Abstract
Atrial fibrillation (AF) is one of the most common arrhythmias seen in clinical cardiology practice. Patients with non-valvular AF have an approximately 5-fold increase in the risk of stroke, with an exponential increase with advancing age. Cardioembolic strokes carry a high mortality risk. Although the potential of warfarin to reduce systemic embolization in AF patients is well established, its use is difficult due to narrow therapeutic windows and additional complications (e.g. increased risk of bleeding), especially for aging patients. Therefore, alternative means of treatment to reduce stroke risk in these patients are needed. The left atrial appendage is the major source of thrombus formation in patients with non-valvular AF. The WATCHMAN device (Boston Scientific, MA) is a percutaneous left atrial appendage closure device which has been tested prospectively in multiple randomized trials. It offers a new stroke risk reduction option for high-risk patients with non-valvular atrial fibrillation who are seeking an alternative to long-term warfarin therapy. Based on the robust WATCHMAN clinical program which consists of numerous studies, with more than 2,400 patients and nearly 6,000 patient-years of follow-up, the WATCHMAN LAAC Device is approved by FDA. In this article we reviewed the preclinical studies and clinical trials, as well as the next generation of the device.
Keywords: Atrial fibrillation, left atrial appendage closure, WATCHMAN device
INTRODUCTION
Atrial fibrillation (AF) is one of the most common arrhythmias seen in clinical cardiology practice. It has been estimated that 2.3 million people in the United States have clinically recognized AF, with the number expected to increase to approximately 5.6 million by 2050. Prevalence of AF increases with age, affecting approximately 9.0% of the population 80 years or older [1]. Hospitalizations associated with AF as the primary diagnosis exceed 460,000 each year. The most serious complication, cardioembolic strokes, carries a high mortality risk.AF is estimated to contribute to more than 80,000 deaths annually according to the American Heart Association Statistics Committee 2009 update [2]. The national incremental cost associated with sequelae of AF is estimated to range from $6 to $26 billion per year [3].
The United States alone is estimated to have a prevalence of 7 million patients with new or recurrent stroke. The overall proportion of strokes thought to be due to AF was reported as 14.7%, a number that steadily increases with age from 6.7% (ages 50 to 59 years) to 36.2% (ages 80 to 89 years) [4, 5]. To assess the stroke risk, there are two commonly used scoring systems: CHADS2 (congestive heart failure, hypertension, age >75 years, diabetes 1 point each prior stroke or transient ischemic attack 2 points) [6] and CHA2DS2-VASC (congestive heart failure, hypertension, age >65=1 point, > 75=2 points, diabetes mellitus, prior strokes 2 points, vascular disease, female gender 1 point each) [7]. Anticoagulation therapy is recommended for patients with a moderate or high score in order to reduce the risk of thromboembolism.
Anatomically, the left atrial appendage (LAA) is the major source of thrombus formation in patients with AF. In their study, Blackshear et al. found that in AF patients with intracardiac thrombi, 90% of the thrombi originated in the LAA [8]. Additionally, a meta-analysis by Mahajan et al. reported a similar incidence of LAA thrombus in patients with non-valvular AF [9]. Presently, oral anticoagulation (OAC) is the mainstay of therapy for thromboembolism prevention in patients with AF; however several challenges limit its use in eligible patients, including bleeding complications, fall risk, and non-compliance. The need for frequent prothrombin time monitoring is a further impediment to usage of the traditional agent, warfarin. Several studies have shown that OAC remains underutilized by as much as 30 to 45% in eligible patients [10-12]. Novel oral anticoagulants (NOAC) (Dabigatran, Rivaroxaban, Apixaban, and Edoxaban) do not currently require regular monitoring and have demonstrated non-inferiority to warfarin in stroke reduction. However, these new agents are costly, lack readily available antidotes, and have reported discontinuation rates as high as 20%. Moreover, these agents have similar or less bleeding complications than warfarin, but are still associated with 2-3% absolute bleeding risk per year, compounding the issue for patients with high bleeding risk [13-16].
These unmet needs have led to emerging therapies involving LAA closure devices to prevent thromboembolism in patients who are poor candidates for OAC. Several percutaneous closure devices have been developed for the exclusion of the LAA. In 2012, [17] the European Society of Cardiology (ESC) Guidelines for the management of atrial fibrillation updated their recommendations to include interventional percutaneous occlusion/closure of the LAA for patients with thromboembolic risk who cannot be managed in the long-term using any form of OAC. Accordingly, the ESC guidelines for the management of atrial fibrillation recommend (Class IIb recommendation) left atrial appendage closure in patients with AF, who have a high stroke risk and contraindications for long term oral anticoagulation therapy.
This review focuses on recent literature regarding LAA anatomy, WATCHMAN preclinical and human trials, and the next generation device, the WATCHMAN FLX.
