Abstract
Chronic total occlusion (CTO), a fascinating and dynamic niche in the realm of coronary artery disease, represents a major technical challenge for interventional cardiologists despite evolution of better guidewires, devices, experience and techniques. Effective wiring technique is the corner stone to success of percutaneous coronary intervention (PCI) in CTO. As a guide for guidewire crossing in CTO, coronary angiography is limited. On the other hand, intravascular ultrasound (IVUS) enhances the ability to identify coronary anatomy, the exact location of the guidewires within an artery, discriminating a true lumen from the false lumen before guidewire crossing. Some angiographic features have been suggested to be predictive of procedural failure, including blunt stump with a side branch at the site of occlusion. Novel use of IVUS can recognize the optimal entry point and evaluate if a guidewire properly penetrates the proximal cap of CTO.
Keywords: Chronic total occlusion, intravascular ultrasound, percutaneous coronary intervention
INTRODUCTION
Percutaneous coronary intervention (PCI) of chronic total occlusion (CTO) poses a major technical challenge and demands for interventional cardiologists despite remarkable advances in expertize, equipment and strategies [1, 2]. Majority of unsuccessful PCIs of CTO are due to failure in crossing the occlusion with guidewires [3]. In recent years, further developments of new techniques and technologies have led to remarkable improvement in procedural success and complication rate [4-8], as well as increased efficiency [9]. Although contrast angiography remains the gold standard for assessing the coronary atherosclerosis and guiding PCI, intravascular ultrasound (IVUS) has become vital adjunctive imaging modality to better recognize the optimal entry point of stumpless CTO with a side branch and evaluate whether a guide wire could properly penetrate proximal cap [10, 11], verify guidewire location within an artery discriminating a true lumen from the false lumen before crossing the occlusion [11].
IVUS may also have an important role in optimizing stent implantation and reducing in-stent restenosis rate.
IVUS NAVIGATED WIRING
The most common reason for CTO PCI failure is inability of the guidewire to cross the occlusion. Because of its ability to depict a cross sectional view of coronary tree, IVUS can focus upon plaque distribution, calcification, reference vessel size, and side branch anatomy. There are mainly two types of IVUS navigated wiring techniques: IVUS-guided wiring at CTO entrance, and IVUS-guided penetration from subintimal space. Initial attempt should be made via the antegrade approach with a single wire. If it fails, the parallel wire technique should be adopted. If this also fails, the third step should be the retrograde approach. IVUS could be used with each of these steps.
IVUS-GUIDANCE WITH THE ANTEGRADE APPROACH
Confirmation of the Entry Point of the CTO
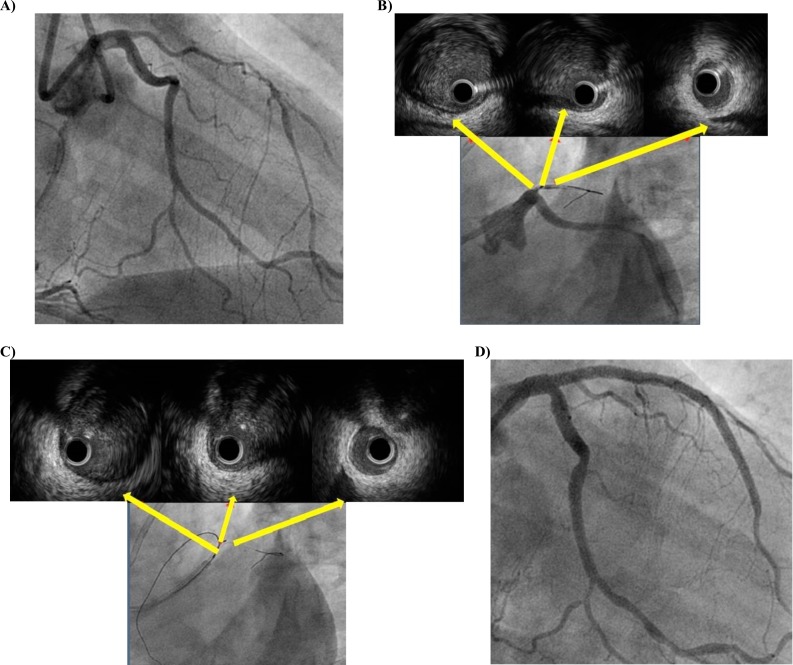
