Abstract
Acute inflammatory lesions of the placenta consist of diffuse infiltration of neutrophils at different sites in the organ. These lesions include acute chorioamnionitis, funisitis, and chorionic vasculitis, and represent a host response (maternal or fetal) to a chemotactic gradient in the amniotic cavity. While acute chorioamnionitis is evidence of a maternal host response, funisitis and chorionic vasculitis represent fetal inflammatory responses. Intra-amniotic infection has been generally considered to be the cause of acute histologic chorioamnionitis and funisitis; however, recent evidence indicates that “sterile” intra-amniotic inflammation, which occurs in the absence of demonstrable microorganisms but can be induced by “danger signals”, is frequently associated with these lesions. In the context of intra-amniotic infection, chemokines (such as interleukin-8 and granulocyte chemotactic protein) establish a gradient favoring the migration of neutrophils from maternal or fetal circulation into the chorioamniotic membranes or umbilical cord, respectively. Danger signals released during the course of cellular stress or cell death can also induce the release of neutrophil chemokines. The prevalence of chorioamnionitis is a function of gestational age at birth, and is present in 3-5% of placentas delivered at term, but in 94% of placentas delivered between 21-24 weeks of gestation. The frequency is higher in patients with spontaneous labor, preterm labor, clinical chorioamnionitis (preterm or term), or ruptured membranes. Funisitis and chorionic vasculitis are the hallmarks for the fetal inflammatory response syndrome, a condition characterized by an elevation in fetal plasma concentrations of interleukin-6, associated with the impending onset of preterm labor, a higher rate of neonatal morbidity (after adjustment for gestational age), and multi-organ fetal involvement. This syndrome is the counterpart of the systemic inflammatory response syndrome in adults; however, in fetuses, it is a risk factor for short- and long-term complications (i.e. neonatal sepsis, bronchopulmonary dysplasia, periventricular leukomalacia, and cerebral palsy). This article reviews the definition, pathogenesis, grading and staging, and clinical significance of the most common lesions in placental pathology. Illustrations of the lesions and diagrams of the mechanisms of disease are provided.
Keywords: acute villitis, ascending intra-amniotic infection, chorionic vasculitis, clinical chorioamnionitis, CXCL6, fetal inflammatory response syndrome, granulocyte chemotactic protein, interleukin (IL)-8, microbial invasion of the amniotic cavity, nosology, pathologic grading, placental pathology, pregnancy, prematurity, staging, sterile inflammation
1. Introduction
Acute chorioamnionitis is the most frequent diagnosis in placental pathology reports, and is generally considered to represent the presence of intra-amniotic infection or “amniotic fluid infection syndrome”1-10. Yet, acute chorioamnionitis can occur with “sterile intra-amniotic inflammation”, which occurs in the absence of demonstrable microorganisms, but can be induced by “danger signals” released under conditions of cellular stress, injury or death 11-15. Therefore, acute chorioamnionitis is evidence of intra-amniotic inflammation, and not intra-amniotic infection. The characteristic feature of acute chorioamnionitis is diffuse infiltration of neutrophils into the chorioamniotic membranes 9. Since obstericians use the term “chorioamnionitis” to refer to a clinical syndrome (the combination of fever, maternal-fetal tachycardia, uterine tenderness, foul-smelling amniotic fluid, etc.) frequently associated with “acute chorioamnionitis” on microscopic examination of the placenta, the word “histologic” has been introduced into the medical lexicon to specify the differences between the clinical syndrome, clinical chorioamnionitis, and the pathologic diagnosis of acute chorioamnionitis. These terms are not synonymous, and confusion is caused when they are used interchangeably. Herein the term “acute chorioamnionitis” will refer to acute histologic chorioamnionitis because the focus of this article is the pathologic condition rather than the clinical syndrome. We will review the acute inflammatory responses deployed by the mother and fetus in response to inflammatory stimuli within the amniotic cavity.
2. Definition
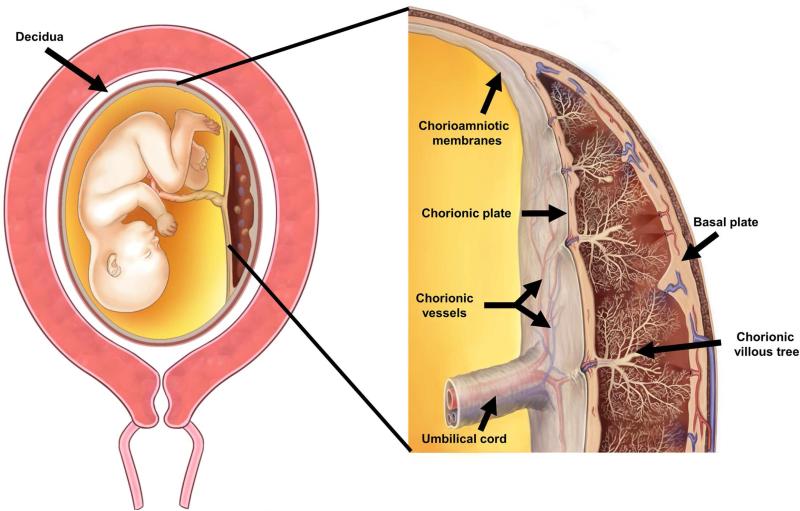
The placenta is composed of three major structures: the placental disc, the chorioamniotic membranes, and the umbilical cord (Figure 1). Acute inflammatory lesions of the placenta are characterized by the infiltration of neutrophils in each of these structures.9 Specifically, when the inflammatory process affects the chorion and amnion, this is termed acute chorioamnionitis9; if it affects the villous tree, this represents acute villitis9. If the inflammatory process involves the umbilical cord (umbilical vein, umbilical artery, and the Wharton's jelly), this is referred to as funisitis, the histological counterpart of the fetal inflammatory response syndrome16 (Figure 1).
Figure 1.
The anatomy of the pregnant uterus with an emphasis on the placenta. The upper part of the figure illustrates the fetus, umbilical cord and placenta. The chorioamniotic membranes include the amnion and chorion. Decidua is of maternal origin (secretory endometrium) and is adjacent to the myometrium. The lower part of the figure represents a cross-section of the human placenta, including the chorionic plate, chorioamniotic membranes, umbilical cord, and the intervillous space. The basal plate of the placenta is formed of decidua, and is traversed by the spiral arteries, which bring maternal blood into the intervillous space. The villous circulation (fetal) is illustrated in a cross-section of the stem villi. The fetal vessels on the surface of the chorionic plate include arteries and veins, which coalesce to form the umbilical vein and umbilical arteries. Modified from Benirschke K, Burton GJ, Baergen RN. Infectious Diseases. Pathology of the Human Placenta. Sixth ed. Berlin Heidelberg: Springer; 2012. p. 33.
3. Prevalence of acute histologic chorioamnionitis
Table 1 shows the frequency of acute chorioamnionitis as a function of gestational age at delivery in a study of 7,505 placentas from singleton pregnancies delivered after 20 weeks of gestation2. It is noteworthy that the frequency of acute chorioamnionitis in patients who delivered between 21 to 24 weeks of gestation was 94.4% (17/18)2. This is consistent with extensive studies subsequently reported by our group17, 18 and others19, 20, and emphasizes the importance of acute inflammation in early preterm deliveries and midtrimester spontaneous abortions.
Table 1.
The frequency of microbial invasion of the amniotic cavity (MIAC) in obstetrical disorders as determined by amniotic fluid studies obtained by transabdominal amniocentesis using cultivation techniques
| Obstetrical disorders | Prevalence of MIAC (%) |
|---|---|
| Spontaneous labor at term with intact membranes | 6.3-18.8 21, 24, 33, 201 |
| Preterm labor with intact membranes | 8.7-34 11, 89-104, 106-114, 327 |
| Prelabor premature rupture of membranes without labor | 17-57.7 13, 97, 98, 115-130, 327 |
| Clinical chorioamnionitis at term | 61 15 |
| Prelabor premature rupture of membranes in labor | 75 122 |
| Spontaneous rupture of membranes at term | 34.3 370 |
| Sonographic short cervix | 2.2-9 14, 136-138 |
| Cervical insufficiency | 8-51.5 131-135 |
| Twin gestations with preterm labor and intact membranes | 11.9-35 371-373 |
| Meconium stained amniotic fluid in preterm gestations | 33 374 |
| Meconium stained amniotic fluid in term gestations | 19.6 375 |
| Placenta previa | 5.7 140 |
| Idiopathic vaginal bleeding | 14 139 |
| Pregnancy with intra-uterine device | 45.9 168 |
| Preeclampsia | 1.6 376 |
| Small for gestational age fetuses | 6 377 |
| Stillbirth | 2.3-13.3 378, 379 |
MIAC: microbial invasion of the amniotic cavity
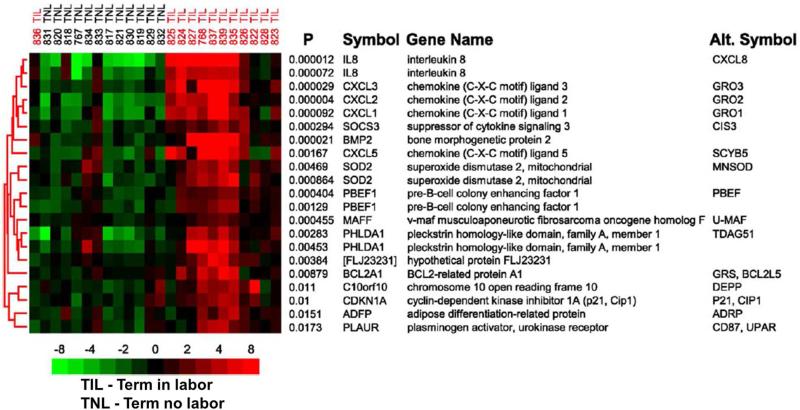
Acute chorioamnionitis is more frequently observed in the placentas of women who delivered after spontaneous labor at term than in the absence of labor21, 22 [early labor with cervical dilatation < 4 cm =11.6% (10/86) vs. no labor=4.4% (34/775); p<0.01]22. Moreover, the frequency of histologic chorioamnionitis is higher with longer the duration of labor and cervical dilatation ≥4 cm [active labor=30.4% (7/23) vs. early labor=11.6% (10/86); p<0.05]23. Two explanations can be invoked from this observation: first, the frequency of microbial invasion of the amniotic cavity is higher in women in spontaneous labor at term with intact membranes than in those without labor (17% vs 1.5%)24. Alternatively, labor per se is an inflammatory state, as demonstrated by the the study of the gene expression profile of the chorioamniotic membranes25. The chorioamniotic membranes obtained from women who experienced labor (even in the absence of any detectable histologic chorioamnionitis) overexpressed neutrophil-specific chemokines [Chemokine (C-X-C motif) ligand 1 (CXCL1), CXCL2, and Interleukin (IL)-8], and monocyte-specific chemokines (C-C motif) ligand 3 (CCL3; Macrophage inflammatory protein (MIP)-1α), CCL4 (MIP-1β), and CCL20 (MIP-3α)25 (Figure 2). This is consistent with reports that the amniotic fluid concentrations of chemokines such as IL-826, Monocyte chemoattractant protein (MCP)-127, Growth-regulated oncogene (GRO)-α 28, MIP-1α 29 and cytokines such as IL-130-32, IL-633, 34 are higher in women in spontaneous labor at term than in those not in labor at term.