LAA ANATOMY AND FUNCTION
The LAA is a trabecular remnant of the original embryonic LA that develops during the third week of gestation [18]. Transesophageal echocardiography (TEE) allows highly accurate imaging of the LAA and is an invaluable tool in the diagnosis of LAA thrombus. The sensitivity and specificity of TEE in the diagnosis of LAA thrombus has been reported to be up to 100% and 99%, respectively in a comparison with intra-operative observation [19]. Recent studies indicate real-time 3D TEE is more accurate than 2D TEE for the assessment of LAA orifice size prior to the percutaneous closure procedure [20]. Pulsed wave examination of LAA in sinus rhythm usually shows four waves, including the early diastolic emptying flow (E wave) and the LAA intrinsic late diastolic contraction (A wave). Furthermore, the filling of the LAA causes an early systolic negative wave following the a-wave and a systolic reflection wave (type I). In AF, two types of emptying waves can be observed. Saw-tooth appearance of high-frequency, low amplitude waves occur during systole; while one or several higher velocity waves (E waves) can be observed during early diastole (Type II). Importantly, AF patients without identifiable flow waves (Type III) have higher incidences of LAA spontaneous echo contrast and thrombus [18, 21]. The LAA is more compliant than rest of the left atrium (LA) and functions as a reservoir. While occlusion of LAA has been shown to increase the LA pressure and dimension, it does not seem to exert any significant impact on overall cardiac output and systolic blood pressure [22]. LAA occlusion with WATCHMAN (WM) has not been associated with impairment of LA function [23]. Experimental studies of LA isolation as an anti-arrhythmic procedure demonstrated that, as long as synchronous activation is present in the right atrium and ventricle, synchronous LA contraction has no effect on the preload, afterload, or forward cardiac output of the left ventricle [24]. The LAA may also serve a role in regulation of fluid balance through secretion of atrial natriuretic peptide [25]. Fluid retention has been commonly observed after the original cut-and-sew maze procedure in which both the LAA and right atrial (RA) appendage were excised. However, sparing of the RA appendage has ameliorated this complication [24]. There are no data to suggest that removal or occlusion of LAA has substantial effect on hormonal balance.
LAA CLOSURE DEVICE: THE WATCHMAN
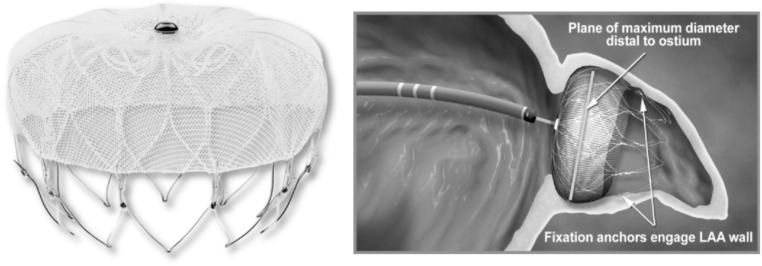
The first-in-man device implantation was performed on 12 August, 2002. It is a parachute shaped implantable nitinol device encased within a trans-septal access sheath and delivery catheter. The device is a self-expanding nitinol frame structure with 10 fixation anchors and a polyethylene terephthalate fabric membrane cap that faces the body of the LA after placement (Fig. 1). The device is available in diameters of 21 mm, 24 mm, 27 mm, 31 mm, and 33 mm. Usually the device size is chosen to be 8-20% larger than the diameter of the LAA ostium to ensure sufficient compression against the LAA wall for stable device positioning. LAA access is achieved by a 14F trans-septal access sheath via the femoral vein approach; the sheath is available in a double, single or anterior curve configuration and serves as a conduit for the delivery catheter. After confirmation of proper device position by cine-angiography and TEE, the device is deployed by retracting the covering sheath. Once deployed, the implant can be retrieved and repositioned.
Fig. (1).
Watchman Device (Boston Scientific in Marlborogh, MA).
PRECLINICAL STUDIES
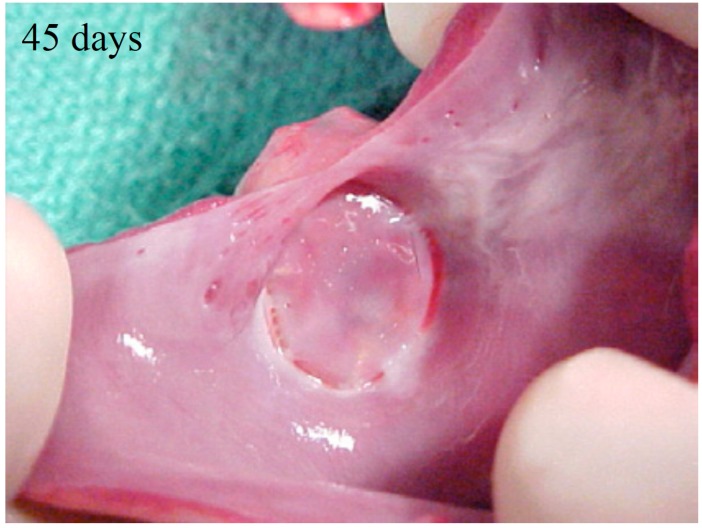
In preclinical studies, the WM device has demonstrated efficacy in closure of the LAA. In a study by Schwartz et al. using canine models, the initial response 3 days after implantation was fibrin deposition on the atrial surfaces which sealed gaps between the LA wall and the device. At 45 days, the endocardial device surface was covered by endothelial cells underlying smooth muscle cells that sealed the device-LA interface. Regions with prior thrombus were replaced by a layer of endocardium (Fig. 2). The LAA body contained disorganized thrombus, along with mild inflammation. By 90 days, a complete endocardial lining covered the former LAA ostium; and the organized thrombus had become connective tissue, with no residual inflammation. Four human hearts (deaths unrelated to device) post implantation at 139, 200, 480, and 852 days were examined. Similar to preclinical healing response, the ostial fabric membrane in these 4 hearts was covered with neo-endocardial tissue. The appendage surface contained organizing thrombus with minimal inflammation. Organizing fibrous tissue was inside the LAA cavity, prominent near the atrial wall. The LAA interior contained organizing thrombus [26].
