Abstract
Human coronary collaterals are inter-coronary communications that are believed to be present from birth. In the presence of chronic total occlusions, recruitment of flow via these collateral anastomoses to the arterial segment distal to occlusion provide an alternative source of blood flow to the myocardial segment at risk. This mitigates the ischemic injury. Clinical outcome of coronary occlusion ie. severity of myocardial infarction/ischemia, impairment of cardiac function and possibly survival depends not only on the acuity of the occlusion, extent of jeopardized myocardium, duration of ischemia but also to the adequacy of collateral circulation. Adequacy of collateral circulation can be assessed by various methods. These coronary collateral channels have been used successfully as a retrograde access route for percutaneous recanalization of chronic total occlusions. Factors that promote angiogenesis and further collateral remodeling ie. arteriogenesis have been identified. Promotion of collateral growth as a therapeutic target in patients with no suitable revascularization option is an exciting proposal.
Keywords: Arteriogenesis, chronic total occlusion, collateral, collateral flow index, percutaneous intervention
INTRODUCTION
Coronary collaterals have been found even in newborn hearts [1]. The extensive channels were repeatedly found to persist into adulthood. These channels appear ‘cork-screw’ with diameters mostly in the range of 40 to 200 μm but can reach up to 800μm in coronary artery disease with lengths ranging from 1 - 2 cm to 4 - 5 cm.
In post-mortem studies, William Fulton demonstrated ubiquitous presence of these arterial anastomoses even in normal hearts [2, 3]. Extensive superficial and deep intercoronary anastomoses were seen, particularly abundant in the interventricular septum and in the subendocardial plexus of the left ventricle.
Collateral arteries are more abundant in the left than in the right ventricle and are uncommon in the subepicardial territory in man.
Zoll et al. [4] demonstrated collaterals in 46% of 1050 post-mortem adult hearts. The prevalence increased from 9% in normal hearts to 63% in specimens with significantly obstructive coronary artery disease(CAD) and up to 95% when chronic total coronary occlusions (CTOs) were present.
In humans, increased presence of functional pre-formed coronary collaterals in the absence of coronary artery disease (CAD) is predicted by low resting heart rate(not pharmacologically reduced) and absence of hypertension [5].
ANATOMY AND PHYSIOLOGY OF CORONARY COLLATERALS
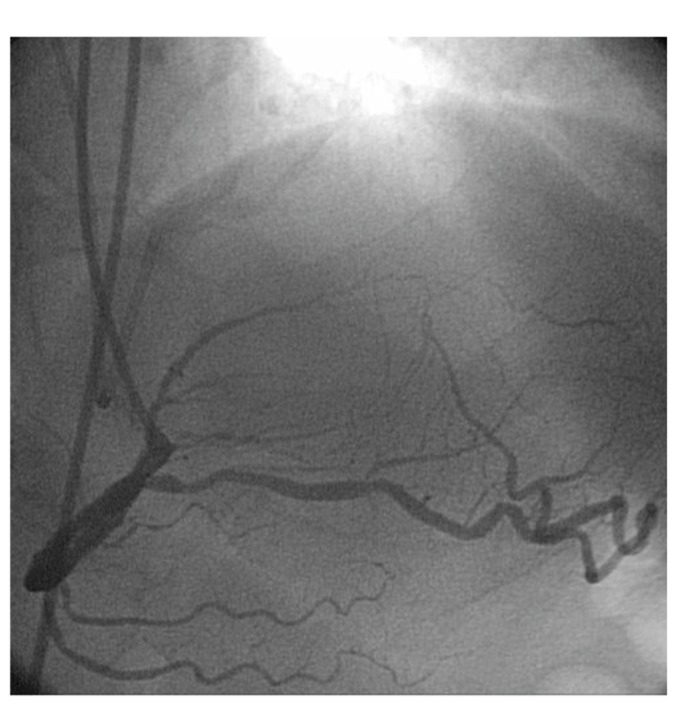
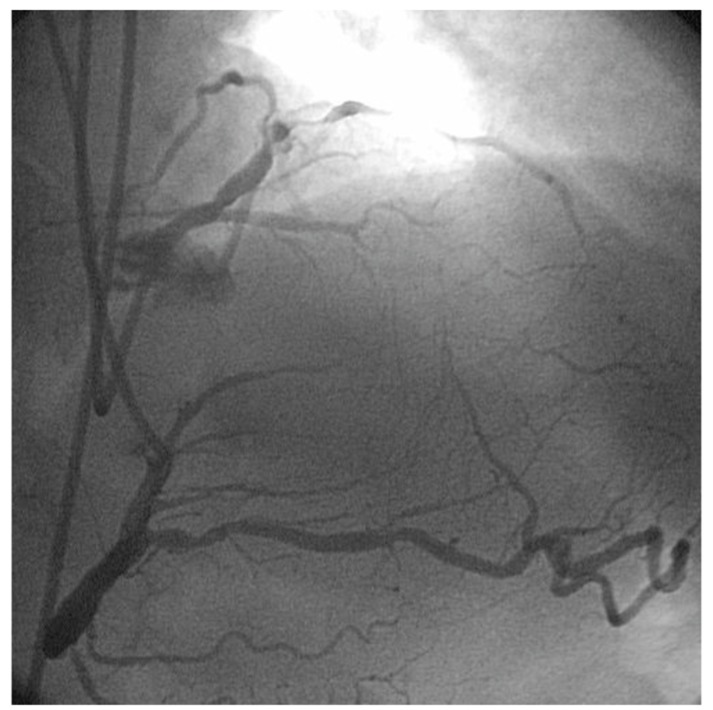
Interventionalists performing coronary angiography are well acquainted with the findings of coronary collaterals in the presence of chronic total occlusions (Figs. 1 and 2).
Fig. (1).
LAD CTO with retrograde filling of distal LAD from RPDA.
Fig. (2).
LAD CTO – bicoronary injection – delineates the CTO segment.
By convention, a total coronary artery occlusion of perceived or known duration of 3 months or longer are labeled as chronic occlusions [6]. However, some of these pre-formed collaterals could be recruited immediately following occlusions (eg. transient balloon occlusion). But with increased chronicity of an occlusion, these small arteriole collaterals undergo remodeling to become muscular arteries, like that of epicardial coronary arteries [7, 8]. This process is termed arteriogenesis.