Figure 2.
The subcluster of genes with the smallest discriminant P values included many genes known to be involved in the inflammatory response. Row labels correspond to the permuted t test P value followed by the HUGO Gene Nomenclature Committee (HGNC) official gene symbol, and include the most commonly used alternative gene symbol. Gene expression levels were median-centered and pseudocolored such that red indicates an increased, green indicates a decreased, and black represents the median expression levels, as indicated by the color bar whose numbers indicate fold change. Labels at the top indicate individual patient samples and their clinical designation. Modified from Figure 2 Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R., Am J Obstet Gynecol. 2006 Aug;195(2):394.e1-24.
4. Pathology
The placenta can be considered as the apposition or fusion of the fetal membranes/placental disc to the uterine mucosa (decidua) for physiologic exchange35. The decidua is of maternal origin, while the chorioamniotic membranes and villous tree are of fetal origin.Thus, the precise origin of the inflammatory process (maternal vs. fetal) can be determined by whether infiltrating neutrophils are of maternal or fetal origin.
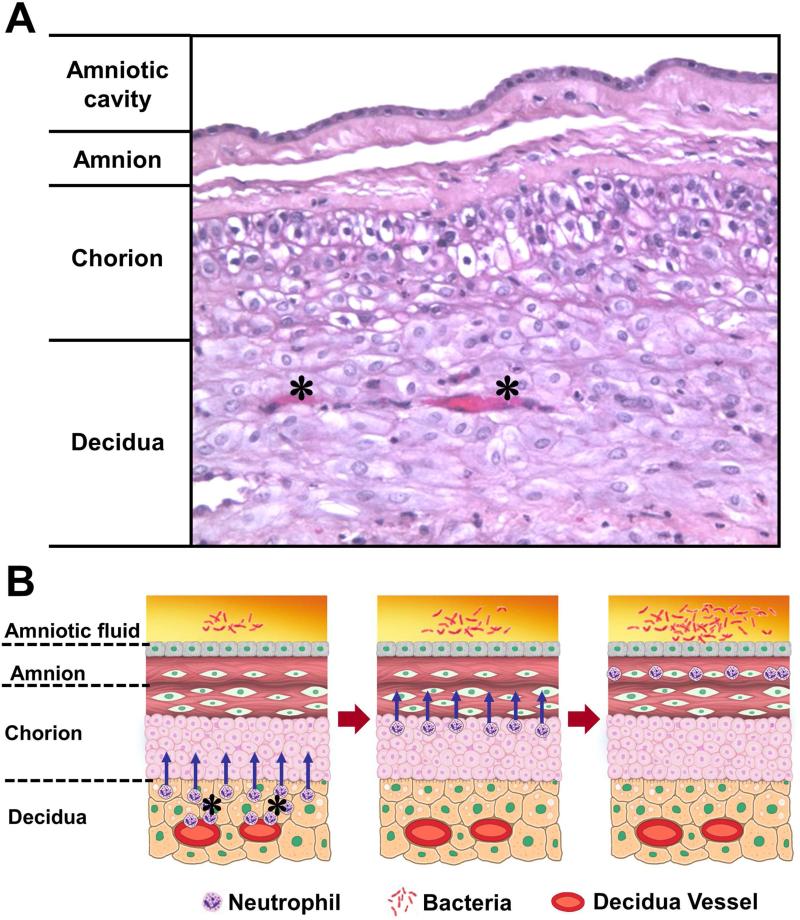
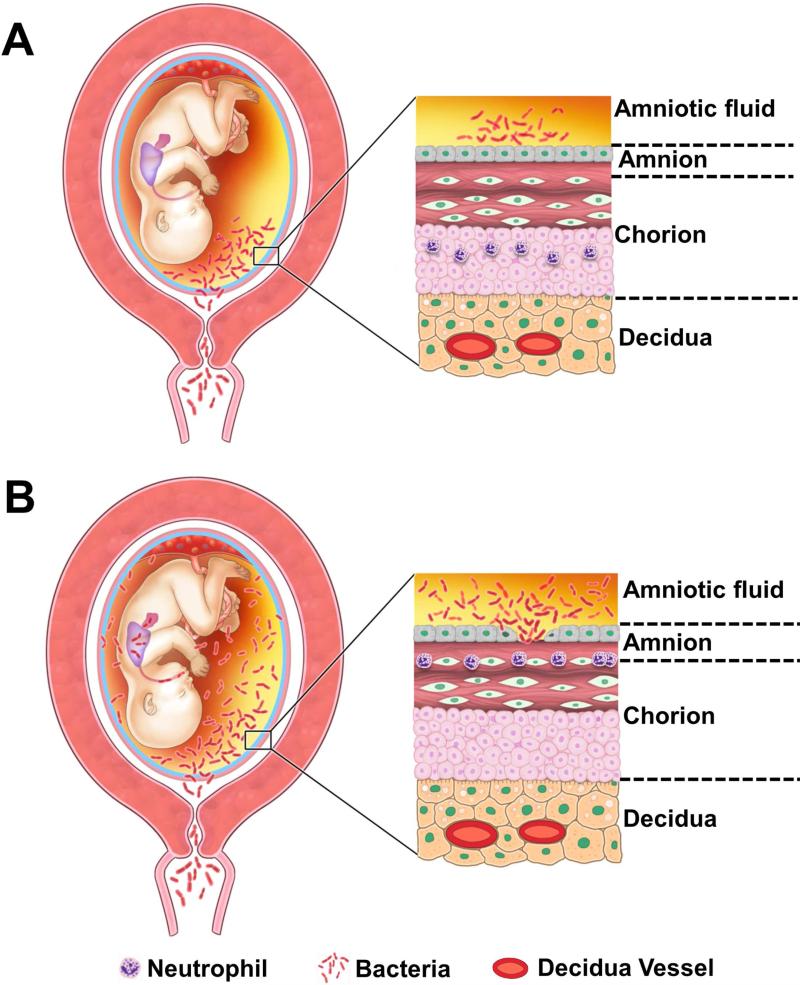
Neutrophils are not normally present in the chorioamniotic membranes, and are thought to migrate from the decidua into the membranes in cases of acute chorioamnionitis36, 37 (Figure 3). On the other hand, neutrophils in the maternal circulation are normally present in the intervillous space (Figure 1). When there is a chemotactic gradient attracting neutrophils toward the amniotic cavity, neutrophils in the intervillous space migrate into the chorionic plate of the placenta, which is also normally devoid of these cells. Thus, inflammation of the chorionic plate is also a maternal inflammatory response.
Figure 3.
Migration of the neutrophils from decidual vessels into the chorioamniotic membranes. (A) Normal histology of the chorioamniotic membranes, which are composed of amnion and chorion laeve. The decidua is adjacent to the chorion and contains maternal capillaries (black asterisk). Neutrophils migrate from the maternal circulation in the presence of chemotactic gradient (increased amniotic fluid neutrophil chemokine concentrations). (B) Progression of neutrophils from the decidual vessels (in red) towards the amnion. The location of bacteria is within the amniotic cavity. Initially, neutrophils accumulate in the choriodecidual interface (B; left); however, in subsequent stages, invade the chorion (B, center) and amnion (B, right).
Neutrophils in acute chorioamnionitis are of maternal origin. Fluorescence in situ hybridization (FISH) with probes for X and Y chromosomes performed in cytospin slides of placentas from male fetuses showed that approximately 90% of neutrophils derived from the membranes were of maternal origin36. Subsequently, FISH combined with immunohistochemistry for CD45 (to identify leukocytes) demonstrated that cells staining for CD45 in the chorioamniotic membranes were of maternal origin37. In contrast, inflammation of the umbilical cord and the chorionic vessels on the chorionic plate of the placenta is of fetal origin38. This conclusion is largely based on the understanding of the anatomy of these tissues, as neutrophils invading the walls of the umbilical vein and arteries must migrate from the fetal circulation to enter the walls of these vessels (Figure 4). Insofar as the origin of white blood cells in the amniotic fluid in cases of intra-amniotic inflammation, the only study reported to date in cases of clinical chorioamnionitis with intact membranes suggested that 99% of neutrophils are of fetal origin 39.
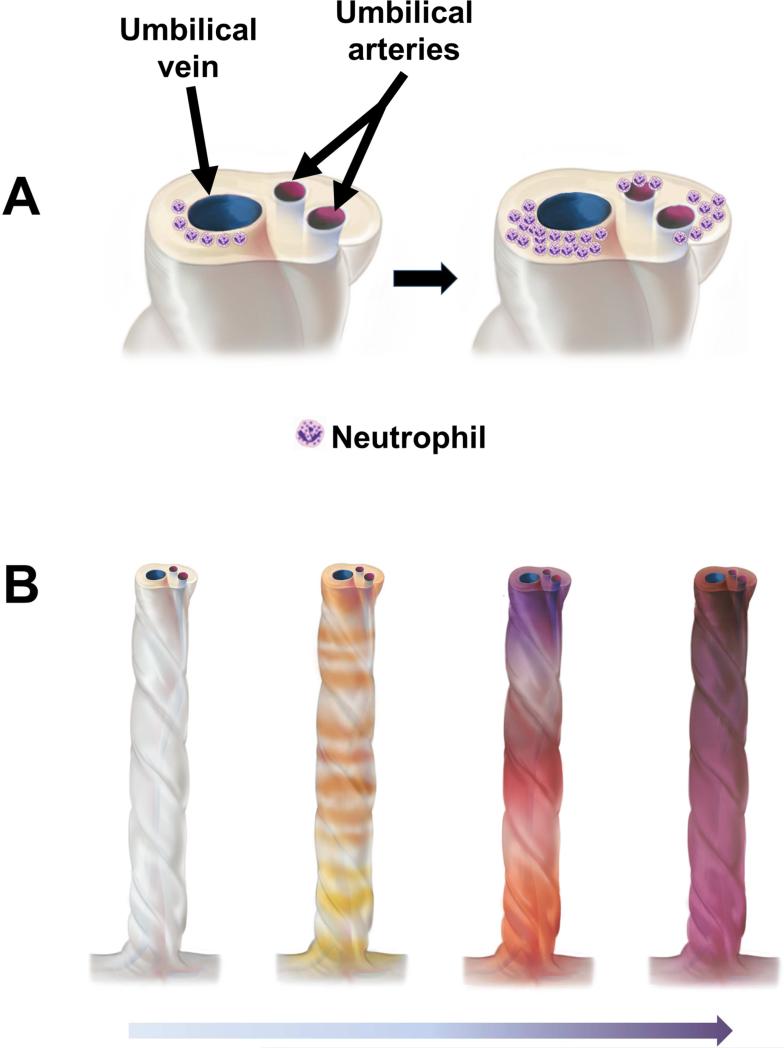
Figure 4.
Topography of the inflammatory process in the umbilical cord. (A) Typically, acute funisitis begins as inflammation of the umbilical vein (umbilical phlebitis; the red vessel represents the umbilical vein), followed by umbilical arteritis involving the umbilical arteries (blue). (B) Progression of inflammation along the length of the umbilical cord. The initial phase is multi-focal, as demonstrated by the yellow/orange rings in the second umbilical cord from left to right in figure 3B. Subsequently, the areas of inflammation coalesce, and funisitis affects the entire umbilical cord.