Fig. (2).
Watchman Device typically endothelializes within 45 to 60 days of implant, as shown in this canine heart (Image provided by Boston Scientific, Marlborough, MA, USA).
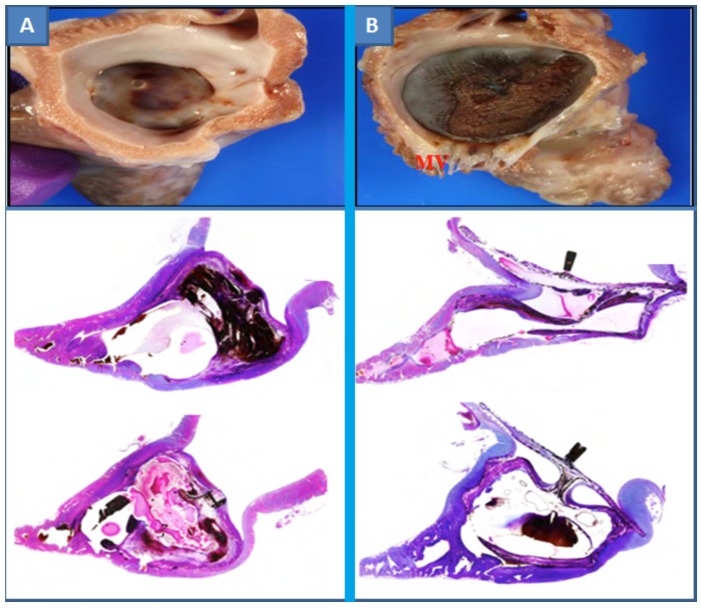
Recently, safety of LAA closure after electrical isolation was also tested by Panikker in a canine model. Of nine dogs undergoing pulmonary vein and LAA isolation followed by WM LAA closure, one animal died of primary intracranial bleed due to anticoagulant hypersensitivity 36 hours after the procedure. At 45 days, 7 of 8 (88%) had persistent LAA electrical isolation. All devices were stable without evidence of erosion. Microscopy revealed complete device-tissue apposition and a mature connective tissue layer overlying the device surface in all cases [27]. To date, there has been no direct comparison of the WM to other LAA occlusion devices in humans. A recent comparative evaluation of the healing response after Watchman (WM) and Amplatzer Cardiac Plug (ACP) (St. Jude Medical, Minneapolis, Minnesota) in a canine LAA model was reported by Kar et al. [28]. The results showed that the WM was properly seated inside the LAA ostium, in comparison to the ACP which the disk was outside of LAA ostium and extended to the edge of the left superior pulmonary vein and mitral valve. These findings may have significant clinical consequences especially when using larger size ACP devices. It is important to pay attention to the disk interference with LAA surrounding structures during the implantation. At 28 days, complete neo-endocardial coverage of the WM was observed; however, the ACP showed an incomplete covering on the disk surface, especially at the lower edge and end-screw hub regions (Fig. 3). There are differences in conformation of LAA surrounding structures with variable healing response between WM and ACP after LAA closure in the canine model. WM does not obstruct or impact the LAA adjacent structures, resulting in favorable surface recovery. In contrast, the disk of ACP could potentially jeopardize LAA neighboring structures, and leading to delayed healing.
Fig. (3).
Gross and Microscopy images of WM and ACP in a canine model at 28 days. The WM device (A) showed the central area of the device covered with a layer of neo-endocardial tissue with tight device apposition to the native LAA wall. The ACP device (B) showed an area of bare flange mesh wires near the inferior edge of the disk; also there was incomplete coverage of the end-screw hub [28].
CLINICAL TRIALS
Since CE mark in 2005, the WATCHMAN device is currently commercially approved in more than 75 countries with over 10,000 implants performed worldwide. So far, this device is the most studied LAA closure device and the only one with long-term clinical data from more than 2,500 patients and 6,000 patient-years of follow-up in clinical trials (Table 1). The initial worldwide experience of the WM device was a prospective study that included 66 patients with non-valvular AF. 93% of devices showed successful sealing of LAA by TEE evaluation at 45 days. No strokes occurred during follow-up despite >90% of patients discontinuing OAC [29].
