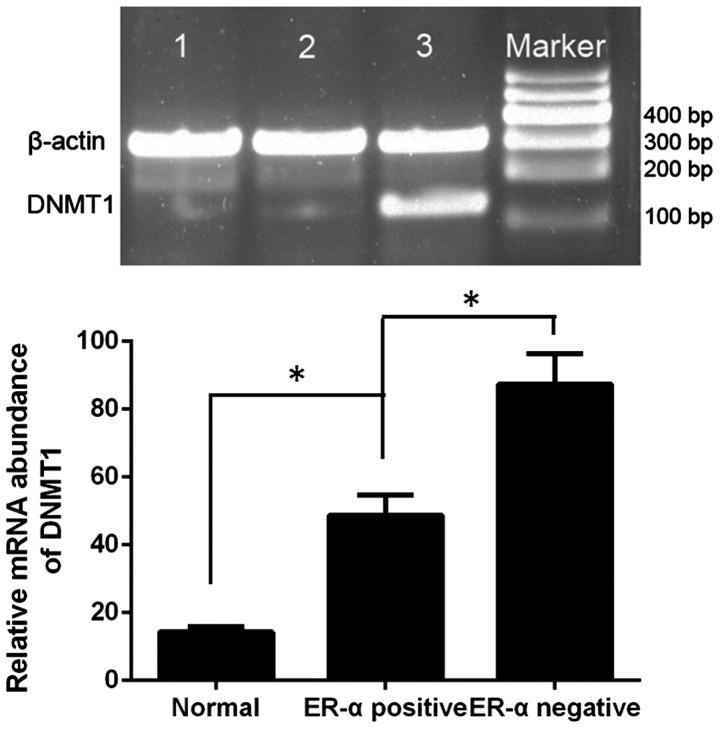
The positive expression rate of ERα mRNA was 100.00 and 70.54% in normal breast and breast cancer specimens, respectively (χ2=7.857; P=0.005; Fig. 3). The positive rate of DNMT1 expression was 15.00, 48.68 and 88.89% in the normal breast tissue group (n=20), ERα-positive breast cancer group (n=76) and ERα-negative breast cancer group (n=36), respectively, and the difference between the three groups was statistically significant (χ2=30.794; P<0.0001; Fig. 4). The mRNA expression levels of DNMT1 were negatively correlated with ERα expression in normal breast and breast cancer specimens [Coefficient of rank correlation (rs)=−0.470; P<0.0001].
Figure 3.
ERα messenger RNA expression in normal breast specimens and breast cancer specimens. 1–3: 1, normal breast specimens; 2, ERα-positive breast cancer specimens and 3, ERα-negative breast cancer specimens. (β-actin, 301 bp; ERα, 168 bp). Normal breast tissue exhibited a higher positive expression rate of ERα mRNA when compared with ERα-positive breast cancer specimens. *P<0.0001. ER, estrogen receptor.
Figure 4.
DNMT1 messenger RNA expression in normal breast specimens and breast cancer specimens. 1, normal breast specimens; 2, ERα-positive breast cancer specimens and 3, ERα-negative breast cancer specimens. (β-actin, 301 bp; DNMT1, 152 bp). The ERα-negative breast cancer group (n=36) exhibited the highest expression rate of DNMT1, when compared with the normal breast tissue (n=20) and ERα-positive breast cancer (n=76) groups. *P<0.0001. ER, estrogen receptor; DNMT, DNA methyltransferase.
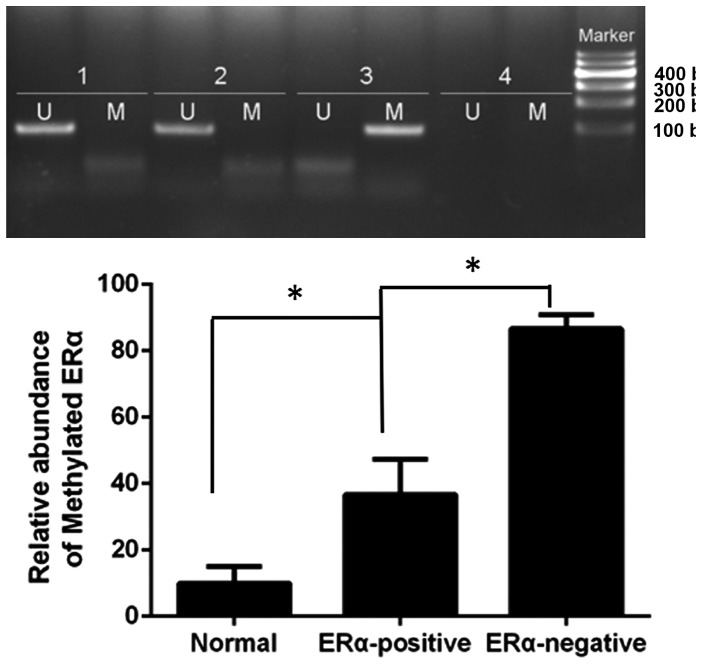
The present study used the MS-PCR method to detect the methylation status of ERα in the normal breast tissue group (n=20), ERα-positive breast cancer group (n=76) and ERα-negative breast cancer group (n=36). The results of the present study revealed that the hypermethylation rates were 10.00, 36.84 and 86.11%, respectively (χ2=36.292; P<0.0001; Fig. 5). Correlation analysis of Spearman rank revealed that DNMT1 expression and the methylation status of ERα were significantly positively correlated in breast cancer (rs =663; P<0.0001).