Inflammation of the umbilical vessels begins in the vein (phlebitis) and is followed by involvement of the arteries (arteritis), then infiltration of neutrophils into the Wharton's jelly 40. The molecular pathogenesis of funisitis has been studied using microarray analysis followed by quantitative real-time PCR of RNA obtained from micro-dissected umbilical arteries and veins. The expression of IL-8 mRNA (the prototypic neutrophil chemokine) is higher in the umbilical vein than in the umbilical artery 40. Moreover, there are substantial differences in the genes expressed by the walls of the umbilical artery and vein. The pattern of gene expression suggests that the wall of the umbilical vein is more prone to a pro-inflammatory response than the umbilical arteries 40. This explains why the umbilical vein is the first vessel to show inflammatory changes, and the presence of arteritis is evidence of a more advanced fetal inflammatory process 40. Indeed, the umbilical cord plasma concentrations of IL-6 (a cytokine used to define systemic inflammation) and the frequency of neonatal complications are higher in cases with umbilical cord arteritis than in those with phlebitis only 41.
Systematic studies of the umbilical cord suggest that acute funisitis begins as multiple, discrete foci, along the umbilical cord, which then merge with the progression of the inflammatory process 40. Figure 4 illustrates the topography of the inflammatory process in several umbilical cords serially sectioned at 1 mm intervals. The chemotactic gradient attracting neutrophils from the lumen of the umbilical vessels into the Wharton's jelly is thought to be dependent on elevated concentrations of chemokines in the amniotic fluid. The severity of funisitis correlates with fetal plasma IL-6 concentrations (an indicator of the severity of the systemic fetal inflammatory response) and amniotic fluid IL-6 – the latter reflects the intensity of the intra-amniotic inflammatory response 41.
5. Histological Grading and Staging of Acute Chorioamnionitis
Several grading and staging systems have been proposed to describe the severity of acute chorioamnionitis 9, 18, 19, 42-47. The most widely used is that recommended by the Amniotic Fluid Infection Nosology Committee of Perinatal Section, the Society for Pediatric Pathology, and reported by Redline et al. in 2003 9. Although that article contains the term “amniotic fluid infection syndrome”, it is now clear that these lesions do not always represent intra-amniotic infection.
Redline et al. classified acute inflammatory lesions of the placenta into two categories: “maternal inflammatory response” and “fetal inflammatory response” 9. The term “stage” refers to the progression of disease based on the anatomical regions infiltrated by neutrophils, while the term “grade” refers to the intensity of the acute inflammatory process at a particular site 9. In the context of a maternal inflammatory response, a stage 1 lesion is characterized by the presence of neutrophils in the chorion or subchorionic space; stage 2 refers to neutrophilic infiltration of the chorionic connective tissue and/or amnion, or the chorionic plate; and stage 3 is necrotizing chorioamnionitis with degenerating neutrophils (karyorrhexis) 9.
Grade 1 (mild to moderate) refers to individual or small clusters of maternal neutrophils, diffusely infiltrating the chorion laeve, chorionic plate, subchorionic fibrin or amnion. Grade 2 (severe) consists of the presence of three or more chorionic microabscesses, which are defined as confluence of neutrophils measuring at least 10×20 cells 9. Microabscesses are typically located between the chorion and decidua, and/or under the chorionic plate 9. Grade 2 is also applied in the presence of a continuous band of confluent neutrophils in the chorion of more than 10 cells in width, occupying more than half of the subchorionic fibrin, or one revolution of the membrane roll. Other staging and grading systems have been used and subsequently modified 18, 19, 42-47.
Staging and grading are also applicable to the fetal inflammatory response 9. Staging (which refers to the location of neutrophil infiltration) is more important and reproducible than grading in the assessment of the severity of the inflammatory process 48. For example, involvement of the amnion (amnionitis) is associated with more intense fetal and intra-amniotic inflammation, measured by the concentration of cytokines, than involvement of the chorion alone 49. The rates of funisitis and positive amniotic fluid culture for microorganisms, as well as the median umbilical cord plasma C-reactive protein, median amniotic fluid Matrix metalloproteinase (MMP)-8 concentration and amniotic fluid white blood cell count are higher when the inflammatory process of the membranes involves amnion and chorion than when neutrophil infiltration is restricted to the chorion/decidua 49. (Figure 5 stage and grade of acute chorioamnionitis, Figure 6 acute funisitis). Moreover, amniotic fluid MMP-8 concentration is correlated with the severity of acute histologic chorioamnionitis (grading) 50.
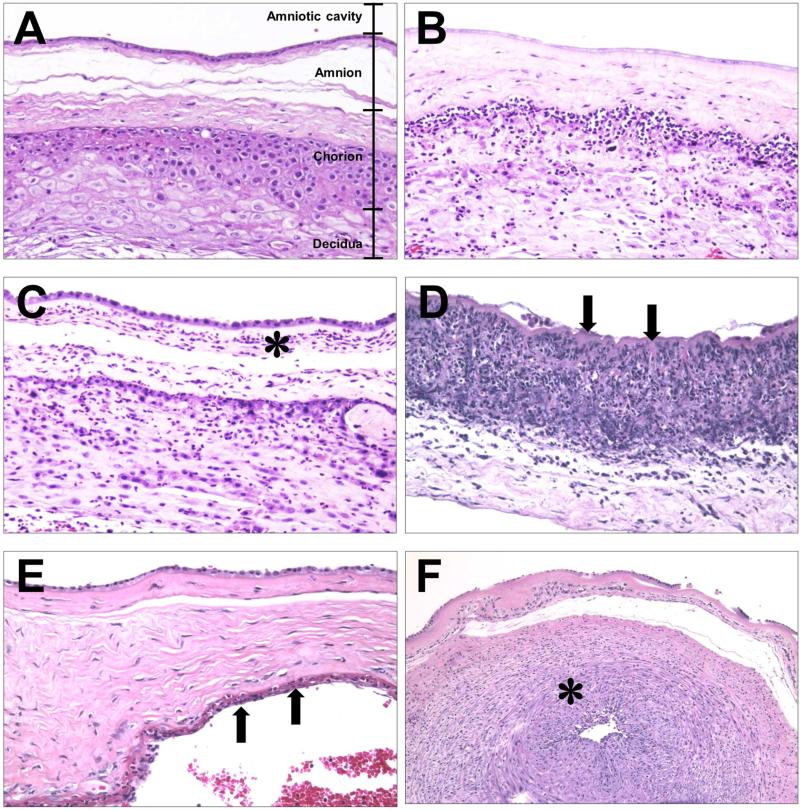
Figure 5.
Staging of acute chorioamnionitis. (A-D) Acute chorioamnionitis of the extraplacental chorioamniotic membranes. (A) Normal chorioamniotic membranes showing the absence of neutrophils. (B) Acute chorionitis is stage 1 acute inflammation of the chorioamniotic membranes, in which neutrophilic infiltration is limited to the chorion. (C) Acute chorioamnionitis is stage 2 acute inflammation of the chorioamniotic membranes, showing neutrophilic migration into the amniotic connective tissue (asterisk). (D) Necrotizing chorioamnionitis is stage 3 acute inflammation of the chorioamniotic membranes, whose characteristic is the amnion epithelial necrosis (arrows). (E, F) Acute inflammation of the chorionic plate. (E) Acute subchorionitis, stage 1 acute inflammation shows neutrophils in the subchorionic fibrin in the chorionic plate (arrows). The area immediately below the arrows represents the intervillous space. (F) Acute chorionic vasculitis (asterisk) is a stage 1 fetal inflammatory response, while acute inflammation of the chorioamniotic membranes (A-F) represents a maternal inflammatory response. Chorionic vasculitis is inflammation on the surface of the fetal vessels within the chorionic plate (see Figure 1 for anatomical location).
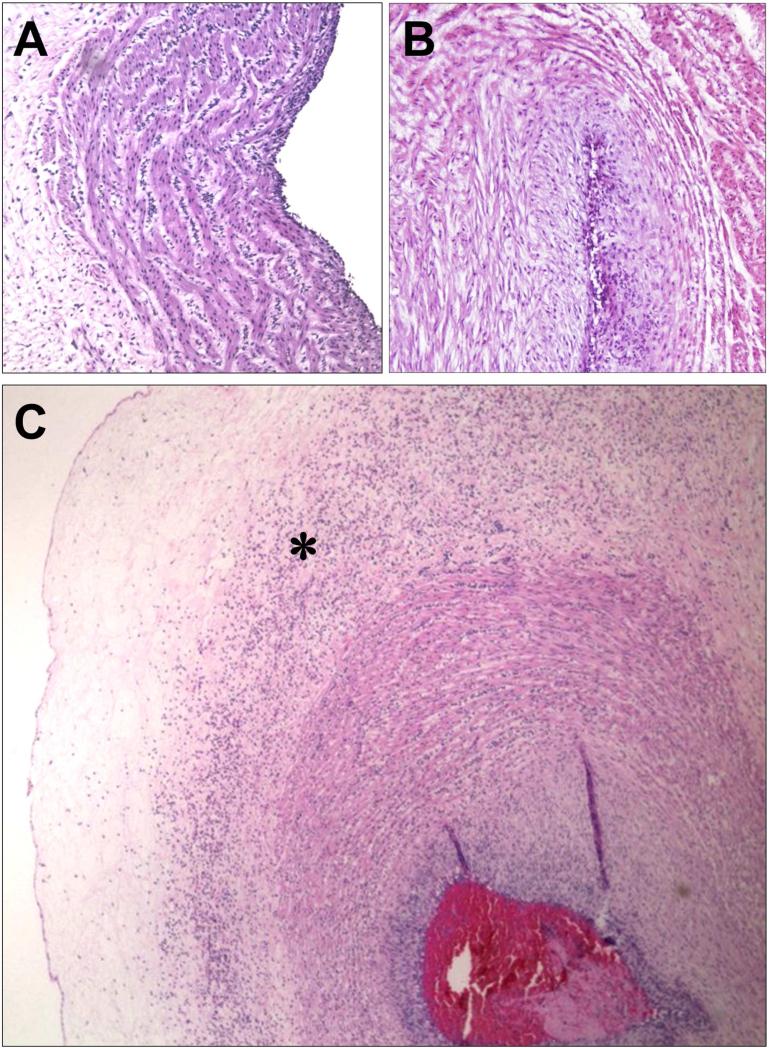
Figure 6.
Staging of acute funisitis. (A) Umbilical phlebitis showing amniotropic migration of fetal neutrophils into the muscle layer of the umbilical vein. Umbilical phlebitis represents stage 1 fetal inflammation. (B) Umbilical arteritis is a stage 2 fetal inflammatory response. (C) Necrotizing funisitis is considered stage 3 fetal inflammatory response. Its characteristic feature is concentric, perivascular distribution of degenerated neutrophils (asterisk). The presence of a thrombus should be considered as a severe fetal inflammatory response.