Table 1.
Watchman clinical studies summary (provided by Boston Scientific, Marlborough, MA, USA).
| WATCHMAN™ Clinical Trials (total of more than 2,500 patients with more than 6,000 patient years follow-up) | ||
|---|---|---|
| Study | Comments | Enrolled |
| Pilot [29] | Early feasibility with >6 years of follow up | 66 |
| PROTECT-AF [33] | Watchman primary efficacy, CV death, and all-cause mortality superior to warfarin at ~4 years | 800 |
| CAP Registry [35] | Significantly improved safety results | 566 |
| ASAP [39] | Expected rate of stroke reduced by 77% in patients contraindicated to warfarin | 150 |
| PREVAIL [37] | Improved implant success procedure safety confirmed with new and experienced operators | 461 |
| CAP2* | Follow-up on-going; Confirmed procedural safety results seen in CAP and PREVAIL | 579 |
The first randomized clinical trial was the Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) trial. It was a prospective, multicenter randomized control trial that enrolled 707 patients (2-to-1 WM device to warfarin control) with non-valvular AF with a CHADS2 score >=1 and eligible for warfarin therapy. These patients underwent randomization in a 2:1 ratio to receive either intervention (intervention group; n=463) with WM device versus continuation of warfarin therapy (control group; n=244). Patients in the intervention arm were initially treated with warfarin for 45 days to facilitate device endothelialization. TEE was performed at 45 days, 6 months, and 12 months to assess residual peri-device flow and device position. If the 45-day TEE showed either complete closure of the LAA or residual peri-device flow (jet<5 mm in width), warfarin therapy was discontinued at that time; and the patients were switched to once daily clopidogrel (75 mg) and aspirin (81–325 mg) for 6 months, from which point aspirin alone was continued indefinitely. The primary efficacy endpoint was defined as a composite of stroke, systemic embolization, or cardiovascular/unexplained death. In a mean 1.5-year, 1065 patient-year follow-up, the annual primary event rates were 3.0% in the device group and 4.9% in warfarin group [30]. In a mean 2.3-year follow up with a 1588 patient-year follow-up, the annual primary event rates were 3.0% and 4.3% in the deviceand warfarin groups, respectively (rate ratio (RR), 0.71; 95% credible interval (CrI), 0.44-1.30), which met the criteria for non-inferiority (posterior probability >99%) [31]. The impact of peri-device flow severity, defined as minor, moderate, or major (<1 mm, 1 mm to 3 mm, or >3 mm, respectively) on the composite primary efficacy endpoint was evaluated and not associated with increased risk of thromboembolism [32]. More recent 3.8 year follow-up study of PROTECT AF trial patients with 2621 patient-years of follow-up continue to demonstrate lower composite event rates in the device group (2.3 events vs 3.8 events per 100 patient-years, RR, 0.60; 95% CrI, 0.41-1.05), meeting pre-specified criteria for both non-inferiority (posterior probability, >99.9%) and superiority (posterior probability, 96.0%) [33]. Device-based therapy was associated less disabling outcomes (relative risk, 0.41; 95% CI, 0.22–0.82) when the functional impact of the primary efficacy and safety events was considered in terms of disability or death. Patients with non-valvular AF at risk for stroke treated with LAA closure have favorable quality of life (QOL) changes at 12 months versus patients treated with warfarin [34]. The analysis of patients included in the PROTECT AF trial who underwent attempted device LAA closure (n=542 patients) and those from a subsequent nonrandomized registry of patients undergoing device implantation (Continued Access Protocol [CAP] Registry; n=460 patients) showed a significant decline in the rate of procedure- or device-related safety events within 7 days of the procedure (7.7% Vs 3.7% P=0.007) [35]. In 2009, based on the results of PROTECT AF at 900 patient years of follow-up, the WM device was reviewed by an FDA Panel and received a positive vote in favor of approval; however the Agency did not grant approval, citing concerns with concomitant medication use, peri-procedural events, and patient risk scores in the trial [36].