In general, contralateral angiography helps precisely to find an entry of CTO despite the absence of stump in total occlusion of a bifurcation lesion. IVUS can be used for this purpose if CTO entrance can not be delineated angiographically. The side branch should be large enough to advance an IVUS catheter. In case of stumpless CTO (Fig. 1A), before starting another guidewire should be placed in the side branch proximal to the CTO lesion. Then, an IVUS catheter is advanced and placed just at the bifurcation and angiography is performed (Fig. 1B). After using IVUS to seek a dimple at the entry, guidewire is navigated successfully into true lumen of CTO (Fig. 1C) and successful PCI (Fig. 1D) is performed. This technique also ascertains the plaque hardness at the entrance. A limitation of this side branch technique is precise anatomical arrangement of a side branch. The angle of side branch relative to parent vessel and CTO must also be favorable for cross-sectional imaging.
Fig. (1).
A. Stumpless LAD ostial CTO. B. LAD entry point marking during pullback of IVUS from small proximal diagonal. C. Confirmation of optimal penetration of proximal cap. D. Final result after implantation of two overlapping drug-eluting stents.
Examination of the Guidewire in the CTO
Even if the first guidewire gets into the true lumen of the CTO lesion, it may slip into subintimal space. It may warrant IVUS examination to check the entry point of first guidewire. When the entry point is located around the center of the entrance circle detected by IVUS, the second wire is negotiated along the first wire in another direction to seek another channel enabling its entry into true lumen. Similarly, the correct entry point of the second wire can be checked by simultaneous wiring with an IVUS catheter. Of course, an 8 F guiding catheter is indispensable for this purpose. This is called the “IVUS-guided parallel wire technique” [12].
IVUS-guided Penetration from Subintimal Space
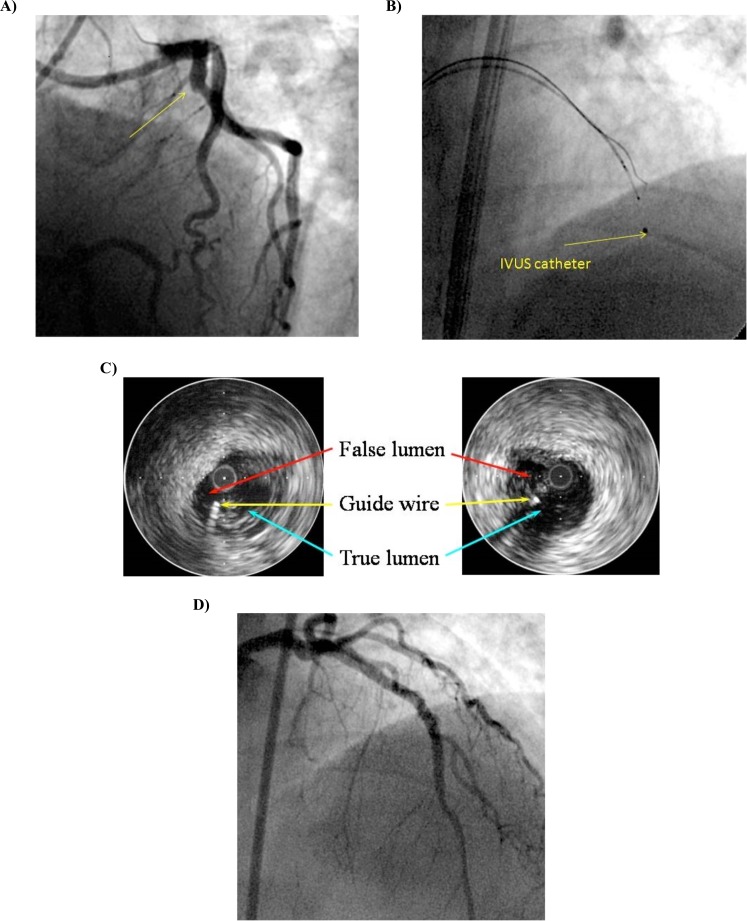
In complex CTO procedure, the guidewires occasionally enlarge the subintimal space while even using parallel wire technique. The distal true lumen can hardly be seen in fluoroscopy once there is significant expansion of subintimal space beyond the distal end of CTO. In this situation, IVUS has the potential to make a breakthrough. It can differentiate a true lumen from a false lumen by identifying the presence of side-branches and intima and media (which surround the true lumen and not the false lumen). Similarly, IVUS can identify if the guidewire has reentered the true lumen from the false lumen [13]. Enlargement of subintimal space, created by first wire often collapses the distal true lumen that can not be observed with angiography. IVUS may be useful to guide the second wire into true lumen. This catheter should be advanced into the presumed subintimal space after 1.5 or 2.0 mm balloon dilatation at the CTO entrance. Then a stiff wire (Conquest or Miracle 12, Asahi Intec, Japan) should be used as second wire. After confirming the second wire, the IVUS catheter should be advanced a couple of millimeters. Then second wire should be advanced simultaneously using fluoroscopic and IVUS guidance. Advancing second wire is oriented to true lumen under IVUS