The reproducibility of the grading and staging of maternal and fetal inflammation has been subject of a rigorous study by Redline et al. 9 in which 20 cases were reviewed by six pathologists who were asked to identify 12 inflammatory lesions. The kappa coefficient was used to measure agreement among observers. In general, the presence or absence of inflammation had a very high kappa value (0.93 for acute chorioamnionitis, and 0.90 for acute chorioamnionitis/fetal inflammatory response). A kappa value between 0.81 and 1 is considered to represent almost perfect agreement. In contrast, the value of kappa was lower for grading and staging. The authors concluded that there is a greater degree of agreement among pathologists in identifying the presence or absence of inflammation, rather than in quantifying grading and staging 9.
6. Pathways of microbial invasion of the amniotic cavity
Under normal conditions, the amniotic cavity is sterile for microorganisms using cultivation 51 and molecular microbiologic techniques, based on the detection of the 16S rRNA gene (present in all bacteria, but not in mammalian cells). Four pathways have been proposed whereby microorganisms reach the amniotic cavity 52-56: 1) ascending from the lower genital tract 1, 7, 57, 58; 2) hematogenous 59-61; 3) accidental introduction at the time of amniocentesis, percutaneous umbilical cord blood sampling, fetoscopy, or another invasive procedure 62-68; and 4) retrograde seeding from the peritoneal cavity from the fallopian tubes 57 . However, there is limited evidence in support of the latter pathway.
Ascending microbial invasion from the lower genital tract appears to be the most frequent pathway for intra-amniotic infection (Figure 7 and Figure 8) 53. While all pregnant women have microorganisms in the lower genital tract, most do not have intra-amniotic infection. The mucus plug represents an anatomical and functional barrier to ascending infection during pregnancy 69-75. In the non-pregnant state, the endometrial cavity is not sterile 76-78, but the decidua is thought to be sterile during pregnancy.
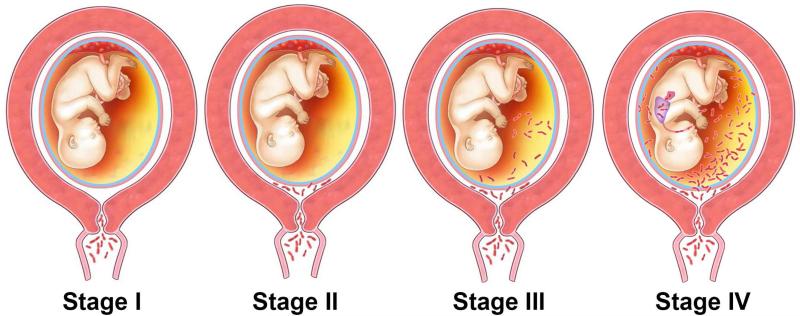
Figure 7.
The stages of ascending infection in preterm labor. Stage I in the process of ascending infection is corresponding to a change in the vaginal/cervical microbial flora or the presence of pathologic organisms in the cervix. Once microorganisms gain access to the amniotic cavity, they reside in the lower pole of the uterus between the membranes and the chorion (Stage II). The microorganisms may invade the fetal vessels (choriovasculitis) or proceed through the amnion (amnionitis) into the amniotic cavity leading to an intra-amniotic infection (Stage III). The microorganisms may invade the fetus by different ports of entry (Stage IV). Modified from Figure 1 in Romero R, Mazor M, Infection and Preterm Labor, Clinical Obstetrics and Gynecology;31:1988:553-584.
Figure 8.
Pathways of intra-amniotic infection. (A) Most cases of microbial invasion of the amniotic cavity are the result of ascending infection from the vagina and cervix. (B) Extensive microbial invasion of the amniotic cavity can result in fetal infection (bacteria are located in the fetal lung) and damaged chorioamniotic membranes (i.e. necrotizing chorioamnionitis). The destruction of the amnion epithelium is a cardinal feature of necrotizing chorioamnionitis. Modified from Figure 5 Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, Mittal P, Erez O, Kusanovic JP, Hassan SS, Kim CJ. Lab Invest. 2009 Aug;89(8):924-36.
A hematogenous pathway can operate during the course of blood-born maternal infections 59-61. Microorganisms such as Listeria monocytogenes 79-81, Treponema pallidum, Yersinia pestis, Cytomegalovirus, Plasmodium species, and others can gain access through the maternal circulation to the intervillous space, from where they invade the villi and the fetal circulation 53. Bacteria involved in periodontal disease may use this pathway to reach the amniotic cavity 82-88.
Intra-amniotic infection has been documented in patients with preterm labor with intact membranes 11, 89-114, prelabor rupture of membranes 13, 115-130, cervical insufficiency 131-135, an asymptomatic short cervix 14, 136-138, idiopathic vaginal bleeding 139, placenta previa 140, and clinical chorioamnionitis at term 15. Rupture of membranes is not necessary for bacteria to reach the amniotic cavity – indeed, there is experimental evidence that bacteria can cross intact membranes 141. Most of these infections are subclinical in nature, and therefore, they occur in the absence of clinical chorioamnionitis 90, 142, 143. Hence, most of these infections are undetected unless the amniotic fluid is analyzed. The most frequent microorganisms found in the amniotic cavity are genital mycoplasmas 93, 103, 122, 142, 144-147, and in particular, Ureaplasma species 135, 148-155, Gardnerella vaginalis 15, 90, 127, 156-158, Fusobacteria species, etc. 11, 110, 127. Fungi can also be found – women who became pregnant with intrauterine contraceptive devices are at high risk for intra-amniotic infection with Candida albicans 159-168. Polymicrobial invasion of the amniotic cavity is present in approximately 30% of cases 11, 13, 93, 110, 127, 169. Table 2 describes the frequency of microbial invasion of the amniotic cavity in different obstetrical syndromes. Table 3 demonstrates the microorganisms detected in amniotic cavity in patients with preterm labor with intact membranes 110 and clinical chorioamnionitis at term 15
Table 2.
Frequency of chorioamnionitis according to gestational age at delivery
| Weeks of gestation | Chorioamnionitis (n) | Total number of patients | Percent (%) |
|---|---|---|---|
| 21–24 | 17 | 18 | 94.4 |
| 25–28 | 19 | 48 | 39.6 |
| 29–32 | 34 | 96 | 35.4 |
| 33–36 | 53 | 497 | 10.7 |
| 37–40 | 233 | 6139 | 3.8 |
| 41–44 | 36 | 707 | 5.1 |
| Total | 392 | 7505 | 5.2 |
Modified from Russell P, Inflammatory lesions of the human placenta I, The American journal of diagnostic gynecology and obstetrics 1979; 1: 127-137
Table 4.
Cytokines implicated in the pathogenesis of intra-amniotic inflammation/infection
| Pro- and anti-inflammatory cytokines | Functions |
|---|---|
| IL-1α (IL1F1) 32 | Alarmin (endogenous molecules that signal tissue and cell damage) Proinflammatory effects by inducing production of cytokines and chemokines Mediates neutrophil recruitment |
| IL-1β (IL1F2) 32 | Proinflammatory cytokine and a major mediator of the inflammatory response |
| IL-6 94, 380 | Key mediator of the acute phase response to infection and tissue injury Activates Tcells and natural killer (NK) cells Stimulates proliferation and immunoglobulin production by B cells |
| Tumor necrosis factor-alpha (TNF-α) 381 | Proinflammatory cytokine and a major mediator of sepsis |
| IL-4 382 | Inhibits production of IL-1β Induces differentiation of helper T cells Stimulates IgG and IgE production |
| IL-10 383 | Inhibits the production of pro-inflammatory cytokines (cytokine inhibitory factor) Downregulates T-cell functions Potent suppressor of the effector functions of macrophages and natural killer (NK) cells |
| Chemokines | Functions |
|---|---|
| IL-8 (Neutrophil activating peptide, CXCL8) 26 | Recruitment and activation of acute inflammatory cells, primarily neutrophils Promote angiogenesis |
| CXCL6 (Granulocyte chemotactic protein-2) 212 | Potent pro-inflammatory chemokine Neutrophil activator |
| CXCL10 (IP-10) 281, 283 | T cell chemotactic cytokine Recruits and potentiates helper T cell responses and pathogenesis of allograft rejection Pro-inflammatory and anti-angiogenic properties |
| CXCL13 (BCA-1) 305 | Induces migration of B and T lymphocytes to areas of infection and inflammation |
| CCL3 (MIP-1α) 29 | Chemotactic cytokine, activates human granulocytes (neutrophils, eosinophils and basophils) in response to inflammation and infection |
| CCL4 (MIP-1β) 196 | Chemotactic cytokine, activates human granulocytes (neutrophils, eosinophils and basophils) in response to inflammation and infection |
| CCL20 (MIP-3α) 384 | Chemotactic activity for immature dendritic cells, effector or memory CD4(+) T lymphocytes, and B lymphocytes |
| Macrophage inhibitory cytokine 298 | Regulates the adaptive immune response, induces cell proliferation, and angiogenesis Inhibits the migration of macrophages and stimulates TNF-α and nitric oxide from macrophages and IL-2 production |
| MCP-1 (CCL2) 300 | Recruits monocytes/macrophages into sites of inflammation Stimulates the respiratory burst required for macrophage activation |
| MCP-2 (CCL8) 303 | Role in the inflammatory response Activates immune cells (including mast cells, eosinophils and basophils, monocytes, T cells, and natural killer (NK) cells) |
| MCP-3 (CCL7) 303 | Monocyte chemoattractant Regulates macrophage function |
| ENA-78 (CXCL5) 306 | Potent neutrophil chemoattractant and activator Ligand for CXCR2 (IL-8 receptor; chemokine receptor that is activated by IL-8) |
| GRO-α (CXCL1) 28 | Recruits and activates neutrophils, lymphocytes and monocytes in host defense Role in wound healing, growth regulation, angiogenesis, tumorigenesis and apoptosis |
| RANTES 307 | Chemoattractant of monocytes, lymphocytes, basophils and eosinophils Regulates the inflammatory response and recruitment of macrophages to the implantation site in early pregnancy Regulates the host response to intrauterine infection |
IL = Interleukin; CXCL = Chemokine (C-X-C motif) ligand; IP = Interferon-gamma-inducible protein; BCA = B cell-attracting chemokine; CCL = chemokine (C-C motif) ligand; MIP = Macrophage inflammatory protein; MCP = Monocyte chemoattractant protein; ENA = Epithelial cell-derived neutrophil-activating peptide; GRO = Growth-regulated oncogene; RANTES = Regulated on activation, normal T cell expressed and secreted
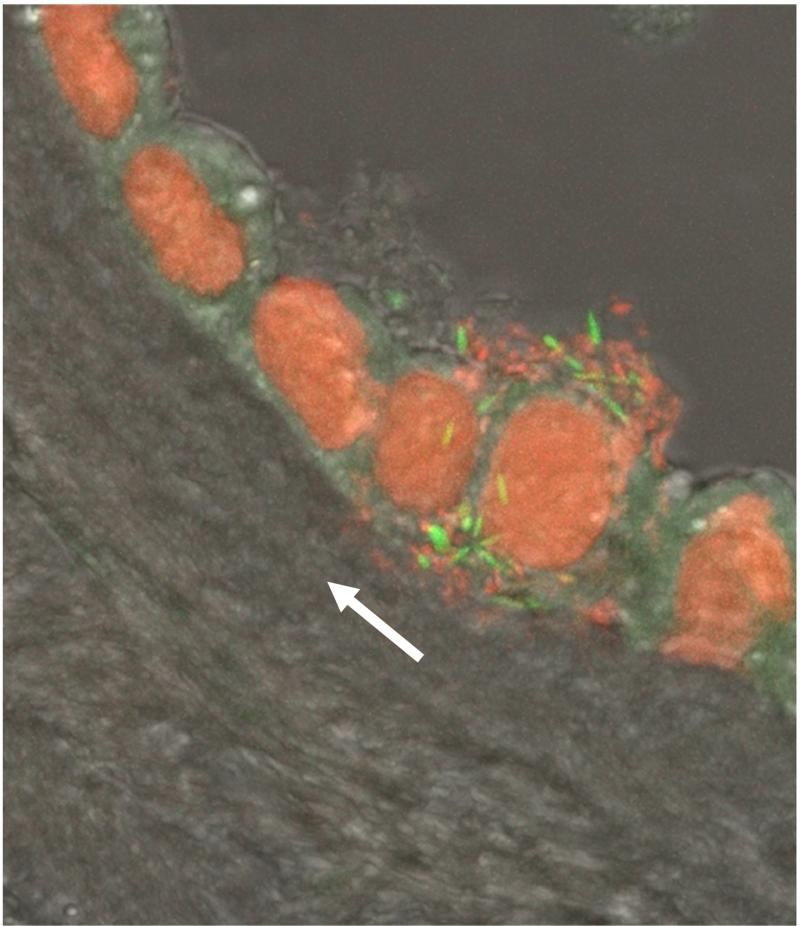
Microorganisms gaining access to the uterine cavity from the lower genital tract are first localized in the decidua of the supracervical region. Subsequent propagation and chorioamniotic passage of the microorganisms can lead to the establishment of microbial invasion of the amniotic cavity 170, 171. Although some investigators believe that there is a stage in which the bacteria are diffusely located in the choriodecidual layer, our studies, using FISH with a bacterial 16S rRNA probe, indicate that there is not extensive involvement of the chorion-decidua in cases with microbial invasion of the amniotic cavity 172. Indeed, bacteria are primarily found in the amnion in cases of intra-amniotic infection, indicating that microbial invasion of the amniotic cavity is a prerequisite for substantial invasion of the amnion and chorion 172. Specifically, bacteria are more frequently detected in the amniotic fluid than in the chorioamniotic membranes of patients with positive amniotic fluid culture (100% vs. 33%; P<0.0001) 172 (Figure 9).