In order to address these questions, a second randomized confirmatory study evaluating the safety and efficacy of the device was started in 2010: the PREVAIL (Patients with Atrial Fibrillation Versus Long Term Warfarin Therapy) trial. This non-inferiority trial added specific requirements for new operators in order to confirm the procedural safety results seen in the latter half of PROTECT AF and CAP, modified the exclusion criteria to include a higher risk population (CHADS2 score ≥2 or CHADS2 score of 1 and another risk factor), and eliminated the use of clopidogrel for the 7 days prior to implant. An adaptive Bayesian statistical method was utilized, and included a portion of the PROTECT AF patients eligible under the new criteria as an informative prior, allowing for a smaller sample size and evaluation of the same composite efficacy endpoint after a minimum follow-up of 6 months for the newly enrolled patients. PREVAIL randomized 407 patients in a 2-to-1 fashion; 269 to the device and 138 to warfarin control. Although 48% were new operators, PREVAIL met the primary safety endpoint acute (7-day) occurrence of death, ischemic stroke, systemic embolism and procedure or device related complications requiring major cardiovascular or endovascular intervention with an event rate of 2.2% (performance goal, 95% upper bound credible interval<2 .67%). At the pre-defined efficacy analysis time point, the composite endpoint (stroke, systemic embolism, and cardiovascular/unexplained death) demonstrated similar 18-month event rates in both device and warfarin groups (0.064 versus 0.063). In spite of the high average CHADS2 score of 2.6 in the warfarin group, the observed rate of stroke per 100 patient years in the PREVAIL control group was much lower than in other published warfarin studies (0.7 for device vs 1.6-2.2 for warfarin [13-16]) causing the endpoint upper boundary to be exceeded (RR 1.07 95% CrI: 0.5-1.88, pre-specified upper bound criteria<1.75). The 18-month rate of stroke or systemic embolism >7 days post randomization was 0.0253 vs 0.0200 (risk difference 0.0053 95% credible interval: –0.0190 to 0.0273, pre-specified upper bound criteria <0.0275), achieving non-inferiority. In the PREVAIL trial, acute safety events requiring a major intervention occurred in 2.2% of patients, significantly lower than in PROTECT AF. Using a broader, more inclusive definition of all serious adverse effects, these still were lower in PREVAIL than in PROTECT AF (4.1% vs. 8.7%; p = 0.004). Pericardial effusions requiring surgical repair decreased from 1.6% to 0.4% (p = 0.027); and those requiring pericardiocentesis decreased from 2.9% to 1.5% (p = 0.36), although the number of events was small [37]. Analyses of time to first major bleed in the PROTECT AF, PREVAIL, and pooled trials such as those performed by Price et al., showed LAA closure with WM significantly reduced bleeding compared with warfarin after post procedural adjunctive therapy was completed (HR 0.30, 95% CI: 0.17-0.53) [38].
These results were reviewed by a second FDA Panel in December of 2013, and received positive votes on safety, efficacy, and benefit-risk profile (13-1). After the Panel, the agency requested an updated analysis for PREVAIL to include any new events occurring since the last data lock for the pre-specified analysis. The new composite efficacy events were equivalent between both arms when accounting for 2 to 1 randomization, however there were more new ischemic strokes in the device arm compared to warfarin (13-1). The warfarin arm continued to maintain an unusually low ischemic stroke rate (0.3 per 100 patient years), causing a greater divergence between the arms and resulting in 2 missed endpoints in the updated analysis. In response, a 3rd FDA Panel was convened in October of 2014 to evaluate the totality of data with the WATCHMAN device. After a thorough discussion, the Panel returned a positive vote (6-5-1) that the benefits of the device outweighed the risks for indicated patients [36], with several members indicating they would have voted positively for a more limited indication than that proposed at the 3rd panel meeting.
The Aspirin Plavix feasibility study with WM LAA closure technology (the ASAP study) is a multicenter nonrandomized trial involving 150 warfarin ineligible patients, with CHADS2 score ≥1, who underwent LAA closure with the WM device. After device implantation, patients were administered 6 months of clopidogrel or ticlopidine and lifelong aspirin. During the mean follow up of 14±8.6 months, serious procedure or device related events occurred in 8.7% of patients. Device group experienced 77% fewer ischemic strokes than that expected (1.7% vs 7.3% year) based on the CHADS2 scores of the patient cohort. Even after discounting the protective effect of clopidogrel, the device therapy resulted in 64% fewer events than expected [39].
Cost effective analysis of the WM device reported by Reddy et al. showed that the cost per composite efficacy endpoint avoided was €98,866 at 5 years. LAAC was dominant (less expensive and more effective) over warfarin by 9 years with a mean cost per patient of €20,227 versus €20,604 [40]. Another economic evaluation of LAA closure performed by Singh et al. reported that the average discounted lifetime cost was $21,429 for a patient taking warfarin, $25,760 for a patient taking dabigatran, and $27,003 for LAA occlusion. Compared with warfarin, the incremental cost-effectiveness ratio for LAA occlusion was $41,565 [41].
The WATCHMAN clinical program provided strong evidence that the WATCHMAN Device can be implanted safely and reduces the risk of stroke in eligible patients while enabling most patients to discontinue warfarin. Additionally, a meta-analysis of all the the randomized trial data demonstrated that while ischemic stroke reduction favored warfarin, the WATCHMAN Device provided patients with a comparable protection against all-cause stroke and statistically superior reductions in hemorrhagic stroke, post-procedure bleeding, and cardiovascular death compared to warfarin over long-term follow-up. Based on the robust WATCHMAN clinical program which consists of numerous studies, with more than 2,400 patients and nearly 6,000 patient-years of follow-up, the WATCHMAN LAAC Device was approved by FDA.