With the formation of larger muscular arteries, the number of collaterals decrease, a process termed “pruning”. As per physics of flow (Hagen Poiseuille law), a larger artery confers less flow resistance and hence, is more efficient for blood flow as opposed to small collaterals with high vascular resistance even though the numbers of the latter may be more [9].
The time course of the development of these collaterals is controversial. Smaller ones may be formed within 10-15 days [10].
Rentrop et al. described presence of collateral circulation in 27% (74 of 272) of patients undergoing acute medical intervention(streptokinase and/or nitroglycerin) for acute myocardial infarction. Repeat angiography 10-14 days later, showed that the prevalence of angiographically visible collateral arteries in patients whose infarct-related artery never recanalised increased from 33 to 90% [11]. In those who had successful and sustained reperfusion at 10-14 days, the prevalence of collaterals decreased from 38% to 7% [10].
Collateral flow can develop regardless of the viability of the area perfused ie. viability is not a pre-requisite to its development [12]. The main stimulus for collateral development is the pressure gradient across the trans-arterial connections [13].
Diabetic patients may have less potential for collateral formation [14]. Reduced expression of vascular endothelial growth factor (VEGF) and hypoxia inducible factor (HIF-1() [15] as well as attenuated monocytes cellular response to VEGF-A [16] may be explanations for this observation.
There are also genetic links that determine the extent of pre-existing collateral network as well as development of functional collateral circulation [17-19].
In 1974, David Lewin published an extensive map of 22 different collateral pathways. These are categorized into four types: septal, atrial, branch-branch in ventricular free walls, and trans-lesion bridging [20].
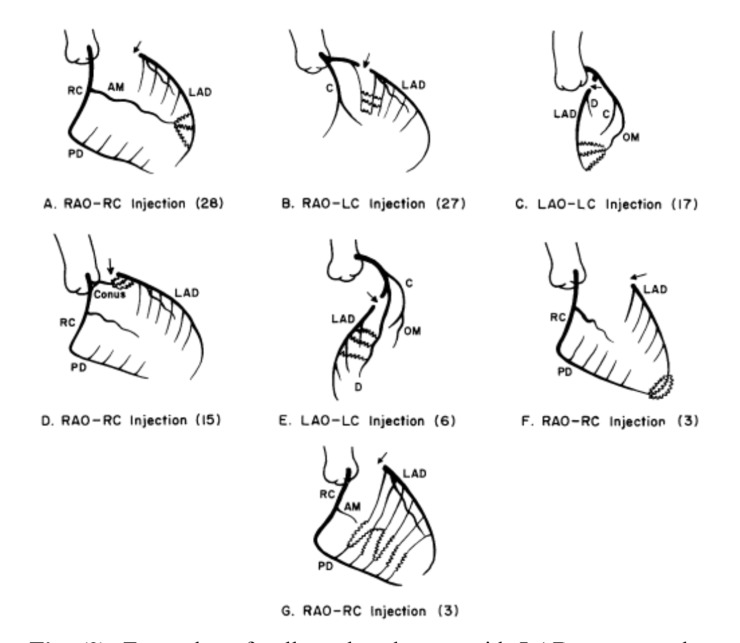
In the presence of a CTO, collateral perfusion to the segment beyond the occlusion often comes from multiple pathways [21]. For example, in the case of a left anterior descending artery occlusion, parallel septal collaterals from the right posterior descending artery, epicardial branches of the right ventricular branches and collaterals from the posterolateral branches of the left circumflex artery often co-exist (Figs. 3 and 4).
Fig. (3).
Examples of collateral pathways with LAD artery occlusion [9].
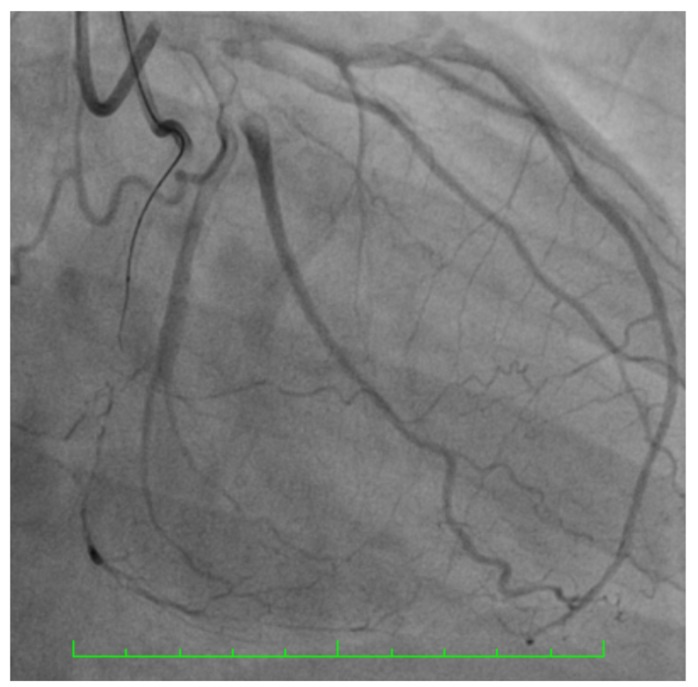
Fig. (4).
RCA CTO - Multiple parallel septal collateral channels and also distal epicardial collaterals from LAD to Right posterolateral branch.
Usually, the bulk of the perfusion comes from the larger of the collaterals as the larger vessels have the least flow resistance. Some of the parallel collateral channels are therefore ‘invisible’ angiographically but operators of the retrograde percutaneous intervention technique are sometimes able to ‘serendipitously’ navigate dedicated guidewires through these unseen tracks [22, 23].
Interestingly, these collaterals appear not to be prone to atherosclerosis [8].
CLASSIFICATION AND ASSESSMENT OF CORONARY COLLATERAL CIRCULATION
There are a number of ways to classify coronary collateral circulation [24].
Interventionalists are familiar with the angiography-based Renthrop classification.
Angiographic Classification of Coronary Collateral Circulation:
Rentrop classification [25]
Grade 0 No visible filling of any collateral channel.
Grade 1 Filling of the side branches of the infarct-related artery.
Grade 2 Partial filling of the epicardial vessel of the infarct-related artery.
Grade 3 Complete collateral filling of the epicardial vessel.
There are a number of factors that affect the angiographic visualization of collaterals eg. speed of injection, contrast concentration, catheter size and spatial resolution of angiographic systems (of up to 100 μm only).