Figure 9.
A cluster of bacteria in amniotic fluid and bacterial invasion of amniotic epithelial cells demonstrated by fluorescent staining. Live bacteria were stained with SYTO 9 (green fluorescence), and dead bacteria were stained with propidium iodide (red fluorescence). Note the lack of bacteria in the chorioamniotic connective tissue indicating bacterial propagation from the amniotic cavity (white arrow). Modified from Figure 3C Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, Mittal P, Erez O, Kusanovic JP, Hassan SS, Kim CJ. Lab Invest. 2009 Aug;89(8):924-36.
In the past, investigators have reported that the space between the chorioamniotic membranes could contain bacteria, even though such bacteria may not be detectable in the amniotic fluid 4, 173. The frequency with which this phenomenon occurs remains to be determined. Studies using a combination of cultivation and molecular microbiologic techniques have not yet been conducted to determine the frequency with which this phenomenon occurs. This question is important for the understanding of the pathogenesis of intra-amniotic infection. Experimental models in nonhuman primates have been generated by the inoculation of bacteria in either the decidua or amniotic cavity. Preterm labor occurs more frequently when bacteria are introduced into the amniotic cavity, rather than between the decidua and chorion 171, 174. Therefore, it seems that intra-amniotic inoculation of bacteria more closely resembles microbial invasion of the amniotic cavity with preterm labor than decidual inoculation 171, 174.
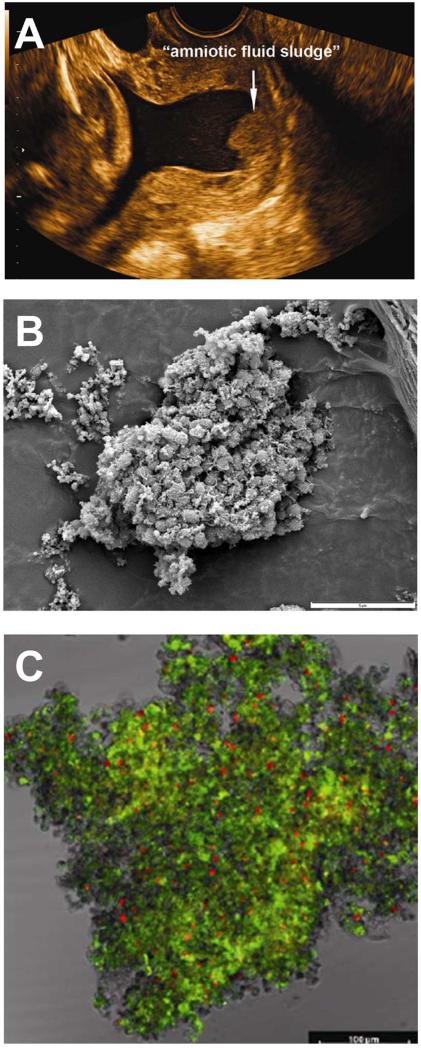
Microbial invasion of the amniotic cavity has been traditionally attributed to planktonic or free-floating bacteria. However, recent evidence suggests that amniotic fluid bacteria can form biofilms 175-182. Biofilms are defined as communities of sessile organisms that attach to a substratum or to each other. The presence of biofilms can be clinically suspected when sludge is detected as particulate matter in the amniotic fluid using ultrasound 175-182 (Figure 10). Bacteria in biofilms are embedded in a hydrated matrix of extracellular polymeric substances, and exhibit an altered phenotype with respect to growth rate and gene transcription in comparison to planktonic (free floating) cells. In addition, more than 99% of the bacteria in biofilms are capable of growing on a wide variety of surfaces 183. Biofilms play a major role in human infections, such as periodontitis otitis media and endocarditis , and are important because bacteria in biofilms are resistant to antibiotic treatment . The formation of biofilms in the amniotic cavity may explain the difficulty in the treatment of intra-amniotic infection. Biofilms are also more common in infections associated with a device (e.g., intra-uterine contraceptive device, prosthetic valves, catheters). Notably, the eradication of intra-amniotic infection diagnosed by amniocentesis in patients with preterm prelabor rupture of membranes (PROM) 184, 185 and those with an asymptomatic short cervix 137 is possible with the administration of intravenous antibiotics to the mother. Success has been documented by demonstrating the absence of microorganisms at the time of a second amniocentesis 137, 184. We believe that the success of this treatment is due to the fact that the infections had been detected early, before the onset of a substantial intra-amniotic inflammatory (see next section). Once microbial invasion of the amniotic cavity leads to an intra-amniotic cytokine storm clinically manifested by preterm labor, parturition is largely irreversible, and eradication of such infection has not been possible with antibiotic treatment.
Figure 10.
Microbial biofilms in the amniotic cavity. (A) Two-dimensional transvaginal ultrasound image showing the presence of “amniotic fluid sludge”. (B) Scanning electron micrograph of a floc of “amniotic fluid sludge” showing the bacterial cells and the exopolymeric matrix material which constitute a biofilm. In the center of the image, cocci are resolved amongst a fibrous mass of matrix material. (C) Confocal laser scanning microscopy displays bacteria (red dots), matrix material (green), and some unstained material which is likely to represent host components trapped by the biofilm. The bar represents 100 microns. Bacteria (red dots) are stained with the EUB338-Cy3probe which reacts with the 16S rRNA (component of bacteria). The matrix material has been stained with wheat germ agglutinin, which reacts with the N-acetylglucosamine of the component of the matrix material that forms the structural framework of the biofilm. Modified from Figure 1,3 and 4 Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, Nhan-Chang CL, Erez O, Kim CJ, Espinoza J, Gonçalves LF, Vaisbuch E, Mazaki-Tovi S, Hassan SS, Costerton JW., Am J Obstet Gynecol. 2008 Jan;198(1):135.e1-5.
7. Inflammatory response to microbial invasion of the amniotic cavity
Microbial invasion of the amniotic cavity induces a robust local inflammatory response, and this is accompanied by a dramatic increase in the concentrations of pro-inflammatory cytokines such as IL-1 31, 32, 34, 106, 186-192, tumor necrosis factor-alpha (TNF-α) 188-190, 193-196, IL-6 12, 34, 94, 129, 188, 197-205, IL-8 (CXCL8) 26, 187-189, 196, 199, 200, 202, 206-211, and CXCL6 212, as well as a cellular response (e.g. increased neutrophil count). Table 4 describes amniotic fluid cytokine/chemokine response to microbial invasion of the amniotic cavity.
Neutrophils express chemokine (C-X-C motif) receptor 2 (CXCR2), which is the receptor for both IL-8 and CXCL6 – potent chemokines for these leukocytes 213-217. The primary cells and tissues responsible for the intra-amniotic inflammatory response include fetal skin, cells comprising the chorioamniotic membranes, and the umbilical cord. The amnion and chorion-decidua respond to bacterial products by increasing the expression of IL-1β 218-220, and TNF-α 221, 222. Amnion cells also synthesize IL-8. 223-225
The temporal relationship between infection or the introduction of inflammatory stimuli (i.e. endotoxin, IL-1, TNFα, IL-6) in the amniotic cavity and the production of cytokines and prostaglandins has been extensively studied using non-human primate models 174, 190, 226-237, sheep 238-245 and other species (rabbits 246-252 and mice 253-261). The works of Gravett and Novy's laboratories, in which maternal blood, amniotic fluid, and fetal blood have been serially sampled, have provided unique information about the relationship between inflammation, prostaglandin production, and myometrial contractility 226, 234. Similar studies have been conducted by the groups of Newham and Jobe using sheep 237-245. Such studies have characterized the complex nature of the fetal immune response after exposure to live bacteria, bacterial products (endotoxin), or inflammatory cytokines (IL-1β) 237-245, 262-265.
The gradient of chemokine concentrations established across the chorioamniotic membranes and the decidua is responsible for diffuse amniotropic infiltration of neutrophils into the chorioamniotic membranes 53. A systematic proteomic analysis of the amniotic fluid in cases of intra-amniotic infection and inflammation reveals dramatic changes in the protein composition, and shows increased availability of matrix-degrading enzymes and other proteins involved in the mechanisms of membrane rupture (i.e. neutrophil elastase) and host defense, such as lactoferrin (an anti-microbial protein), calgranulins, and alarmins such as heat shock protein and S100 proteins 266, 267.
The concentrations of cytokines, matrix-degrading enzymes, and other products released during the course of inflammation have been extensively studied to determine if they have diagnostic and prognostic value in cases of suspected intra-amniotic inflammation/infection. Thus far, amniotic fluid concentrations of MMP-8 268, 269 and IL-6 101, 111, 124, 198, 270-272 appear to be the best predictors of pregnancy outcome and neonatal complications in patients with preterm labor and intact membranes 11, 12, 109, 112, 273, 274, preterm PROM 13, 275, as well as in those undergoing genetic amniocentesis for standard clinical indications 276-282. Originally tested as researched methods, rapid analysis with point-of-care tests to identify intra-amniotic inflammation with cytokines 113, 130, 205, 283, 284 and MMP-8 is now possible 285-293.