CONCOMITANT PROCEDURES WITH WATCHMAN IMPLANTATION
The safety of the LAA closure with the WM device along with catheter ablation has been studied by Swaans et al. Thirty patients underwent AF ablation using multi-electrode catheters. All patients underwent LAA occlusion with the device. There were 3 minor complications, including one tongue hematoma and 2 small groin hematomas. At sixty days, all patients met criteria for LAA closure by TEE. The recurrence rate of AF was 30% at the 12 month follow-up visit. The device did not interfere with repeated pulmonary vein isolation in 4 patients. No thromboembolic events occurred during 1-year follow-up [42].
AF is a common problem in patients with mitral valve disease. LAA closure along with mitral valve clip has been described. In selected patients with high bleeding risk, the combined procedure shortens the period of early post-interventional antithrombotic therapy. Furthermore, periprocedural risks associated with transseptal puncture are minimized to one procedure [43].
A case of LAA closure with the WM device along, with mitral valvuloplasty, has been described in a 62-year old woman with the rheumatic mitral stenosis and atrial fibrillation. The exact location of the septal puncture can be challenging and is ideally in the mid to lower part of the posterior inter-atrial septum for LAA closure; however this site may complicate mitral valve procedures in certain patients [44]. LAA closure through a patent atrial septal defect followed by atrial septal defect closure has also been described [45].
NEXT GENERATION OF WATCHMAN FLX DEVICE
The next generation WATCHMAN FLX (WM FLX) device is designed to further minimize the risk of device embolization and the risk of periprocedural pericardial effusion. The new design includes 18 struts (vs. 10 in current generation WATCHMAN device (CG-WM)), an atraumatic closed distal end (vs. open end in CG-WM), and a reduced device length. The WM FLX is the first closure device which can be redeployed after either full or partial recapture; in contrast, the CG-WM must be replaced if a full recapture is needed. The WM FLX has shown an improvement in the ease of implantation. In animal models involving 14 dogs, 100% of WM FLX (6/6) compared to 75% of CG-WM (6/8) were successfully deployed. The number of required full and partial captures were less (0 vs. 4 and 1 vs. 3, respectively) with WM FLX compared to CG-WM [46].
CONCLUSION
The WATCHMAN Device has been commercially available internationally since 2009 and is the leading device in percutaneous left atrial appendage closure globally. It is registered in 75 countries and more than 10,000 patients have been treated with the WATCHMAN Device. The WATCHMAN Device is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with non-valvular atrial fibrillation who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AF
= Atrial fibrillation
- LAA
= Left Atrial Appendage
- LA
= Left Atrium
- RA
= Right Atrium
- OAC
= Oral Anticoagulation
- TEE
= Trans-Esophageal Echocardiography
- WM
= WATCHMAN
CONFLICT OF INTEREST
Pflugfelder A, Gordon N, Stein K, Hubregtse B & Hou D are full-time employee of Boston Scientific. The study was funded by Boston Scientific.
REFERENCES
- 1.Go A.S., Hylek E.M., Phillips K.A., Chang Y., Henault L.E., Selby J.V., Singer D.E. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D., Adams R., Carnethon M., De Simone G., Ferguson T.B., Flegal K., Ford E., Furie K., Go A., Greenlund K., Haase N., Hailpern S., Ho M., Howard V., Kissela B., Kittner S., Lackland D., Lisabeth L., Marelli A., McDermott M., Meigs J., Mozaffarian D., Nichol G., O’Donnell C., Roger V., Rosamond W., Sacco R., Sorlie P., Stafford R., Steinberger J., Thom T., Wasserthiel-Smoller S., Wong N., Wylie-Rosett J., Hong Y., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Kim M.H., Johnston S.S., Chu B-C., Dalal M.R., Schulman K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 4.Petty G.W., Brown R.D., Jr, Whisnant J.P., Sicks J.D., O’Fallon W.M., Wiebers D.O. Ischemic stroke subtypes : a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31(5):1062–1068. doi: 10.1161/01.STR.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 5.Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Makuc D.M., Marcus G.M., Marelli A., Matchar D.B., Moy C.S., Mozaffarian D., Mussolino M.E., Nichol G., Paynter N.P., Soliman E.Z., Sorlie P.D., Sotoodehnia N., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 7.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 8.Blackshear J.L., Odell J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996;61(2):755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 9.Mahajan R., Brooks A.G., Sullivan T., Lim H.S., Alasady M., Abed H.S., Ganesan A.N., Nayyar S., Lau D.H., Roberts-Thomson K.C., Kalman J.M., Sanders P. Importance of the underlying substrate in determining thrombus location in atrial fibrillation: implications for left atrial appendage closure. Heart. 2012;98(15):1120–1126. doi: 10.1136/heartjnl-2012-301799. [DOI] [PubMed] [Google Scholar]