2. A more recently described angiographic classification describes the size of the collateral connections.
Collateral connection (CC) grades-size based classification [26].
CC0 No continuous connection between donor and recipient artery
CC1 Continuous, threadlike connection
CC2 Continuous, side branch-like size throughout its course
The adequacy of collateral circulation can be assessed with these few methods:
Collateral Flow Index (CFI)
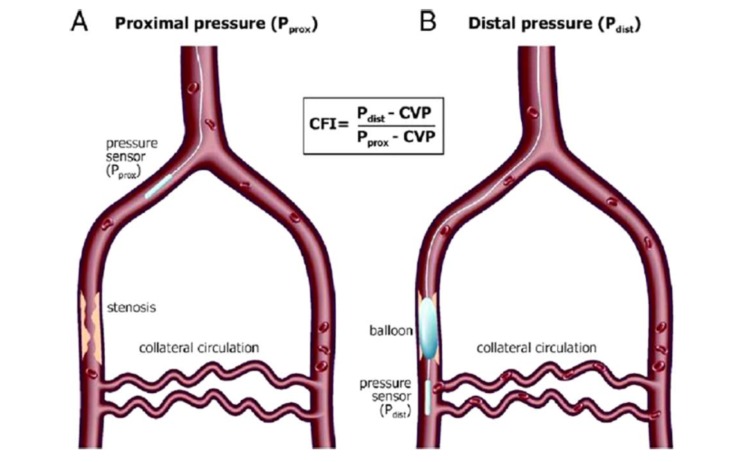
First described by Pijls et al. [27], this allows a quantitative assessment of the adequacy of collateral circulation (Fig. 5) [21]. In the initial papers, a value of ≤ 23% predicts presence of inducible ischemia during balloon inflation and it could predict future ischemic events [28].
Fig. (5).
Measurement of collateral flow index (CFI) requires measurements of mean aortic pressure or proximal pressure (P prox), mean distal coronary pressure (P distal) and central venous pressure (CVP) [29].
Collateral supply should be assessed during maximal coronary hyperemia which is best achieved by systemic intravenous infusion of adenosine (140 mcg/kg/min). In a large cohort of stable CAD patients undergoing PCI, a CFI of < 0.25 predicts likelihood of ischaemia during balloon occlusion [30].
A higher CFI value correlates with the development of hemodynamically significant obstructive lesion. Hence, in the presence of CTO, the occluded vascular bed has a higher CFI compared to those without [31].
CFI of ( 25% is a threshold that is required to prevent myocardial ischemia and limit infarct size during coronary occlusion [32].
2. Intracoronary ECG lead
Using the coronary angioplasty or coronary pressure guidewire as an ECG lead, presence of ST-segment elevation > 0.1mv is used as a tool for detection of ischemia at the region of interest [33]. In fact, a strong inverse relationship between CFI and the intracoronary occlusive ECG ST-segment shift (elevation or depression) during coronary occlusion has been described. A good collateral perfusion with CFI ≥ 0.217 can be accurately detected with an ST-segment shift of ≤ 0.1mV [34].
3. Presence of angina during balloon occlusion is a crude way of determining presence of ischaemia owing to inadequate collateral support.
4. Myocardial perfusion imaging with 99mTc-Sestamibi scan can be used to quantify collateral circulation by assessment of the extent of ischemia [35].
5. Myocardial contrast echocardiography (MCE) is a tool that may allow the demonstration of spatial extent of perfusion from collateral circulation by selectively injecting sonicated microbubbles into the donor artery [36]. However, direct intra-arterial injection of these contrast agents are not FDA approved.
Realtime non-invasive assessment of collateral supply using MCE and systemic administration of contrast is feasible. In one series, a close relationship between the pressure-derived CFI and absolute collateral-derived myocardial blood flow (MBFc) measured by echocardiography in 32 PCI cases was demonstrated [37].
Non-invasive MCE demonstrates the importance of collateral circulation to predict potential for LV function recovery post-myocardial infarction [38, 39].
6. Washout collaterometry [40]. Following injection of contrast into the coronary artery immediately before coronary balloon occlusion, the number of heart beats needed to wash out the angiographic medium is calculated. Washout time of ≤ 11 heart beats accurately predicts adequacy of collaterals sufficient to prevent ischemia.
CLINICAL IMPORTANCE OF CORONARY COLLATERAL CIRCULATION
During acute coronary occlusion, collaterals are recruited to mitigate regional myocardial hypoperfusion and ischemia.
Interventionalists are well aware of ischemic manifestations during angioplasty balloon inflation eg. chest pain, ischemic ST-segment depressions. However, there are many patients who do not manifest such ischemia and every patient is different in his/her ischemic duration thresholds. These differences may be accounted for by the presence of collateral circulation.
Antoniucci et al. found at least grade 2 Renthrop’s collateral circulation in 23% of 1164 patients undergoing primary PCI [41].
In experimental occlusion of coronary artery with angioplasty balloon of 100 individuals, the flow provided by collateral circulation is 18% of baseline flow – as measured by collateral flow index, CFI. During this 1 minute coronary occlusion time, about 25% of individuals do not develop angina pectoris and 20% do not develop intracoronary ECG signs of ischemia [42].
In the setting of acute ST-elevation myocardial infarction (STEMI), collateral circulation have the following potential beneficial effects:
Reduction of infarct size. This was seen in pre-clinical animal studies [43] as well as in human studies. Human studies demonstrated lower peak serum creatine kinase (CK) levels post-STEMI in patients with collateral circulation compared to those without [44]. Following anteroseptal MI, presence of well developed collateral minimised infarct size and predicted more viable myocardium [45]. One MRI study showed that collateral circulation reduced transmurality of infarction but not the extent of lateral boundaries of infarct area [46]. Microvascular obstruction is also less extensive in the presence of well-developed collateral circulation [47].
The preservation of myocardium from the reduced infarct area, ensures higher residual cardiac function (left ventricular ejection fraction) – which is a great prognostic indicator [48].
In addition, decreased myocardial death reduces associated mechanical complications eg. cardiogenic shock [49], myocardial rupture [50] or ventricular aneurysm [51] formation. The reduction of cardiogenic shock translates to improved survival rates [52].
Presence of coronary collaterals maintains viability for a longer period of time [53]. This may extend the time buffer for successful reperfusion be it with thrombolysis or primary PCI [54].