Detection of microorganisms has traditionally relied on cultivation methods. However, novel approaches allow identifications of genes and species within 8 hours 11. Increased amniotic fluid IL-6 195, 294, 295 and MMP-8 in 269, 295 patients at risk for preterm delivery is a risk factor for neonatal brain white matter lesions and the subsequent risk of cerebral palsy.
8. Pathogenesis: Chemotactic signals in the amniotic cavity are responsible for chorioamnionitis and funisitis
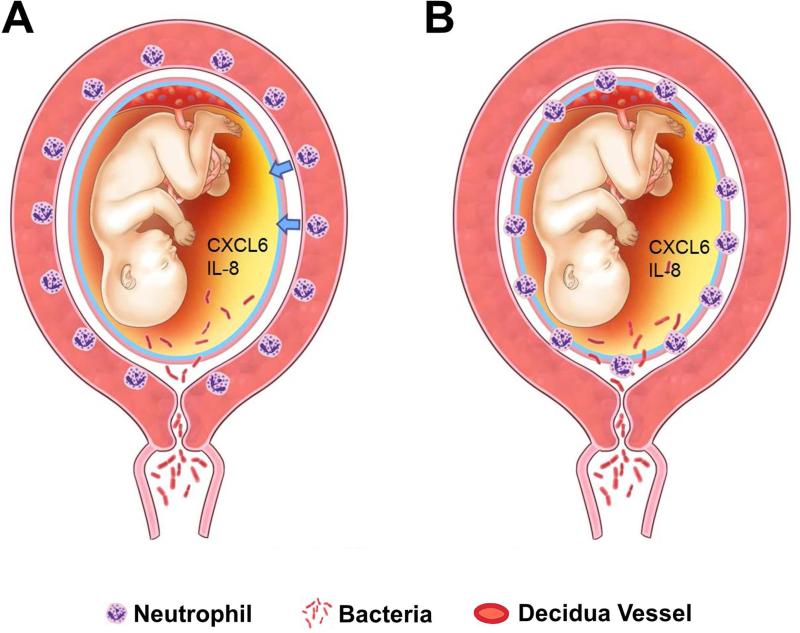
Chemotactic stimuli are required for neutrophils to migrate into tissue (Figure 11) 215, 216. Such stimuli are provided by neutrophil chemokines (e.g. IL-8, also known as neutrophil activating peptide, and CXCL6 – granulocyte chemotactic protein) 215, 216, 296. Intra-amniotic inflammation due to microorganisms or “danger signals” can result in the production of the following chemokines: IL-8 26, 187-189, 196, 199, 200, 202, 206-210, Macrophage inhibitory cytokine 297, 298, MCP 27, 299-302, MCP-2, MCP-3 303, MIP-1α 29, 196, 302, 304, CXCL6 212, CXCL10 281, CXCL13 305, ENA-78 306, RANTES 307 and GRO-α 28, 208. Therefore, amniotic fluid chemokine concentrations are elevated and establish a chemotactic gradient favoring migration of neutrophils. In the absence of microorganisms, danger signals released by cells under stress conditions or cell death can induce intra-amniotic inflammation (“sterile inflammation”)308-319. The diagnosis of this condition is one of exclusion and requires examination of the amniotic fluid with both cultivation and molecular microbiologic techniques11-15.
Figure 11.
Chemotactic stimuli for neutrophil migrate into tissue. (A) With the increase in the amniotic fluid concentrations of chemokines such as CXCL6 and IL-8, CXCR2 positive neutrophils show amniotropic migration (arrows). (B) As a consequence maternal neutrophils show infiltration into the chorioamniotic membranes from the decidual vessels.
9. Acute chorioamnionitis should not be equated with intra-amniotic infection
Acute inflammatory lesions of the placenta have been traditionally considered as reflective of amniotic fluid infection1-10, 149, 320-322. In 1987, Dong et al reported that acute histologic chorioamnionitis was present in 97% (32/33) of patients with intra-amniotic infection, defined as the presence of microorganisms using cultivation techniques323. However, amniotic fluid samples for microbiologic studies in that study were obtained by transcervical collection323. Indeed, acute chorioamnionitis was found in 37% (18/49) of patients with negative amniotic fluid cultures, suggesting that contamination of samples retrieved by a transcervical route is difficult, if not impossible323.
The most rigorous evidence that intra-amniotic infection is associated with acute chorioamnionitis is derived from studies in which a trans-abdominal amniocentesis was performed in patients with preterm labor and intact membranes, and the placenta was examined within 48 hours of the procedure7. Placentas with acute chorioamnionitis and acute funisitis were from mothers who had intra-amniotic infection proven by culture in 71.1% and 78.7% of cases respectively7. The prevalence of microbial invasion of the amniotic cavity was 38%. The negative predictive values of acute chorioamnionitis and funisitis for intra-amniotic infection were 87% and 82%, respectively7.
Recently, we reported a new type of intra-amniotic inflammation termed “sterile inflammation”, which is more frequent than intra-amniotic infection (microbial-associated intra-amniotic inflammation) in patients with preterm labor with intact membranes12, preterm PROM13 and an asymptomatic short cervix14. Interestingly, sterile intra-amniotic inflammation is associated with acute histologic chorioamnionitis (40-60% of cases)11-15. Moreover, acute inflammatory lesions of the placenta are present in a small subset of patients without intraamniotic inflammation in the context of preterm labor11, 13, preterm PROM13, short cervix14, and clinical chorioamnionitis15. Potential explanations are: 1) inflammation of chorioamniotic membranes is a non-specific mechanism of host defense against “danger signals” of non-microbial origin; 2) extra-amniotic infection, which is probably rare; 3) non-viable microorganisms which may release chemotactic factors leading to inflammation7. These organisms may have invaded the amniotic cavity and been cleared by the immune system.
The observation that acute histologic chorioamnionitis is present without demonstrable intra-amniotic infection is now well-established11-15, 324. Roberts et al reported, using both cultivation and molecular microbiologic techniques, that only 4% of patients with acute histologic chorioamnionitis at term have microorganisms in the placenta324. Therefore, acute histologic chorioamnionitis should not be considered synonymous with amniotic fluid infection. The characterization of any biological fluid as “sterile” is dependent on the sensitivity of the assays used to detect microorganisms. Cultivation can be very sensitive, and even one microorganism can grow into a colony under optimal conditions; however, such conditions are rarely provided in clinical laboratories. Molecular microbiologic techniques are considered more sensitive; yet, sufficient microbial DNA must be present for this methodology to provide a positive result. PCR assays with specific primers for a microorganism are considered superior to broad range PCR assays based on conserved regions of the bacterial genome (e.g. 16S gene). The use of deep sequencing can change what is known about the microbiologic landscape of biological fluids. Extreme caution must be used when interpreting the results of sequencing studies, as contamination during metagenomics can occur.
10. The host response to microbial invasion of the amniotic cavity is stronger in preterm than in term gestations
The frequency of microbial invasion of the amniotic cavity is similar in patients with spontaneous labor at term and those with preterm labor and intact membranes who subsequently deliver a preterm neonate (17% vs. 22%, respectively) 24, 93. Yet, preterm neonates born to mothers with microbial invasion of the amniotic cavity have a higher frequency of neonatal sepsis, a systemic inflammatory response (defined as an elevated umbilical cord IL-6 concentration), and funisitis than those born to mothers at term with microbial invasion of the amniotic cavity. Why? Microbial invasion of the amniotic cavity in women in spontaneous labor at term is of shorter duration and can occur after the initiation of parturition 201. For example, bacteria can be introduced when the chorioamniotic membranes are exposed to the vaginal microbiota during the course of digital examinations performed during labor to determine cervical dilatation and effacement. Such microbial invasion typically has a low inoculum size which elicits a mild intra-amniotic inflammatory response and rarely leads to fetal microbial invasion (hence, the low frequency of funisitis and neonatal sepsis).
On the other hand, in preterm labor with intact membranes or preterm PROM, microbial invasion is established before the initiation of preterm labor. Such infections have a higher microbial burden than those observed in most women in spontaneous labor at term, have probably lasted longer, and therefore, result in a more intensive intra-amniotic inflammatory response 201.Given the longer duration of infection, the likelihood of a fetal attack is higher, and thus, not surprisingly, the rate of congenital neonatal sepsis is greater in preterm than in term neonates (2.27-5.14/1000 in preterm neonates versus 0.04-0.89/1000 term neonates) 325.
11. The Fetal Inflammatory Response Syndrome (FIRS)
Microbial invasion of the amniotic cavity can progress to fetal invasion. The ports of entry for bacteria into the fetus include the respiratory tract (fetal breathing), gastrointestinal tract (swallowing), skin, and ear. Amniotic fluid fills the external auditory canal and bacteria can invade the tympanic membrane and middle ear. Similarly, depending upon the gestational age, microorganisms may gain access to the conjunctiva.
Once microorganisms gain access to the fetal mucosa, they are recognized by pattern recognition receptors such as Toll-like receptors (TLRs), and ligation of such receptors can induce the production of transcription factors such as NFκB and elicit a localized (and subsequently systemic) inflammatory response 326. For example, fetuses exposed to bacteria can develop severe dermatitis or pneumonitis. Subsequently, microorganisms reaching the fetal circulation could lead to a systemic inflammatory response.
The frequency with which microorganisms invade the human fetus is difficult to ascertain; however, studies in which amniocentesis and cordocentesis have been performed in patients with preterm PROM indicate that 30% of patients with microbial invasion of the amniotic cavity have positive fetal blood cultures for microorganisms (i.e. bacteremia) 327, 328. Similar findings have been reported when cultures for genital mycoplasmas have been performed in umbilical cord blood at the time of birth 144, 329. Therefore, the frequency of congenital microbial invasion of the fetus is likely to be higher than that reported in the pediatric literature – the reasons for this are multiple (e.g. bacteremia may not be continuous in the neonatal period; the inoculum size may be small, leading to a high rate of negative blood cultures; and the lack of detection of the most common microorganisms, genital mycoplasmas, may reflect that cultures for these organisms require special media, and such cultures are not routinely performed in neonatal intensive care units) 330-332.
We have defined a fetal systemic inflammatory response syndrome (FIRS) using the fetal plasma concentrations of IL-6 16, 327, 333-343. This cytokine is a major mediator of the acute phase response, and its concentration can be readily determined using immunoassays. It is noteworthy that the systemic inflammatory response syndrome (SIRS, in adults) was originally defined using clinical criteria such as fever, tachycardia, respiratory rate, and white blood cell count 344-346. However, this definition cannot be used in the human fetus, because the vital signs (with the exception of heart rate) cannot readily be determined before birth or during the intrapartum period 347. Our definition of FIRS was based on the concentration of fetal plasma IL-6 associated with adverse outcome (in samples obtained by cordocentesis) 327, and was introduced in 1997 348. Subsequently, in 2001, the American College of Chest Physicians and the Society of Critical Care Medicine noted that an elevated plasma concentration of IL-6 was associated with the likelihood of SIRS, and proposed that the concentrations of this cytokine may be useful in its diagnosis 349.