- 10.Waldo A.L., Becker R.C., Tapson V.F., Colgan K.J., NABOR Steering Committee Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J. Am. Coll. Cardiol. 2005;46(9):1729–1736. doi: 10.1016/j.jacc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 11.Birman-Deych E., Radford M.J., Nilasena D.S., Gage B.F. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37(4):1070–1074. doi: 10.1161/01.STR.0000208294.46968.a4. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava A., Hudson M., Hamoud I., Cavalcante J., Pai C., Kaatz S. Examining warfarin underutilization rates in patients with atrial fibrillation: Detailed chart review essential to capture contraindications to warfarin therapy. Thromb. J. 2008;6:6. doi: 10.1186/1477-9560-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., Pogue J., Reilly P.A., Themeles E., Varrone J., Wang S., Alings M., Xavier D., Zhu J., Diaz R., Lewis B.S., Darius H., Diener H.C., Joyner C.D., Wallentin L., RE-LY Steering Committee and Investigators Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 14.Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W., Breithardt G., Halperin J.L., Hankey G.J., Piccini J.P., Becker R.C., Nessel C.C., Paolini J.F., Berkowitz S.D., Fox K.A., Califf R.M., ROCKET AF Investigators Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 15.Giugliano R.P., Ruff C.T., Braunwald E., Murphy S.A., Wiviott S.D., Halperin J.L., Waldo A.L., Ezekowitz M.D., Weitz J.I., Špinar J., Ruzyllo W., Ruda M., Koretsune Y., Betcher J., Shi M., Grip L.T., Patel S.P., Patel I., Hanyok J.J., Mercuri M., Antman E.M., ENGAGE AF-TIMI 48 Investigators Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 16.Granger C.B., Alexander J.H., McMurray J.J., Lopes R.D., Hylek E.M., Hanna M., Al-Khalidi H.R., Ansell J., Atar D., Avezum A., Bahit M.C., Diaz R., Easton J.D., Ezekowitz J.A., Flaker G., Garcia D., Geraldes M., Gersh B.J., Golitsyn S., Goto S., Hermosillo A.G., Hohnloser S.H., Horowitz J., Mohan P., Jansky P., Lewis B.S., Lopez-Sendon J.L., Pais P., Parkhomenko A., Verheugt F.W., Zhu J., Wallentin L., ARISTOTLE Committees and Investigators Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 17.Camm A.J., Lip G.Y., De Caterina R., Savelieva I., Atar D., Hohnloser S.H., Hindricks G., Kirchhof P., ESC Committee for Practice Guidelines (CPG) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 18.Donal E., Yamada H., Leclercq C., Herpin D. The left atrial appendage, a small, blind-ended structure: a review of its echocardiographic evaluation and its clinical role. Chest. 2005;128(3):1853–1862. doi: 10.1378/chest.128.3.1853. [DOI] [PubMed] [Google Scholar]
- 19.Manning W.J., Weintraub R.M., Waksmonski C.A., Haering J.M., Rooney P.S., Maslow A.D., Johnson R.G., Douglas P.S. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann. Intern. Med. 1995;123(11):817–822. doi: 10.7326/0003-4819-123-11-199512010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Nucifora G., Faletra F.F., Regoli F., Pasotti E., Pedrazzini G., Moccetti T., Auricchio A. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: implications for catheter-based left atrial appendage closure. Circ Cardiovasc Imaging. 2011;4(5):514–523. doi: 10.1161/CIRCIMAGING.111.963892. [DOI] [PubMed] [Google Scholar]
- 21.Al-Saady N.M., Obel O.A., Camm A.J. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82(5):547–554. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabata T., Oki T., Yamada H., Iuchi A., Ito S., Hori T., Kitagawa T., Kato I., Kitahata H., Oshita S. Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am. J. Cardiol. 1998;81(3):327–332. doi: 10.1016/S0002-9149(97)00903-X. [DOI] [PubMed] [Google Scholar]
- 23.Almeida P., Infante de Oliveira E., Marques J., et al. TCT-763 Impact of left atrial appendage occlusion, with percutaneous device on left atrial function. J. Am. Coll. Cardiol. 2012;60:B222. doi: 10.1016/j.jacc.2012.08.806. [DOI] [Google Scholar]
- 24.Cox J.L. Mechanical closure of the left atrial appendage: is it time to be more aggressive? J. Thorac. Cardiovasc. Surg. 2013;146(5):1018–1027.e2. doi: 10.1016/j.jtcvs.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Tabata T., Oki T., Yamada H., Abe M., Onose Y., Thomas J.D. Relationship between left atrial appendage function and plasma concentration of atrial natriuretic peptide. Eur. J. Echocardiogr. 2000;1(2):130–137. doi: 10.1053/euje.2000.0019. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz R.S., Holmes D.R., Van Tassel R.A., Hauser R., Henry T.D., Mooney M., Matthews R., Doshi S., Jones R.M., Virmani R. Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc. Interv. 2010;3(8):870–877. doi: 10.1016/j.jcin.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Panikker S., Virmani R., Sakakura K., Kolodgie F., Francis D.P., Markides V., Walcott G., McElderry H.T., Wong T. Left atrial appendage electrical isolation and concomitant device occlusion: A safety and feasibility study with histologic characterization. Heart Rhythm. 2015;12(1):202–210. doi: 10.1016/j.hrthm.