CHRONIC CAD AND COLLATERAL CIRCULATION
Majority of studies correlated improved survival in the presence of well-developed collateral circulation in chronic CAD patients.
Using collateral flow index (CFI) to determine adequacy of collateral circulation, cumulative 10-year survival rates (from all-cause and cardiac mortality) were 71% and 88%, respectively, in patients with low CFI and 89% and 97% in the group with high CFI (p = 0.04, p = 0.01, respectively) [55].
The primary author, Dr. Meier of this paper and other co-authors, later performed a meta-analysis of 12 studies with 6,529 stable CAD or acute MI patients. High collateralization was associated with less mortality especially in the stable CAD group (RR 0.59; CI 0.39-0.89; p = 0.012) [56].
COLLATERAL CIRCULATION IN CHRONIC TOTAL OCCLUSIONS [57]
Development of collateral circulation to the territory of occluded artery is related to the distribution extent of the donor artery [58], microvascular function [59], chronicity of occlusion [60] and left ventricular function.
The collateral circulation may be bidirectional [61].
Despite the development of large collateral channels in CTO, this alternative supply may be sufficient at rest but this is not so during demand periods eg. exercise [62, 63].
Pressure changes and coronary flow velocity distal to CTO are significantly impaired (ie. FFR < 0.75) during stress provocation with systemic adenosine infusion [64].
PERCUTANEOUS RECANALISATION OF CHRONIC TOTAL OCCLUSIONS(CTOS)
Percutaneous coronary intervention (PCI) of CTOs remain one of the most challenging procedures for interventional cardiologists. Successful recanalization rates have improved to >80% in many reported series and may be in excess of 90% in the hands of CTO masters. In addition to refinement of techniques and devices (eg. CTO wires, IVUS guidance, support catheters, etc) , one other important advance has been the introduction of retrograde approach for wire crossing of CTO). This essentially uses the ‘back door’ to pass the guidewire from the donor artery via collateral channels to penetrate the distal CTO cap.
This is an option in cases of antegrade crossing failure. Collateral channel crossing with a guidewire is successfully achieved in 73% to 87% of cases in experienced hands [65, 66].
Collateral channels can be as large as 800μm (0.031”) [3]. Only those > 100μm are visible angiographically. Specialised polymer jacket wires eg. non-tapered 360μm (0.014”) eg. Fielder FC( (Asahi Intecc, Japan), 360 μm (0.014”) Whisper( (Abbott Vascular, USA) or tapered wires 230μm (0.009”) eg. Fielder XT( (Asahi Intecc, Japan) are device options designed to cross these channels.
The guidewires’ collateral crossing is supported by microcatheters. One of the most successful support catheter is the Corsair ((Asahi Intecc, Japan). It’s atraumatic tip is a tapered soft cone of 0.016” (420μm) profile. The distal shaft diameter is 870μm (2.6 Fr / 0.034”) and the proximal shaft diameter is 930μm (2.8Fr / 0.037”). The kink-resistant tungsten braiding and distal hydrophilic polymer coating enables crossing of tortuous micro-channels. Finecross( Terumo, Japan is another micro-catheter that may be used in contemporary PCI for collateral tracking. It has an outer diameter of 600μm (1.8Fr; 0.024”).
There are 2 forms of coronary collaterals. The capillary collaterals and the larger muscular-walled collaterals that develop from pre-existing arterioles through a process called arteriogenesis [67, 68].
Hence, the smaller collateral channels may be stretched/expanded with the passage of the microcatheters. Together with the tortuosity of collaterals and device manouvering for collateral tracking, the potential for injury is real especially with the smaller capillary type of collaterals. The risks includes septal hematoma, collateral channel rupture causing pericardial tamponade, equipment entrapment and donor artery injury.
Following successful recanalization of a CTO, collateral circulation regresses and loses its functional ability [69]. However, they remain recruitable rapidly, within minutes if re-occlusion occurs soon after.
Diabetic patients may have less pronounced collateral recruitment compared to non-diabetics early after angioplasty especially when the occlusion had only occurred less than 3 months earlier, a factor that may account for the poorer outcome in these patient subset [70].
With time, the collaterals regress further (up to 5 months) [71]. If the re-occlusion then occurs gradually, these collaterals may recover but for acute occlusions eg. acute myocardial infarction, they may not be recruited early enough for significant myocardial salvage.
However, myocardial infarction occurrence is less common than re-occlusion. It is believed that there are persistent collaterals that protect the post-occluded segment. It could also be that MI is less common because the culprit artery is more likely to reocclude gradually rather than abruptly, hence allowing time for recruitment and development of collateral circulation [10].
There are some suggestions that paclitaxel and rapamycin drugs from drug-eluting stents (DES) may impair the function and development of collaterals. Although, DES improves patency rates after successful revascularization, acute occlusion eg. stent thrombosis may have worse consequences without adequate collateral support [72].
THERAPEUTIC PROMOTION OF COLLATERAL FORMATION
There are patients that have such extensive CAD especially occlusive and diffuse atherosclerotic disease that there is no revascularization option. With our understanding of the various stimuli for angiogenesis, arteriogenesis and collateral function, the prospect of ‘growing’ or augmenting collateral circulation is an attractive investigation subject.
Granulocte macrophage-colony stimulating factor (GM-CSF) in small randomized placebo-controlled trials [73, 74] have been shown to improve collateral function (CFI) in patients with CAD. However, these trials were stopped owing to potential harm by the induction of plaque rupture.
Granulocyte-colony-stimulating factory (G-CSF) has been showed to have similar benefits in enhancing collateral function (CFI) [75].
Increased coronary blood flow and tangential shear stress stimulate arteriogenesis. Hence, physical exercise is a logical physical stimulus to promote functional collateral development. However, exercise therapy failed to increase collateral circulation as assessed by Rentrop’s classification in a prospective 1 year trial of 113 patients, despite improvements in CAD severity [76]. The criticism of this paper noted that this angiographic classification is an insensitive way to assess collateral growth and the improvements in the CAD severity may have deterred collateral development.
In another report, following 3-months of cardiac rehabilitation utilizing exercise-based regimen, collateral function improved in CAD patients when compared to a sedentary control group [77].
Augmentation of vascular shear stress via enhance external counter-pulsation (EECP) to promote collateral function may be the mechanism for some of the positive outcomes of this therapy [78].