Despite the similarities between FIRS and SIRS, the unique circumstances of the patient 330 and its environment (uterus) pose challenges which are sui generis for the diagnosis, management, and treatment of FIRS 56, 143, 350, 351. Importantly, FIRS and SIRS share an important feature – both can be caused by non-microbial-related insults. Although the consequences of microbial invasion/proliferation in adult and neonatal medicine are well-known, and the evolution of FIRS/SIRS to sepsis, septic shock and death has been well-characterized, SIRS can occur in cases of sterile inflammation (e.g. pancreatitis or burns) 346, 352. Since our original report of FIRS, we identified that some cases of this syndrome are observed without demonstrable microbial invasion of the amniotic cavity, and have proposed that such cases represent the response to danger signals which cause cellular stress in the amniotic cavity and fetus 11-13. The precise nature of the danger signals in sterile intra-amniotic inflammation and corresponding cases of FIRS has not been elucidated; yet, we have proposed that this may result from insults that trigger cell death (necrosis, pyroptosis, etc.) 308, 310, 311, 314, 316, 318.
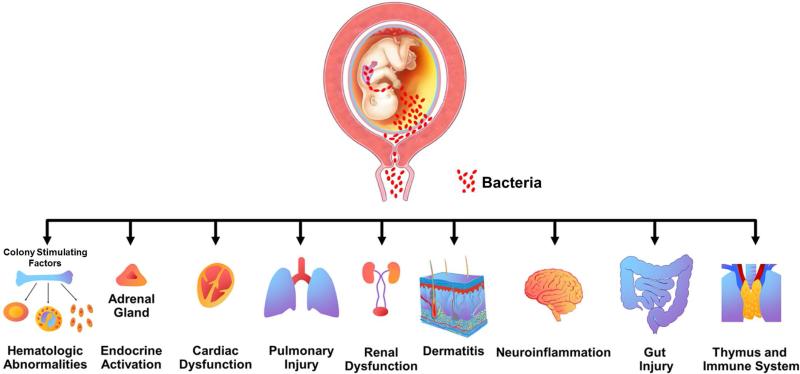
The presence of FIRS was originally described in fetuses presenting with preterm labor and preterm PROM 327, and was associated with three major consequences: 1) a shorter interval-to-delivery 327; 2) higher neonatal morbidity after adjustment for gestational age at birth 327; and 3) multi- organ involvement 351, including hematopoietic system 336, 338, 339, 353, immune system 336, 353-356 thymus 357-361, heart 362, adrenal glands (e.g. alteration in cortisol) 363, skin 335, lung 188, 333, brain 195, 294, 364-366, kidney 367 and gut 46, 368, 369 (Figure 12). Although these observations were originally made in humans, subsequent experimental studies in non-human primates as well as sheep have demonstrated the involvement of multiple organ systems when the fetus is exposed to inflammatory stimuli 242. A full description of fetal immune response to chorioamnionitis/intra-amniotic infection in animal model is available in a review by Kallapur and Jobe et al 242.
Figure 12.
Fetal target organs during the fetal inflammatory response syndrome. Modified from Figure 2 in Gotsch F, Romero R, Kusanovic JP et al, The fetal inflammatory response syndrome, Clinical Obstetrics and Gynecology; 50: 2007: 652-683
12. Conclusions
Acute chorioamnionitis, acute funisitis and chorionic vasculitis are acute inflammatory lesions with important short- and long-term clinical significance. Substantial progress has been made in the understanding of the mechanisms responsible for maternal and fetal inflammation in the context of infection. The causes of sterile intra-amniotic inflammation are unknown, and represent important clinical and scientific challenges.
Table 3.
Microorganisms detected in the amniotic fluid of patients with spontaneous preterm labor with intact membranes and patients with clinical chorioamnionitis at term using cultivation and molecular microbiologic technique
| Patients with spontaneous preterm labor with intact membranes 110 | Patients with clinical chorioamnionitis at term 15 1515 |
|---|---|
| Fusobacterium nucleatum | Ureaplasma species |
| Sneathia sanguinegens | Gardnerella vaginalis |
| Ureaplasma species | Mycoplasma hominis |
| Streptococcus mitis | Streptococcus agalactiae |
| Gardnerella vaginalis | Lactobacillus species |
| Peptostreptococcus species | Bacteroides species |
| Leptotrichia amnionii | Acinetobacter species |
| Mycoplasma hominis | Sneathia |
| Streptococcus agalactiae | Streptococcus viridans |
| Lactobacillus species | Porphyromonas species |
| Bacillus species | Veillonella species |
| Coagulase-negative Staphylococcus species | Peptostreptococcus species |
| Prevotella species | Escherichia coli |
| Others: Uncultivated Bacteroidetes, Delftia acidovorans, Neisseria cinerea | Pseudomonas aeruginosa |
| Staphylococcus aureus | |
| Eubacterium species | |
| Gram (–) bacilli | |
| Enterococcus species | |
| Others: Fusobacterium species, Candida species, Abiotrophia defective, Micrococcus luteus, Staphylococcus epidermidis, Firmicute, Propionibacterium acnes | |
Acknowledgement
This work was supported, in part, by the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blanc WA. Amniotic infection syndrome; pathogenesis, morphology, and significance in circumnatal mortality. Clin Obstet Gynecol. 1959;2:705–34. [PubMed] [Google Scholar]
- 2.Russell P. Inflammatory lesions of the human placenta: Clinical significance of acute chorioamnionitis. Am J Diagn Gynecol Obstet. 1979;2:127–37. [Google Scholar]
- 3.Blanc WA. Pathology of the placenta and cord in ascending and in haematogenous infection. Ciba Found Symp. 1979;(77):17–38. doi: 10.1002/9780470720608.ch3. [DOI] [PubMed] [Google Scholar]
- 4.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319(15):972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 5.Benirschke K, Burton GJ, Baergen RN. Pathology of the Human Placenta. Sixth ed. Springer; Berlin Heidelberg: 2012. Infectious Diseases. pp. 557–656. [Google Scholar]
- 6.Fox H, Sebire NJ. Pathology of the Placenta. Third ed. ELSEVIER; China: 2007. Infections and Inflammatory Lesions of the Placenta. pp. 303–54. [Google Scholar]
- 7.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166(5):1382–8. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 8.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165(4 Pt 1):955–61. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 9.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435–48. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 10.Redline RW. Placental inflammation. Semin Neonatol. 2004;9(4):265–74. doi: 10.1016/j.siny.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71(4):330–58. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–74. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014:1–16. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17. doi: 10.3109/14767058.2014.954243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43(1):19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11(1):18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 17.Lee SM, Park JW, Kim BJ, Park CW, Park JS, Jun JK, et al. Acute histologic chorioamnionitis is a risk factor for adverse neonatal outcome in late preterm birth after preterm premature rupture of membranes. PLoS One. 2013;8(12):e79941. doi: 10.1371/journal.pone.0079941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SM, Romero R, Park JW, Oh KJ, Jun JK, Yoon BH. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med. 2014:1–10. doi: 10.3109/14767058.2014.961009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Hoeven KH, Anyaegbunam A, Hochster H, Whitty JE, Distant J, Crawford C, et al. Clinical significance of increasing histologic severity of acute inflammation in the fetal membranes and umbilical cord. Pediatr Pathol Lab Med. 1996;16(5):731–44. [PubMed] [Google Scholar]
- 20.Srinivas SK, Ernst LM, Edlow AG, Elovitz MA. Can placental pathology explain second-trimester pregnancy loss and subsequent pregnancy outcomes? Am J Obstet Gynecol. 2008;199(4):402, e1–5. doi: 10.1016/j.ajog.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Seong HS, Lee SE, Kang JH, Romero R, Yoon BH. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol. 2008;199(4):375 e1–5. doi: 10.1016/j.ajog.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HS, Romero R, Lee SM, Park CW, Jun JK, Yoon BH. Histologic chorioamnionitis is more common after spontaneous labor than after induced labor at term. Placenta. 2010;31(9):792–5. doi: 10.1016/j.placenta.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SM, Lee KA, Kim SM, Park CW, Yoon BH. The risk of intra-amniotic infection, inflammation and histologic chorioamnionitis in term pregnant women with intact membranes and labor. Placenta. 2011;32(7):516–21. doi: 10.1016/j.placenta.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38(7):543–8. [PubMed] [Google Scholar]
- 25.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195(2):394 e1–24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165(4 Pt 1):813–20. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 27.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med. 2003;14(1):51–6. doi: 10.1080/jmf.14.1.51.56. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J, Ghezzi F, Romero R, Ghidini A, Mazor M, Tolosa JE, et al. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol. 1996;35(1):23–9. doi: 10.1111/j.1600-0897.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 29.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstet Gynecol. 1996;87(1):94–8. doi: 10.1016/0029-7844(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35(3):235–8. [PubMed] [Google Scholar]
- 31.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160(5 Pt 1):1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27(3-4):117–23. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 33.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32(3):200–10. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 34.Cox SM, Casey ML, Macdonald PC. Accumulation of interleukin-1beta and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Hum Reprod Update. 1997;3(5):517–27. doi: 10.1093/humupd/3.5.517. [DOI] [PubMed] [Google Scholar]
- 35.Mossman HW. Classics revisited: Comparative morphogenesis of the fetal membranes and accessory uterine structures. Placenta. 1991;12(1):1–5. doi: 10.1016/0143-4004(91)90504-9. [DOI] [PubMed] [Google Scholar]
- 36.Mcnamara MF, Wallis T, Qureshi F, Jacques SM, Gonik B. Determining the maternal and fetal cellular immunologic contributions in preterm deliveries with clinical or subclinical chorioamnionitis. Infect Dis Obstet Gynecol. 1997;5(4):273–9. doi: 10.1155/S1064744997000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steel JH, O'donoghue K, Kennea NL, Sullivan MH, Edwards AD. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta. 2005;26(8-9):672–7. doi: 10.1016/j.placenta.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Lee SD, Kim MR, Hwang PG, Shim SS, Yoon BH, Kim CJ. Chorionic plate vessels as an origin of amniotic fluid neutrophils. Pathol Int. 2004;54(7):516–22. doi: 10.1111/j.1440-1827.2004.01659.x. [DOI] [PubMed] [Google Scholar]
- 39.Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol. 1997;176(1 Pt 1):77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 40.Kim CJ, Yoon BH, Kim M, Park JO, Cho SY, Chi JG. Histo-topographic distribution of acute inflammation of the human umbilical cord. Pathol Int. 2001;51(11):861–5. doi: 10.1046/j.1440-1827.2001.01284.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim CJ, Yoon BH, Romero R, Moon JB, Kim M, Park SS, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol. 2001;185(2):496–500. doi: 10.1067/mob.2001.116689. [DOI] [PubMed] [Google Scholar]
- 42.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73(3 Pt 1):383–9. [PubMed] [Google Scholar]
- 43.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172(3):960–70. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 44.Miyano A, Miyamichi T, Nakayama M, Kitajima H, Shimizu A. Differences among acute, subacute, and chronic chorioamnionitis based on levels of inflammation-associated proteins in cord blood. Pediatr Dev Pathol. 1998;1(6):513–21. doi: 10.1007/s100249900070. [DOI] [PubMed] [Google Scholar]