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Kar S., Hou D., Jones R., Werner D., Swanson L., Tischler B., Stein K., Huibregtse B., Ladich E., Kutys R., Virmani R. Impact of Watchman and Amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC Cardiovasc. Interv. 2014;7(7):801–809. doi: 10.1016/j.jcin.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Sick P.B., Schuler G., Hauptmann K.E., Grube E., Yakubov S., Turi Z.G., Mishkel G., Almany S., Holmes D.R. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J. Am. Coll. Cardiol. 2007;49(13):1490–1495. doi: 10.1016/j.jacc.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 30.Holmes D.R., Reddy V.Y., Turi Z.G., Doshi S.K., Sievert H., Buchbinder M., Mullin C.M., Sick P., PROTECT AF Investigators Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374(9689):534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 31.Reddy V.Y., Doshi S.K., Sievert H., Buchbinder M., Neuzil P., Huber K., Halperin J.L., Holmes D., PROTECT AF Investigators Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127(6):720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 32.Viles-Gonzalez J.F., Kar S., Douglas P., et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients Wit. J. Am. Coll. Cardiol. 2012;59:923–929. doi: 10.1016/j.jacc.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Reddy V.Y., Sievert H., Halperin J., Doshi S.K., Buchbinder M., Neuzil P., Huber K., Whisenant B., Kar S., Swarup V., Gordon N., Holmes D., PROTECT AF Steering Committee and Investigators Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312(19):1988–1998. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 34.Alli O., Doshi S., Kar S., Reddy V., Sievert H., Mullin C., Swarup V., Whisenant B., Holmes D., Jr Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J. Am. Coll. Cardiol. 2013;61(17):1790–1798. doi: 10.1016/j.jacc.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 35.Reddy V.Y., Holmes D., Doshi S.K., Neuzil P., Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123(4):417–424. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 36.Waksman R., Pendyala L.K. Overview of the Food and Drug Administration circulatory system devices panel meetings on WATCHMAN left atrial appendage closure therapy. Am. J. Cardiol. 2015;115(3):378–384. doi: 10.1016/j.amjcard.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Holmes D.R., Jr, Kar S., Price M.J., Whisenant B., Sievert H., Doshi S.K., Huber K., Reddy V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J. Am. Coll. Cardiol. 2014;64(1):1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Price M.J., Valderrabano M., Huber K., et al. TCT-171 Avoidance of Major Bleeding with WATCHMAN Left Atrial Appendage Closure compared with Long-Term Oral Anticoagulation: A Pooled Analysis of Randomized Trials. J. Am. Coll. Cardiol. 2014;64:B50–B51. doi: 10.1016/j.jacc.2014.07.210. [DOI] [Google Scholar]
- 39.Reddy V.Y., Möbius-Winkler S., Miller M.A., Neuzil P., Schuler G., Wiebe J., Sick P., Sievert H. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J. Am. Coll. Cardiol. 2013;61(25):2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 40.Reddy V.Y., Akehurst R.L., Amorosi S.L., Armstrong S., Holmes D.R. Abstract 17478: Cost Effectiveness of Left Atrial Appendage Closure in Atrial Fibrillation Patients With Previous Stroke or Transient Ischemic Attack. Circulation. 2013;128:A174–A178. [Google Scholar]
- 41.Singh S.M., Micieli A., Wijeysundera H.C. Economic evaluation of percutaneous left atrial appendage occlusion, dabigatran, and warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. Circulation. 2013;127(24):2414–2423. doi: 10.1161/CIRCULATIONAHA.112.000920. [DOI] [PubMed] [Google Scholar]
- 42.Swaans M.J., Post M.C., Rensing B.J., Boersma L.V. Ablation for atrial fibrillation in combination with left atrial appendage closure: first results of a feasibility study. J. Am. Heart Assoc. 2012;1(5):e002212. doi: 10.1161/JAHA.112.002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schade A., Kerber S., Hamm K. Two in a single procedure: combined approach for MitraClip implantation and left atrial appendage occlusion using the Watchman device. J. Invasive Cardiol. 2014;26(3):E32–E34. [PubMed] [Google Scholar]
- 44.Murdoch D., McAulay L., Walters D.L. Combined percutaneous balloon mitral valvuloplasty and left atrial appendage occlusion device implantation for rheumatic mitral stenosis and atrial fibrillation. Cardiovasc. Revasc. Med. 2014;15(8):428–431. doi: 10.1016/j.carrev.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Gafoor S., Franke J., Boehm P., Lam S., Bertog S., Vaskelyte L., Hofmann I., Sievert H. Leaving no hole unclosed: left atrial appendage occlusion in patients having closure of patent foramen ovale or atrial septal defect. J. Interv. Cardiol. 2014;27(4):414–422. doi: 10.1111/joic.12138. [DOI] [PubMed] [Google Scholar]
- 46.Hou D, Tischler B, Alex P, et al. TCT-175 Preclinical Study of Novel Generation of Watchman for Left Atrial Appendage Occlusion. . J Am Coll Cardiol . 2014;64 : B51–2. [Google Scholar]