Figure 5.
Methylation status of ERα in normal breast specimens and breast cancer specimens. 1, normal breast specimens; 2, ERα-positive breast cancer specimens; 3, ERα-negative breast cancer specimens; 4, H2O. The ERα-negative breast cancer group (n=36) exhibited the highest methylation rate when compared with the normal breast tissue (n=20) and ERα-positive breast cancer (n=76) groups. *P<0.0001. M, methylated band (170bp); U, unmethylated band (170bp); ER, estrogen receptor.
In conclusion, the results of the present study indicated that DNMT1 protein and mRNA expression levels were negatively correlated with the expression of ERα, but positively correlated with methylation of ERα.
Discussion
Currently, there is an increasing risk of developing breast cancer for woman globally according to the present estimates of breast cancer incidence (19). Cumulative evidence has provided an extensive understanding of the underlying factors that contribute to the development of breast cancer (20). In recent years, epigenetic regulation, rather than loss of heterozygosity or homozygous deletion, has been considered to be a significant cause of transcriptional silencing (21). Epigenetic changes of gene happened within a defined region with high frequency (22). The low expression and inactivation of cancer suppressor genes is caused by hypermethylation in CpG islands of gene promoters (11). Notably, in the ERα gene there is a cluster of sites for methylation-sensitive restriction endonucleases in the CpG island of the promoter and first exon (23). Hypermethylation of the ERα gene has been well studied in breast cancer cell lines or tissues, and leads to the loss of expression of ERα (24). Studies have demonstrated that methylation of the ERα gene is not present in normal breast tissue samples and ERα-positive breast cancer cell lines; however, extensive methylation is exhibited in ERα-negative breast cancer cell lines (10,12). Epigenetic changes may be responsible for inactivation of the ERα gene.
In eukaryotic cells, the DNMT family includes DNMT1, DNMT2, DNMT3a and DNMT3b. These enzymes transfer methyl cytosine nucleotides to the first five carbon atoms in DNA using S-methionine as a methyl donor (13). The expression and activity levels of DNMTs are two important factors that affect the level of genomic methylation. DNMT1 is an essential member of the DNMT family. It has been demonstrated that DNMT1 is associated with the methylation of a number of tumor suppressor genes (18).
In the present study, the positive rates of DNMT1 protein and mRNA were low in normal breast specimens and ERα-positive breast cancer specimens, which were 10.00 and 46.05, and 15.00 and 48.68%, respectively. DNMT1 protein and mRNA levels were increased in ERα-negative breast cancer specimens, and were 81.11 and 88.89%, respectively (P<0.05). The positive expression of ERα protein was associated with PR expression (P<0.05), but not with age, menopause state, tumor size, number of positive nodes, TNM stage and tumor type (P>0.05). The protein and mRNA expression levels of DNMT1 were negatively correlated with the expression of ERα (P<0.05). However, the mRNA expression levels of DNMT1 were positively correlated with methylation of ERα (P<0.05). The methylation rates of ERα were increased in normal tissue, ERα-positive and ERα-negative breast cancer specimens, and were 10.00, 36.84 and 86.11%, respectively (P<0.05).
In conclusion, the protein and mRNA expression levels of DNMT1 were negatively correlated with the expression of ERα in breast cancer specimens, and the expression of DNMT1 was positively correlated with the methylation of CpG islands in the ERα gene. The protein expression of DNMT1 was increased in ERα-negative breast cancer compared with normal breast tissue and ERα-positive breast cancer, which may be significant for the diagnosis and typing of breast cancer.
Acknowledgments
The present study was supported by the Henan Science and Technology Committee (grant no. 052SGYS33209) and The Young Foundation of the First Affiliated Hospital of Zhengzhou University (grant no. 2011YN01014).