CONCLUSION
Coronary collateral network is present in both normal as well as CAD hearts. They mitigate ischemic insults during acute and chronic coronary events eg. acute myocardial infarction, and after the development of chronic total occlusions. The extent of collateral network depends on acuity of occlusion, duration of ischemia, genetic influence and co-morbid states eg. diabetes status. The adequacy of collateral circulation may be assessed and graded anatomically or via functional measurements eg. collateral flow index(CFI). Success of percutaneous recanalization of chronic total occlusions have improved with the use of collateral channels as retrograde access routes for intervention. Knowledge of the collateral anatomy, physiology coupled with the development of appropriate devices and techniques has enabled the conduct of these procedures successfully and safely. Future research targeting collateral enhancement may provide therapeutic opportunity for patients without revascularization options.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENT
Declared none.
REFERENCES
- 1.Baroldi G., Mantero O., Scomazzoni G. The collaterals of the coronary arteries in normal and pathologic hearts. Circ. Res. 1956;4(2):223–229. doi: 10.1161/01.RES.4.2.223. [DOI] [PubMed] [Google Scholar]
- 2.Fulton W.F. Arterial anastomoses in the coronary circulation. I. Anatomical features in normal and diseased hearts demonstrated by stereoarteriography. Scott. Med. J. 1963;8:420–434. doi: 10.1177/003693306300801102. [DOI] [PubMed] [Google Scholar]
- 3.Fulton W.F. Arterial anastomoses in the coronary circulation. Ii. Distribution, enumeration and measurement of coronary arterial anastomoses in health and disease. Scott. Med. J. 1963;8:466–474. doi: 10.1177/003693306300801202. [DOI] [PubMed] [Google Scholar]
- 4.Zoll P.M., Wessler S., Schlesinger M.J. Interarterial coronary anastomoses in the human heart, with particular reference to anemia and relative cardiac anoxia. Circulation. 1951;4(6):797–815. doi: 10.1161/01.CIR.4.6.797. [DOI] [PubMed] [Google Scholar]
- 5.de Marchi S.F., Streuli S., Haefeli P., Gloekler S., Traupe T., Warncke C., Rimoldi S.F., Stortecky S., Steck H., Seiler C. Determinants of prognostically relevant intracoronary electrocardiogram ST-segment shift during coronary balloon occlusion. Am. J. Cardiol. 2012;110(9):1234–1239. doi: 10.1016/j.amjcard.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Di Mario C., Werner G.S., Sianos G., Galassi A.R., Büttner J., Dudek D., Chevalier B., Lefevre T., Schofer J., Koolen J., Sievert H., Reimers B., Fajadet J., Colombo A., Gershlick A., Serruys P.W., Reifart N. European perspective in the recanalisation of Chronic Total Occlusions (CTO): consensus document from the EuroCTO Club. EuroIntervention. 2007;3(1):30–43. [PubMed] [Google Scholar]
- 7.Schaper W. Collateral vessel growth in the human heart. Role of fibroblast growth factor-2. Circulation. 1996;94(4):600–601. doi: 10.1161/01.CIR.94.4.600. [DOI] [PubMed] [Google Scholar]
- 8.Schaper W. The Collateral Circulation of the Heart. New York: Elsevier; 1971. [Google Scholar]
- 9.Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. 2013 doi: 10.1093/eurheartj/eht195. Published June 5, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Bourassa M.G., Solignac A., Goulet C., Lespérance J. Regression and appearance of coronary collaterals in humans during life. Circulation. 1974;50(2) Suppl.:II127–II135. [PubMed] [Google Scholar]
- 11.Rentrop K.P., Feit F., Sherman W., Thornton J.C. Serial angiographic assessment of coronary artery obstruction and collateral flow in acute myocardial infarction. Report from the second Mount Sinai-New York University Reperfusion Trial. Circulation. 1989;80(5):1166–1175. doi: 10.1161/01.CIR.80.5.1166. [DOI] [PubMed] [Google Scholar]
- 12.Heil M., Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ. Res. 2004;95(5):449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 13.Fulton W.F., van Royen N. The coronary collateral circulation in man. In: Schaper W., Schaper J., editors. Arteriogenesis. Dordrecht: Kluwer Academic; 2004. [DOI] [Google Scholar]
- 14.Abaci A., Oğuzhan A., Kahraman S., Eryol N.K., Unal S., Arinç H., Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99(17):2239–2242. doi: 10.1161/01.CIR.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 15.Marfella R., Esposito K., Nappo F., Siniscalchi M., Sasso F.C., Portoghese M., Di Marino M.P., Baldi A., Cuzzocrea S., Di Filippo C., Barboso G., Baldi F., Rossi F., D’Amico M., Giugliano D. Expression of angiogenic factors during acute coronary syndromes in human type 2 diabetes. Diabetes. 2004;53(9):2383–2391. doi: 10.2337/diabetes.53.9.2383. [DOI] [PubMed] [Google Scholar]
- 16.Waltenberger J., Lange J., Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: A potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102(2):185–190. doi: 10.1161/01.CIR.102.2.185. [DOI] [PubMed] [Google Scholar]
- 17.de Marchi S.F. Determinants of human coronary collaterals. Curr. Cardiol. Rev. 2014;10(1):24–28. doi: 10.2174/1573403X1001140317114411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier P., Antonov J., Zbinden R., Kuhn A., Zbinden S., Gloekler S., Delorenzi M., Jaggi R., Seiler C. Non-invasive gene-expression-based detection of well-developed collateral function in individuals with and without coronary artery disease. Heart. 2009;95(11):900–908. doi: 10.1136/hrt.2008.145383. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J., Regieli J.J., Schipper M., Entius M.M., Liang F., Koerselman J., Ruven H.J., van der Graaf Y., Grobbee D.E., Doevendans P.A. Inflammatory gene haplotype-interaction networks involved in coronary collateral formation. Hum. Hered. 2008;66(4):252–264. doi: 10.1159/000143407. [DOI] [PubMed] [Google Scholar]