guidance (Fig. 2A-D)). This process of “crawling forward” should be repeated to advance IVUS catheter and second wire alternately. Key points are: a) ability to translate cross-sectional images of IVUS into 3 dimensions is required; b) second wire should be stiffer, tapered tip supported by a microcatheter; therefore, an 8 F guide catheter is indispensable; c) re-entry point should be closer to the proximal cap, and d) contrast injection should be withheld after balloon dilatation in subintimal space to avoid hydraulic dissection. Multiple stenting is mandatory to fully cover the enlarged subintimal space. IVUS is pivotal in helping to recanalyze CTOs after initial failure under angiographic guidance [11, 14]. However, the need for subintimal dilatation creates an unwanted larger false lumen, and the monorail design of existing IVUS catheters precludes wire exchanges. This technique should be performed as a last option in the antegrade approach, when standard wiring procedures fail in cases without a chance for retrograde approach.
IVUS-guidance with Retrograde Approach
Retrograde approach involving antegrade access to proximal cap and retrograde access to distal cap of a CTO, has got a great potential in the event of antegrade failure or in ambiguous or difficult anatomic subsets. Knowledge and expertize of this technique has become an essential adjunct for interventional cardiologists to improve procedural success [15]. Also this technical skill set is likely to shorten procedural time and reduce radiation [16]. The controlled antegrade and retrograde subintimal tracking (CART) involves antegrade wiring through the CTO after a local subintimal dissection is created by a retrograde balloon. It allows limited subintimal tracking at the site of CTO lesion and avoids the difficulty of reentering the distal true lumen [17, 18]. It is important to use the closest sized balloon inside the CTO to create sufficient wire reentry space. The major limitations are empiric estimation of the retrograde balloon size, and overall unpredictable procedure time [19]. Reverse CART technique is a modified version of CART the steps of which include retrograde dissection in the subintimal space past the distal cap, negotiation of a retrograde support catheter over the dissecting wire to near the proximal cap, antegrade subintimal space creation distal to the retrograde support catheter, and subintimal dilatation of antegrade balloon next to the retrograde support catheter with subsequent connection of two dissections and finally the retrograde wiring from the support catheter into the proximal true lumen [20].
Several limitations are encountered while using this technique: intimal rather than subintimal position of retrograde wire; bilateral wires are not in the same subintimal space; antegrade ballooning does not always lead to successful bilateral connection; the histopathology of proximal and distal plaques within CTO is quite different, and also the degree of difficulty in navigating a retrograde wire through antegrade dissection is usually different. However, IVUS-guided approach could overcome these pitfalls [21].