References
- 1.Drageset S, Lindstrøm TC, Underlid K. Coping with breast cancer: Between diagnosis and surgery. J Adv Nurs. 2010;66:149–158. doi: 10.1111/j.1365-2648.2009.05210.x. [DOI] [PubMed] [Google Scholar]
- 2.Hou N, Huo D. A trend analysis of breast cancer incidence rates in the United States from 2000 to 2009 shows a recent increase. Breast Cancer Res Treat. 2013;138:633–641. doi: 10.1007/s10549-013-2434-0. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu JQ, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, Ohuchi N, Sasano H. Estrogen-related receptor alpha in human breast carcinoma as a potent prognostic factor. Cancer Res. 2004;64:4670–4676. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- 5.Clark GM, McGuire WL. Steroid receptors and other prognostic factors in primary breast cancer. Semin Oncol. 1988;15:20–25. [PubMed] [Google Scholar]
- 6.Lapidus RG, Nass SJ, Davidson NE. The loss of estrogen and progesterone receptor gene expression in human breast cancer. J Mammary Gland Biol Neoplasia. 1998;3:85–94. doi: 10.1023/A:1018778403001. [DOI] [PubMed] [Google Scholar]
- 7.Barone I, Brusco L, Fuqua SA. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin Cancer Res. 2010;16:2702–2708. doi: 10.1158/1078-0432.CCR-09-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapidus RG, Nass SJ, Butash KA, Parl FF, Weitzman SA, Graff JG, Herman JG, Davidson NE. Mapping of ER gene CpG island methylation-specific polymerase chain reaction. Cancer Res. 1998;58:2515–2519. [PubMed] [Google Scholar]
- 9.Weigel RJ, deConinck EC. Transcriptional control of estrogen receptor in estrogen receptor-negative breast carcinoma. Cancer Res. 1993;53:3472–3474. [PubMed] [Google Scholar]
- 10.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 11.Esteller M. CpG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 12.Ross SA. Diet and DNA methylation interactions in cancer prevention. Ann NY Acad Sci. 2003;983:197–207. doi: 10.1111/j.1749-6632.2003.tb05974.x. [DOI] [PubMed] [Google Scholar]
- 13.Brenner C, Fuks F. DNA methyltransferases: facts, clues, mysteries. Curr Top Microbiol Immunol. 2006;301:45–66. doi: 10.1007/3-540-31390-7_3. [DOI] [PubMed] [Google Scholar]
- 14.Katz TA, Vasilatos SN, Harrington E, Oesterreich S, Davidson NE, Huang Y. Inhibition of histone demethylase, LSD2 (KDM1B), attenuates DNA methylation and increases sensitivity to DNMT inhibitor-induced apoptosis in breast cancer cells. Breast Cancer Res Treat. 2014;146:99–108. doi: 10.1007/s10549-014-3012-9. [DOI] [PubMed] [Google Scholar]
- 15.Gopisetty G, Ramachandran K, Singal R. DNA methylation and apoptosis. Mol Immunol. 2006;43:1729–1740. doi: 10.1016/j.molimm.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- 17.Agrawal A, Murphy RF, Agrawal DK. DNA methylation in breast and colorectal cancers. Mod Pathol. 2007;20:711–721. doi: 10.1038/modpathol.3800822. [DOI] [PubMed] [Google Scholar]
- 18.Yan L, Yang XW, Davidson NE. Role of DNA methylation and histone acetylation in steroid receptor expression in breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:183–192. doi: 10.1023/A:1011308707512. [DOI] [PubMed] [Google Scholar]
- 19.Schwarzenbach H, Pantel K. Circulating DNA as biomarker in breast cancer. Breast Cancer Res. 2015;17:136. doi: 10.1186/s13058-015-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell GR, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baxter E, Windloch K, Gannon F, Lee JS. Epigenetic regulation in cancer progression. Cell Biosci. 2014;4:45. doi: 10.1186/2045-3701-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widschwendter M, Jones PA. DNA methylation and breast carcinogenesis. Oncogene. 2002;21:5462–5482. doi: 10.1038/sj.onc.1205606. [DOI] [PubMed] [Google Scholar]
- 23.Piva R, Gambari R, Zorzato F, Kumar L, del Senno L. Analysis of upstream sequences of the human estrogen receptor gene. Biochem Biophys Res Commun. 1992;183:996–1002. doi: 10.1016/S0006-291X(05)80289-X. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Wang L, Jin F, Ma W, Ren J, Wen X, He M, Sun M, Tang H, Wei M. Silencing of estrogen receptor alpha (ERalpha) gene by promoter hypermethylation is a frequent even in Chinese women with sporadic breast cancer. Breast Cancer Res Treat. 2009;117:253–259. doi: 10.1007/s10549-008-0192-1. [DOI] [PubMed] [Google Scholar]