- 20.Levin D.C. Pathways and functional significance of the coronary collateral circulation. Circulation. 1974;50(4):831–837. doi: 10.1161/01.CIR.50.4.831. [DOI] [PubMed] [Google Scholar]
- 21.van Royen N., Piek J.J., Schaper W., Fulton W.F. A critical review of clinical arteriogenesis research. J. Am. Coll. Cardiol. 2009;55(1):17–25. doi: 10.1016/j.jacc.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 22.Surmely J.F., Katoh O., Tsuchikane E., Nasu K., Suzuki T. Coronary septal collaterals as an access for the retrograde approach in the percutaneous treatment of coronary chronic total occlusions. Catheter. Cardiovasc. Interv. 2007;69(6):826–832. doi: 10.1002/ccd.20816. [DOI] [PubMed] [Google Scholar]
- 23.Sianos G., Barlis P., Di Mario C., Papafaklis M.I., Büttner J., Galassi A.R., Schofer J., Werner G., Lefevre T., Louvard Y., Serruys P.W., Reifart N., EuroCTO Club European experience with the retrograde approach for the recanalisation of coronary artery chronic total occlusions. A report on behalf of the euroCTO club. EuroIntervention. 2008;4(1):84–92. doi: 10.4244/EIJV4I1A15. [DOI] [PubMed] [Google Scholar]
- 24.Karrowni W., El Accaoui R.N., Chatterjee K. Coronary collateral circulation: its relevance. Catheter. Cardiovasc. Interv. 2013;82(6):915–928. doi: 10.1002/ccd.24910. [DOI] [PubMed] [Google Scholar]
- 25.Rentrop K.P., Cohen M., Blanke H., Phillips R.A. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J. Am. Coll. Cardiol. 1985;5(3):587–592. doi: 10.1016/S0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 26.Werner G.S., Ferrari M., Heinke S., Kuethe F., Surber R., Richartz B.M., Figulla H.R. Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation. 2003;107(15):1972–1977. doi: 10.1161/01.CIR.0000061953.72662.3A. [DOI] [PubMed] [Google Scholar]
- 27.Pijls N.H., van Son J.A., Kirkeeide R.L., De Bruyne B., Gould K.L. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354–1367. doi: 10.1161/01.CIR.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 28.Pijls N.H., Bech G.J., el Gamal M.I., Bonnier H.J., De Bruyne B., Van Gelder B., Michels H.R., Koolen J.J. Quantification of recruitable coronary collateral blood flow in conscious humans and its potential to predict future ischemic events. J. Am. Coll. Cardiol. 1995;25(7):1522–1528. doi: 10.1016/0735-1097(95)00111-G. [DOI] [PubMed] [Google Scholar]
- 29.Karrowni W., El Accaoui R.N., Chatterjee K. Coronary collateral circulation: its relevance. Catheter. Cardiovasc. Interv. 2013;82(6):915–928. doi: 10.1002/ccd.24910. [DOI] [PubMed] [Google Scholar]
- 30.Seiler C., Fleisch M., Garachemani A., Meier B. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J. Am. Coll. Cardiol. 1998;32(5):1272–1279. doi: 10.1016/S0735-1097(98)00384-2. [DOI] [PubMed] [Google Scholar]
- 31.Seiler C. Collateral circulation of the heart. London: Springer; 2009. [DOI] [Google Scholar]
- 32.Lee C.W., Park S.W., Cho G.Y., Hong M.K., Kim J.J., Kang D.H., Song J.K., Lee H.J., Park S.J. Pressure-derived fractional collateral blood flow: a primary determinant of left ventricular recovery after reperfused acute myocardial infarction. J. Am. Coll. Cardiol. 2000;35(4):949–955. doi: 10.1016/S0735-1097(99)00649-X. [DOI] [PubMed] [Google Scholar]
- 33.Meier B., Rutishauser W. Coronary pacing during percutaneous transluminal coronary angioplasty. Circulation. 1985;71(3):557–561. doi: 10.1161/01.CIR.71.3.557. [DOI] [PubMed] [Google Scholar]
- 34.de Marchi S.F., Streuli S., Haefeli P., Gloekler S., Traupe T., Warncke C., Rimoldi S.F., Stortecky S., Steck H., Seiler C. Determinants of prognostically relevant intracoronary electrocardiogram ST-segment shift during coronary balloon occlusion. Am. J. Cardiol. 2012;110(9):1234–1239. doi: 10.1016/j.amjcard.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo H., Watanabe S., Kadosaki T., Yamaki T., Tanaka S., Miyata S., Segawa T., Matsuno Y., Tomita M., Fujiwara H. Validation of collateral fractional flow reserve by myocardial perfusion imaging. Circulation. 2002;105(9):1060–1065. doi: 10.1161/hc0902.104719. [DOI] [PubMed] [Google Scholar]
- 36.Mills J.D., Fischer D., Villanueva F.S. Coronary collateral development during chronic ischemia: serial assessment using harmonic myocardial contrast echocardiography. J. Am. Coll. Cardiol. 2000;36(2):618–624. doi: 10.1016/S0735-1097(00)00739-7. [DOI] [PubMed] [Google Scholar]
- 37.Vogel R., Zbinden R., Indermühle A., Windecker S., Meier B., Seiler C. Collateral-flow measurements in humans by myocardial contrast echocardiography: validation of coronary pressure-derived collateral-flow assessment. Eur. Heart J. 2006;27(2):157–165. doi: 10.1093/eurheartj/ehi585. [DOI] [PubMed] [Google Scholar]
- 38.Sabia P.J., Powers E.R., Ragosta M., Sarembock I.J., Burwell L.R., Kaul S. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N. Engl. J. Med. 1992;327(26):1825–1831. doi: 10.1056/NEJM199212243272601. [DOI] [PubMed] [Google Scholar]
- 39.Sabia P.J., Powers E.R., Jayaweera A.R., Ragosta M., Kaul S. Functional significance of collateral blood flow in patients with recent acute myocardial infarction. A study using myocardial contrast echocardiography. Circulation. 1992;85(6):2080–2089. doi: 10.1161/01.CIR.85.6.2080. [DOI] [PubMed] [Google Scholar]