When retrograde wire fails to advance into the proximal true lumen even after dilatation with 2.5-3.0 mm antegrade balloon, IVUS should be used to ascertain vessel size, wire position, and plaque morphology. The position of each of the bilateral wires could affect success of reverse CART technique. IVUS is rarely required if both the bilateral wires exist in the subintimal space. It may be required if either the antegrade or retrograde wire is located in true lumen. As both the wires need to be very close to each other, the retrograde wire is advanced as far as possible, and then the positional relation of both the antegrade and retrograde wires is identified. The partition of the tissue still remains between each wire even after dilatation with antegrade balloon. In such circumstances, additional dilatation with larger balloons is required after IVUS evaluation. Coronary calcification is predictor of failure of reverse CART technique. At the calcified segment, this technique seems to be difficult due to difficulty in connecting bilateral wires. In such a situation, this should be performed at the area with least calcification after IVUS evaluation. IVUS could also be useful while attempting to manipulate a stiffer retrograde wire puncturing inflated antegrade balloon directly. Additionally, this guidance could be useful to minimize the risk of CTO segment perforation caused by wires. It allows guiding deployment of stents and identifying the result of stent expansion; it also reduces radiation exposure and decreases contrast use [21].
FUTURE GOALS: FORWARD LOOKING (FL)-IVUS
IVUS-guided wiring technique has two potential limitations. First, it fails to provide information on the course of the vessel distal to occlusion. Therefore, one needs to depend on bilateral coronary angiography to visualize the entire course of the vessel distal to the occlusion. Second, IVUS guidance may not be applicable to cases with inappropriate side branches, such as those with diameter smaller than those of IVUS catheters. In addition, the distal end of the current IVUS catheter must reside approximately 10.5 to 23 mm distal to the imaging element, requiring relatively longer subintimal space to be made [22].
The limitations of conventional IVUS are attributable to fundamental shortcomings of the side-viewing equipment. The main innovation with FL-IVUS is the ability to image in antegrade (forward) manner distal to the catheter tip obviating the need for the side branch to advance the IVUS catheter. It can provide adequate information of the CTO entrance, combined with 3-dimensional reconstruction techniques; allow visualizing the lesion distal to the occlusion and providing real time road maps of the occluded vessels [23]. By keeping the catheter centered in the vessel, the guidewire can be directed to maintain a true lumen position (Table 1) [22]. A second-generation Fl-IVUS catheter integrating a radiofrequency (RF) ablation element at the catheter tip helps in steering and tissue ablation in addition to imaging [22].
Table 1.
Forward looking IVUS technology: features and advantages.
|
CONCLUSION
CTO is frequently encountered in clinical practice and remains the most challenging and technically demanding subset of PCI. These procedures tend to be time-intensive and mandate operator skill, experience and patience. The commonest reason for failure of CTO PCI is inability to cross the lesion with a guide wire. Even with advanced operator skills, entering, maintaining or re-entering a true lumen might be a formidable challenge. IVUS-guided wiring technique for stumpless CTO appears to be useful for recognizing the optimal entry point and evaluating the appropriate penetration of the proximal cap by a guidewire. True lumen reentry can also be assisted with IVUS when the guidewire has entered into a subintimal space. FL-IVUS technology holds particular promise in facilitating CTO PCI by direct visualization of the CTO distal to catheter tip. Whether with guidewire assisted or RF energy crossing, FL-IVUS may assume paramount importance in improving the ease and success of CTO PCI in the near future.
Fig. (2).
A. Stumpless CTO of proximal LAD. B. IVUS catheter in subintimal space and guide wire in true lumen. C. IVUS guided penetration technique. D. Final result after implantation of two overlapping drug-eluting stents.