- 40.Seiler C., Billinger M., Fleisch M., Meier B. Washout collaterometry: a new method of assessing collaterals using angiographic contrast clearance during coronary occlusion. Heart. 2001;86(5):540–546. doi: 10.1136/heart.86.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antoniucci D., Valenti R., Moschi G., Migliorini A., Trapani M., Santoro G.M., Bolognese L., Cerisano G., Buonamici P., Dovellini E.V. Relation between preintervention angiographic evidence of coronary collateral circulation and clinical and angiographic outcomes after primary angioplasty or stenting for acute myocardial infarction. Am. J. Cardiol. 2002;89(2):121–125. doi: 10.1016/S0002-9149(01)02186-5. [DOI] [PubMed] [Google Scholar]
- 42.Wustmann K., Zbinden S., Windecker S., Meier B., Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation. 2003;107(17):2213–2220. doi: 10.1161/01.CIR.0000066321.03474.DA. [DOI] [PubMed] [Google Scholar]
- 43.Cheirif J., Narkiewicz-Jodko J.B., Hawkins H.K., Bravenec J.S., Quinones M.A., Mickelson J.K. Myocardial contrast echocardiography: relation of collateral perfusion to extent of injury and severity of contractile dysfunction in a canine model of coronary thrombosis and reperfusion. J. Am. Coll. Cardiol. 1995;26(2):537–546. doi: 10.1016/0735-1097(95)80034-E. [DOI] [PubMed] [Google Scholar]
- 44.Habib G.B., Heibig J., Forman S.A., Brown B.G., Roberts R., Terrin M.L., Bolli R., The TIMI Investigators Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. Circulation. 1991;83(3):739–746. doi: 10.1161/01.CIR.83.3.739. [DOI] [PubMed] [Google Scholar]
- 45.Fukai M., Ii M., Nakakoji T., Kawakatsu M., Nariyama J., Yokota N., Negoro N., Kojima S., Ohkubo T., Hoshiga M., Nakajima O., Ishihara T. Angiographically demonstrated coronary collaterals predict residual viable myocardium in patients with chronic myocardial infarction: a regional metabolic study. J. Cardiol. 2000;35(2):103–111. [PubMed] [Google Scholar]
- 46.Ortiz-Pérez J.T., Meyers S.N., Lee D.C., Kansal P., Klocke F.J., Holly T.A., Davidson C.J., Bonow R.O., Wu E. Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur. Heart J. 2007;28(14):1750–1758. doi: 10.1093/eurheartj/ehm212. [DOI] [PubMed] [Google Scholar]
- 47.Desch S., de Waha S., Eitel I., Koch A., Gutberlet M., Schuler G., Thiele H. Effect of coronary collaterals on long-term prognosis in patients undergoing primary angioplasty for acute ST-elevation myocardial infarction. Am. J. Cardiol. 2010;106(5):605–611. doi: 10.1016/j.amjcard.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Nicolau J.C., Pinto M.A., Nogueira P.R., Lorga A.M., Jacob J.L., Garzon S.A. The role of antegrade and collateral flow in relation to left ventricular function post-thrombolysis. Int. J. Cardiol. 1997;61(1):47–54. doi: 10.1016/S0167-5273(97)00134-4. [DOI] [PubMed] [Google Scholar]
- 49.Elsman P., van ’t Hof A.W., de Boer M.J., Hoorntje J.C., Suryapranata H., Dambrink J.H., Zijlstra F., Zwolle Myocardial Infarction Study Group Role of collateral circulation in the acute phase of ST-segment-elevation myocardial infarction treated with primary coronary intervention. Eur. Heart J. 2004;25(10):854–858. doi: 10.1016/j.ehj.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Nakatani D., Sato H., Kinjo K., Mizuno H., Hishida E., Hirayama A., Mishima M., Ito H., Matsumura Y., Hori M., Osaka Acute Coronary Insufficiency Study Group Effect of successful late reperfusion by primary coronary angioplasty on mechanical complications of acute myocardial infarction. Am. J. Cardiol. 2003;92(7):785–788. doi: 10.1016/S0002-9149(03)00883-X. [DOI] [PubMed] [Google Scholar]
- 51.Hirai T., Fujita M., Nakajima H., Asanoi H., Yamanishi K., Ohno A., Sasayama S. Importance of collateral circulation for prevention of left ventricular aneurysm formation in acute myocardial infarction. Circulation. 1989;79(4):791–796. doi: 10.1161/01.CIR.79.4.791. [DOI] [PubMed] [Google Scholar]
- 52.Pérez-Castellano N., García E.J., Abeytua M., Soriano J., Serrano J.A., Elízaga J., Botas J., López-Sendón J.L., Delcán J.L. Influence of collateral circulation on in-hospital death from anterior acute myocardial infarction. J. Am. Coll. Cardiol. 1998;31(3):512–518. doi: 10.1016/S0735-1097(97)00521-4. [DOI] [PubMed] [Google Scholar]
- 53.Charney R., Cohen M. The role of the coronary collateral circulation in limiting myocardial ischemia and infarct size. Am. Heart J. 1993;126(4):937–945. doi: 10.1016/0002-8703(93)90710-Q. [DOI] [PubMed] [Google Scholar]
- 54.Waldecker B., Waas W., Haberbosch W., Voss R., Wiecha J., Tillmanns H. [Prevalence and significance of coronary collateral circulation in patients with acute myocardial infarct]. Z. Kardiol. 2002;91(3):243–248. doi: 10.1007/s003920200018. [DOI] [PubMed] [Google Scholar]
- 55.Meier P., Gloekler S., Zbinden R., Beckh S., de Marchi S.F., Zbinden S., Wustmann K., Billinger M., Vogel R., Cook S., Wenaweser P., Togni M., Windecker S., Meier B., Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116(9):975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 56.Meier P., Hemingway H., Lansky A.J., Knapp G., Pitt B., Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur. Heart J. 2012;33(5):614–621. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 57.Berry C., Balachandran K.P., L’Allier P.L., Lespérance J., Bonan R., Oldroyd K.G. Importance of collateral circulation in coronary heart disease. Eur. Heart J. 2007;28(3):278–291. doi: 10.1093/eurheartj/ehl446. [DOI] [PubMed] [Google Scholar]
- 58.Gatzov P., Manginas A., Voudris V., Pavlides G., Genchev G.D., Cokkinos D.V. Blood flow velocity in donor coronary artery depends on the degree and pattern of collateral vessel development: a study using thrombolysis in myocardial infarction frame count method. Catheter. Cardiovasc. Interv. 2003;60(4):462–468. doi: 10.1002/ccd.10694. [DOI] [PubMed] [Google Scholar]