ACKNOWLEDGEMENT
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Noguchi T., Miyazaki MD S., Morii I., Daikoku S., Goto Y., Nonogi H. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Determinants of primary success and long-term clinical outcome. Catheter. Cardiovasc. Interv. 2000;49(3):258–264. doi: 10.1002/(SICI)1522-726X(200003)49:3<258::AID-CCD7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Olivari Z., Rubartelli P., Piscione F., Ettori F., Fontanelli A., Salemme L., Giachero C., Di Mario C., Gabrielli G., Spedicato L., Bedogni F., TOAST-GISE Investigators Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE). J. Am. Coll. Cardiol. 2003;41(10):1672–1678. doi: 10.1016/S0735-1097(03)00312-7. [DOI] [PubMed] [Google Scholar]
- 3.Saito S., Tanaka S., Hiroe Y., Miyashita Y., Takahashi S., Satake S., Tanaka K. Angioplasty for chronic total occlusion by using tapered-tip guidewires. Catheter. Cardiovasc. Interv. 2003;59(3):305–311. doi: 10.1002/ccd.10505. [DOI] [PubMed] [Google Scholar]
- 4.Brilakis E.S., editor. Manual of Coronary Chronic Total Occlusion Interventions. A Step-By-Step Approach. Waltham, MA: Elsevier; 2013. [Google Scholar]
- 5.Brilakis E.S., Karmpaliotis D., Vo M.N., Garcia S., Michalis L., Alaswad K., Doshi P., Lombardi W.L., Banerjee S. Advances in the management of coronary chronic total occlusions. J. Cardiovasc. Transl. Res. 2014;7(4):426–436. doi: 10.1007/s12265-014-9556-6. [DOI] [PubMed] [Google Scholar]
- 6.Garcia S., Abdullah S., Banerjee S., Brilakis E.S. Chronic total occlusions: patient selection and overview of advanced techniques. Curr. Cardiol. Rep. 2013;15(2):334. doi: 10.1007/s11886-012-0334-2. [DOI] [PubMed] [Google Scholar]
- 7.Patel V.G., Brayton K.M., Tamayo A., Mogabgab O., Michael T.T., Lo N., Alomar M., Shorrock D., Cipher D., Abdullah S., Banerjee S., Brilakis E.S. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc. Interv. 2013;6(2):128–136. doi: 10.1016/j.jcin.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Michael T.T., Karmpaliotis D., Brilakis E.S., Fuh E., Patel V.G., Mogabgab O., Alomar M., Kirkland B.L., Lembo N., Kalynych A., Carlson H., Banerjee S., Lombardi W., Kandzari D.E. Procedural outcomes of revascularization of chronic total occlusion of native coronary arteries (from a multicenter United States registry). Am. J. Cardiol. 2013;112(4):488–492. doi: 10.1016/j.amjcard.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Michael T.T., Karmpaliotis D., Brilakis E.S., Alomar M., Abdullah S.M., Kirkland B.L., Mishoe K.L., Lembo N., Kalynych A., Carlson H., Banerjee S., Luna M., Lombardi W., Kandzari D.E. Temporal trends of fluoroscopy time and contrast utilization in coronary chronic total occlusion revascularization: insights from a multicenter united states registry. Catheter. Cardiovasc. Interv. 2015;85(3):393–399. doi: 10.1002/ccd.25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuichi S., Airoldi F., Colombo A. Intravascular ultrasound-guided wiring for chronic total occlusion. Catheter. Cardiovasc. Interv. 2007;70(6):856–859. doi: 10.1002/ccd.21219. [DOI] [PubMed] [Google Scholar]
- 11.Ito S., Suzuki T., Ito T., Katoh O., Ojio S., Sato H., Ehara M., Suzuki T., Kawase Y., Myoishi M., Kurokawa R., Ishihara Y., Suzuki Y., Sato K., Toyama J., Fukutomi T., Itoh M. Novel technique using intravascular ultrasound-guided guidewire cross in coronary intervention for uncrossable chronic total occlusions. Circ. J. 2004;68(11):1088–1092. doi: 10.1253/circj.68.1088. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchikane E. IVUS-guided recanalization of CTO. In: Waksman R., Saito S., editors. Chronic Total Occlusion: A Guide to Recanalization. New York: John Wiley & Sons; 2013. pp. 105–108. [DOI] [Google Scholar]