- 59.Werner G.S., Emig U., Bahrmann P., Ferrari M., Figulla H.R. Recovery of impaired microvascular function in collateral dependent myocardium after recanalisation of a chronic total coronary occlusion. Heart. 2004;90(11):1303–1309. doi: 10.1136/hrt.2003.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Werner G.S., Ferrari M., Betge S., Gastmann O., Richartz B.M., Figulla H.R. Collateral function in chronic total coronary occlusions is related to regional myocardial function and duration of occlusion. Circulation. 2001;104(23):2784–2790. doi: 10.1161/hc4801.100352. [DOI] [PubMed] [Google Scholar]
- 61.Werner G.S., Richartz B.M., Gastmann O., Ferrari M., Figulla H.R. Immediate changes of collateral function after successful recanalization of chronic total coronary occlusions. Circulation. 2000;102(24):2959–2965. doi: 10.1161/01.CIR.102.24.2959. [DOI] [PubMed] [Google Scholar]
- 62.Werner G.S., Figulla H.R. Direct assessment of coronary steal and associated changes of collateral hemodynamics in chronic total coronary occlusions. Circulation. 2002;106(4):435–440. doi: 10.1161/01.CIR.0000022848.92729.33. [DOI] [PubMed] [Google Scholar]
- 63.Werner G.S., Fritzenwanger M., Prochnau D., Schwarz G., Ferrari M., Aarnoudse W., Pijls N.H., Figulla H.R. Determinants of coronary steal in chronic total coronary occlusions donor artery, collateral, and microvascular resistance. J. Am. Coll. Cardiol. 2006;48(1):51–58. doi: 10.1016/j.jacc.2005.11.093. [DOI] [PubMed] [Google Scholar]
- 64.Werner G.S., Surber R., Ferrari M., Fritzenwanger M., Figulla H.R. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur. Heart J. 2006;27(20):2406–2412. doi: 10.1093/eurheartj/ehl270. [DOI] [PubMed] [Google Scholar]
- 65.Surmely J.F., Tsuchikane E., Katoh O., Nishida Y., Nakayama M., Nakamura S., Oida A., Hattori E., Suzuki T. New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART technique. J. Invasive Cardiol. 2006;18(7):334–338. [PubMed] [Google Scholar]
- 66.Rathore S., Katoh O., Matsuo H., Terashima M., Tanaka N., Kinoshita Y., Kimura M., Tsuchikane E., Nasu K., Ehara M., Asakura K., Asakura Y., Suzuki T. Retrograde percutaneous recanalization of chronic total occlusion of the coronary arteries: procedural outcomes and predictors of success in contemporary practice. Circ. Cardiovasc. Interv. 2009;2(2):124–132. doi: 10.1161/CIRCINTERVENTIONS.108.838862. [DOI] [PubMed] [Google Scholar]
- 67.Ito W.D., Arras M., Winkler B., Scholz D., Schaper J., Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ. Res. 1997;80(6):829–837. doi: 10.1161/01.RES.80.6.829. [DOI] [PubMed] [Google Scholar]
- 68.Seiler C. The human coronary collateral circulation. Heart. 2003;89(11):1352–1357. doi: 10.1136/heart.89.11.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Werner G.S., Emig U., Mutschke O., Schwarz G., Bahrmann P., Figulla H.R. Regression of collateral function after recanalization of chronic total coronary occlusions: a serial assessment by intracoronary pressure and Doppler recordings. Circulation. 2003;108(23):2877–2882. doi: 10.1161/01.CIR.0000100724.44398.01. [DOI] [PubMed] [Google Scholar]
- 70.Werner G.S., Richartz B.M., Heinke S., Ferrari M., Figulla H.R. Impaired acute collateral recruitment as a possible mechanism for increased cardiac adverse events in patients with diabetes mellitus. Eur. Heart J. 2003;24(12):1134–1142. doi: 10.1016/S0195-668X(03)00187-8. [DOI] [PubMed] [Google Scholar]
- 71.Werner G.S., Emig U., Mutschke O., Schwarz G., Bahrmann P., Figulla H.R. Regression of collateral function after recanalization of chronic total coronary occlusions: a serial assessment by intracoronary pressure and Doppler recordings. Circulation. 2003;108(23):2877–2882. doi: 10.1161/01.CIR.0000100724.44398.01. [DOI] [PubMed] [Google Scholar]
- 72.Meier P., Zbinden R., Togni M., Wenaweser P., Windecker S., Meier B., Seiler C. Coronary collateral function long after drug-eluting stent implantation. J. Am. Coll. Cardiol. 2007;49(1):15–20. doi: 10.1016/j.jacc.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 73.Seiler C., Pohl T., Wustmann K., Hutter D., Nicolet P.A., Windecker S., Eberli F.R., Meier B. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation. 2001;104(17):2012–2017. doi: 10.1161/hc4201.097835. [DOI] [PubMed] [Google Scholar]
- 74.Zbinden S., Zbinden R., Meier P., Windecker S., Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J. Am. Coll. Cardiol. 2005;46(9):1636–1642. doi: 10.1016/j.jacc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 75.Meier P., Gloekler S., de Marchi S.F., Indermuehle A., Rutz T., Traupe T., Steck H., Vogel R., Seiler C. Myocardial salvage through coronary collateral growth by granulocyte colony-stimulating factor in chronic coronary artery disease: a controlled randomized trial. Circulation. 2009;120(14):1355–1363. doi: 10.1161/CIRCULATIONAHA.109.866269. [DOI] [PubMed] [Google Scholar]
- 76.Niebauer J., Hambrecht R., Marburger C., Hauer K., Velich T., von Hodenberg E., Schlierf G., Kübler W., Schuler G. Impact of intensive physical exercise and low-fat diet on collateral vessel formation in stable angina pectoris and angiographically confirmed coronary artery disease. Am. J. Cardiol. 1995;76(11):771–775. doi: 10.1016/S0002-9149(99)80224-0. [DOI] [PubMed] [Google Scholar]
- 77.Zbinden R., Zbinden S., Meier P., Hutter D., Billinger M., Wahl A., Schmid J.P., Windecker S., Meier B., Seiler C. Coronary collateral flow in response to endurance exercise training. Eur. J. Cardiovasc. Prev. Rehabil. 2007;14(2):250–257. doi: 10.1097/HJR.0b013e3280565dee. [DOI] [PubMed] [Google Scholar]
- 78.Gloekler S., Meier P., de Marchi S.F., Rutz T., Traupe T., Rimoldi S.F., Wustmann K., Steck H., Cook S., Vogel R., Togni M., Seiler C. Coronary collateral growth by external counterpulsation: a randomised controlled trial. Heart. 2010;96(3):202–207. doi: 10.1136/hrt.2009.184507. [DOI] [PubMed] [Google Scholar]