- 13.Stone G.W., Colombo A., Teirstein P.S., Moses J.W., Leon M.B., Reifart N.J., Mintz G.S., Hoye A., Cox D.A., Baim D.S., Strauss B.H., Selmon M., Moussa I., Suzuki T., Tamai H., Katoh O., Mitsudo K., Grube E., Cannon L.A., Kandzari D.E., Reisman M., Schwartz R.S., Bailey S., Dangas G., Mehran R., Abizaid A., Serruys P.W. Percutaneous recanalization of chronically occluded coronary arteries: procedural techniques, devices, and results. Catheter. Cardiovasc. Interv. 2005;66(2):217–236. doi: 10.1002/ccd.20489. [DOI] [PubMed] [Google Scholar]
- 14.Matsubara T., Murata A., Kanyama H., Ogino A. IVUS-guided wiring technique: promising approach for the chronic total occlusion. Catheter. Cardiovasc. Interv. 2004;61(3):381–386. doi: 10.1002/ccd.10796. [DOI] [PubMed] [Google Scholar]
- 15.Rathore S., Katoh O., Matsuo H., Terashima M., Tanaka N., Kinoshita Y., Kimura M., Tsuchikane E., Nasu K., Ehara M., Asakura K., Asakura Y., Suzuki T. Retrograde percutaneous recanalization of chronic total occlusion of the coronary arteries: procedural outcomes and predictors of success in contemporary practice. Circ. Cardiovasc. Interv. 2009;2(2):124–132. doi: 10.1161/CIRCINTERVENTIONS.108.838862. [DOI] [PubMed] [Google Scholar]
- 16.Joyal D., Thompson C.A., Grantham J.A., Buller C.E., Rinfret S. The retrograde technique for recanalization of chronic total occlusions: a step-by-step approach. JACC Cardiovasc. Interv. 2012;5(1):1–11. doi: 10.1016/j.jcin.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Surmely J.F., Tsuchikane E., Katoh O., Nishida Y., Nakayama M., Nakamura S., Oida A., Hattori E., Suzuki T. New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART technique. J. Invasive Cardiol. 2006;18(7):334–338. [PubMed] [Google Scholar]
- 18.Kimura M., Katoh O., Tsuchikane E., Nasu K., Kinoshita Y., Ehara M., Terashima M., Matsuo H., Matsubara T., Asakura K., Asakura Y., Nakamura S., Oida A., Takase S., Reifart N., Di Mario C., Suzuki T. The efficacy of a bilateral approach for treating lesions with chronic total occlusions the CART (controlled antegrade and retrograde subintimal tracking) registry. JACC Cardiovasc. Interv. 2009;2(11):1135–1141. doi: 10.1016/j.jcin.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Yamane M. Current percutaneous recanalization of coronary chronic total occlusion. Rev. Esp. Cardiol. (Engl. Ed.) 2012;65(3):265–277. doi: 10.1016/j.rec.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.DeMartini T.J. Retrograde dissection reentry for coronary chronic total occlusions. Intervent Cardiol Clin. 2012;1:339–344. doi: 10.1016/j.iccl.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Dai J., Katoh O., Kyo E., Tsuji T., Watanabe S., Ohya H. Approach for chronic total occlusion with intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking technique: single center experience. J. Interv. Cardiol. 2013;26(5):434–443. doi: 10.1111/joic.12066. [DOI] [PubMed] [Google Scholar]
- 22.Rogers J. Forward-looking IVUS in chronic total occlusions.
- 23.Park Y., Park H.S., Jang G.L., Lee D.Y., Lee H., Lee J.H., Kang H.J., Yang D.H., Cho Y., Chae S.C., Jun J.E., Park W.H. Intravascular ultrasound guided recanalization of stumpless chronic total occlusion. Int. J. Cardiol. 2011;148(2):174–178. doi: 10.1016/j.ijcard.2009.10.052. [DOI] [PubMed] [Google Scholar]