- 45.Ohyama M, Itani Y, Yamanaka M, Goto A, Kato K, Ijiri R, et al. Re-evaluation of chorioamnionitis and funisitis with a special reference to subacute chorioamnionitis. Hum Pathol. 2002;33(2):183–90. doi: 10.1053/hupa.2002.31291. [DOI] [PubMed] [Google Scholar]
- 46.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–8. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 47.Torricelli M, Voltolini C, Toti P, Vellucci FL, Conti N, Cannoni A, et al. Histologic chorioamnionitis: different histologic features at different gestational ages. J Matern Fetal Neonatal Med. 2014;27(9):910–3. doi: 10.3109/14767058.2013.846313. [DOI] [PubMed] [Google Scholar]
- 48.Park CW, Yoon BH, Kim SM, Park JS, Jun JK. Which is more important for the intensity of intra-amniotic inflammation between total grade or involved anatomical region in preterm gestations with acute histologic chorioamnionitis? Obstet Gynecol Sci. 2013;56(4):227–33. doi: 10.5468/ogs.2013.56.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park CW, Moon KC, Park JS, Jun JK, Romero R, Yoon BH. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications. Placenta. 2009;30(1):56–61. doi: 10.1016/j.placenta.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MS, Romero R, Park JW, Oh KJ, Jun JK, Yoon BH. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J matern Fetal Neonatal Med (Accepted) 2014 doi: 10.3109/14767058.2014.961009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris JW, Brown H. Bacterial content of the uterus at cesarean section. Am J Obstet Gynecol. 1927;(13):133. [Google Scholar]
- 52.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31(3):553–84. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 55.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8(1):3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 56.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benirschke K. Routes and types of infection in the fetus and the newborn. AMA J Dis Child. 1960;99:714–21. doi: 10.1001/archpedi.1960.02070030716003. [DOI] [PubMed] [Google Scholar]
- 58.Naeye RL, Dellinger WS, Blanc WA. Fetal and maternal features of antenatal bacterial infections. J Pediatr. 1971;79(5):733–9. doi: 10.1016/s0022-3476(71)80383-9. [DOI] [PubMed] [Google Scholar]
- 59.Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: A clinical review. Obstet Gynecol. 1973;42(1):112–7. [PubMed] [Google Scholar]
- 60.Benedetti TJ, Valle R, Ledger WJ. Antepartum pneumonia in pregnancy. Am J Obstet Gynecol. 1982;144(4):413–7. doi: 10.1016/0002-9378(82)90246-0. [DOI] [PubMed] [Google Scholar]
- 61.Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun. 1999;67(11):5958–66. doi: 10.1128/iai.67.11.5958-5966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romero R, Jeanty P, Hobbins JC. Invasive techniques for antenatal diagnosis: Chorion villous biopsy, fetoscopy and amniocentesis in prenatal diagnosis. Semin Ultra-sound. 1984;(5):3. [Google Scholar]
- 63.Fray RE, Davis TP, Brown EA. Clostridium welchii infection after amniocentesis. Br Med J (Clin Res Ed) 1984;288(6421):901–2. doi: 10.1136/bmj.288.6421.901-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romero R, Jeanty P, Reece EA, Grannum P, Bracken M, Berkowitz R, et al. Sonographically monitored amniocentesis to decrease intraoperative complications. Obstet Gynecol. 1985;65(3):426–30. [PubMed] [Google Scholar]
- 65.Romero R, Hobbins JC, Mahoney MJ. Fetal blood sampling and fetoscopy. In: Aubrey Milunsky, ed. Genetic Disorders of the Fetus. Plenum Publishing. 1986:571. [Google Scholar]
- 66.Mccolgin SW, Hess LW, Martin RW, Martin JN, Jr., Morrison JC. Group B streptococcal sepsis and death in utero following funipuncture. Obstet Gynecol. 1989;74(3 Pt 2):464–5. [PubMed] [Google Scholar]
- 67.Hamoda H, Chamberlain PF. Clostridium welchii infection following amniocentesis: a case report and review of the literature. Prenat Diagn. 2002;22(9):783–5. doi: 10.1002/pd.409. [DOI] [PubMed] [Google Scholar]
- 68.Li Kim Mui SV, Chitrit Y, Boulanger MC, Maisonneuve L, Choudat L, De Bievre P. Sepsis due to Clostridium perfringens after pregnancy termination with feticide by cordocentesis: a case report. Fetal Diagn Ther. 2002;17(2):124–6. doi: 10.1159/000048022. [DOI] [PubMed] [Google Scholar]
- 69.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001;185(3):586–92. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 70.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187(1):137–44. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 71.Habte HH, De Beer C, Lotz ZE, Tyler MG, Schoeman L, Kahn D, et al. The inhibition of the Human Immunodeficiency Virus type 1 activity by crude and purified human pregnancy plug mucus and mucins in an inhibition assay. Virol J. 2008;5:59. doi: 10.1186/1743-422X-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Becher N, Adams Waldorf K, Hein M, Uldbjerg N. The cervical mucus plug: structured review of the literature. Acta Obstet Gynecol Scand. 2009;88(5):502–13. doi: 10.1080/00016340902852898. [DOI] [PubMed] [Google Scholar]
- 73.Becher N, Hein M, Danielsen CC, Uldbjerg N. Matrix metalloproteinases in the cervical mucus plug in relation to gestational age, plug compartment, and preterm labor. Reprod Biol Endocrinol. 2010;8:113. doi: 10.1186/1477-7827-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee DC, Hassan SS, Romero R, Tarca AL, Bhatti G, Gervasi MT, et al. Protein profiling underscores immunological functions of uterine cervical mucus plug in human pregnancy. J Proteomics. 2011;74(6):817–28. doi: 10.1016/j.jprot.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hansen LK, Becher N, Bastholm S, Glavind J, Ramsing M, Kim CJ, et al. The cervical mucus plug inhibits, but does not block, the passage of ascending bacteria from the vagina during pregnancy. Acta Obstet Gynecol Scand. 2014;93(1):102–8. doi: 10.1111/aogs.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril. 2004;82(4):799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 77.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194(3):630–7. doi: 10.1016/j.ajog.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 78.Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol. 2015;212(5):611, e1–9. doi: 10.1016/j.ajog.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rivera-Alsina ME, Saldana LR, Kohl S, Arias JW. Listeria monocytogenes. An important pathogen in premature labor and intrauterine fetal sepsis. J Reprod Med. 1983;28(3):212–4. [PubMed] [Google Scholar]
- 80.Romero R, Winn HN, Wan M, Hobbins JC. Listeria monocytogenes chorioamnionitis and preterm labor. Am J Perinatol. 1988;5(3):286–8. doi: 10.1055/s-2007-999705. [DOI] [PubMed] [Google Scholar]
- 81.Mazor M, Froimovich M, Lazer S, Maymon E, Glezerman M. Listeria monocytogenes. The role of transabdominal amniocentesis in febrile patients with preterm labor. Arch Gynecol Obstet. 1992;252(2):109–12. doi: 10.1007/BF02389637. [DOI] [PubMed] [Google Scholar]
- 82.Offenbacher S, Lieff S, Boggess KA, Murtha AP, Madianos PN, Champagne CM, et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001;6(1):164–74. doi: 10.1902/annals.2001.6.1.164. [DOI] [PubMed] [Google Scholar]
- 83.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG. 2002;109(5):527–33. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 84.Offenbacher S. Maternal periodontal infections, prematurity, and growth restriction. Clin Obstet Gynecol. 2004;47(4):808–21. doi: 10.1097/01.grf.0000141894.85221.f7. discussion 81-2. [DOI] [PubMed] [Google Scholar]
- 85.Boggess KA, Moss K, Madianos P, Murtha AP, Beck J, Offenbacher S. Fetal immune response to oral pathogens and risk of preterm birth. Am J Obstet Gynecol. 2005;193(3 Pt 2):1121–6. doi: 10.1016/j.ajog.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 86.Boggess KA, Madianos PN, Preisser JS, Moise KJ, Jr., Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol. 2005;192(2):554–7. doi: 10.1016/j.ajog.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Newnham JP, Shub A, Jobe AH, Bird PS, Ikegami M, Nitsos I, et al. The effects of intra-amniotic injection of periodontopathic lipopolysaccharides in sheep. Am J Obstet Gynecol. 2005;193(2):313–21. doi: 10.1016/j.ajog.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 88.Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, et al. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007;78(7):1249–55. doi: 10.1902/jop.2007.060368. [DOI] [PubMed] [Google Scholar]
- 89.Hameed C, Tejani N, Verma UL, Archbald F. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am J Obstet Gynecol. 1984;149(7):726–30. doi: 10.1016/0002-9378(84)90111-x. [DOI] [PubMed] [Google Scholar]
- 90.Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986;67(2):229–37. doi: 10.1097/00006250-198602000-00013. [DOI] [PubMed] [Google Scholar]
- 91.Leigh J, Garite TJ. Amniocentesis and the management of premature labor. Obstet Gynecol. 1986;67(4):500–6. [PubMed] [Google Scholar]
- 92.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159(1):114–9. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 93.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161(3):817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 94.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85(5):1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163(3):968–74. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 96.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165(4 Pt 1):821–30. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 97.Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol. 1991;165(4 Pt 1):1105–10. doi: 10.1016/0002-9378(91)90480-f. [DOI] [PubMed] [Google Scholar]
- 98.Coultrip LL, Grossman JH. Evaluation of rapid diagnostic tests in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1992;167(5):1231–42. doi: 10.1016/s0002-9378(11)91694-9. [DOI] [PubMed] [Google Scholar]
- 99.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79(3):351–7. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 100.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169(4):805–16. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 101.Coultrip LL, Lien JM, Gomez R, Kapernick P, Khoury A, Grossman JH. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1994;171(4):901–11. doi: 10.1016/s0002-9378(94)70057-5. [DOI] [PubMed] [Google Scholar]
- 102.Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol. 1996;87(2):231–7. doi: 10.1016/0029-7844(95)00380-0. [DOI] [PubMed] [Google Scholar]
- 103.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92(1):77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 104.Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, Curet LB. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol. 1998;179(1):172–8. doi: 10.1016/s0002-9378(98)70269-8. [DOI] [PubMed] [Google Scholar]
- 105.Oyarzun E, Gomez R, Rioseco A, Gonzalez P, Gutierrez P, Donoso E, et al. Antibiotic treatment in preterm labor and intact membranes: a randomized, double-blinded, placebo-controlled trial. J Matern Fetal Med. 1998;7(3):105–10. doi: 10.1002/(SICI)1520-6661(199805/06)7:3<105::AID-MFM1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 106.Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, Gasser I, Bermejo B, Cabero L. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med. 1999;8(4):155–8. doi: 10.1002/(SICI)1520-6661(199907/08)8:4<155::AID-MFM3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 107.Locksmith GJ, Clark P, Duff P, Schultz GS. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet Gynecol. 1999;94(1):1–6. doi: 10.1016/s0029-7844(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 108.Ovalle A, Martinez MA, Gomez R, Saez J, Menares I, Aspillaga C, et al. [Premature labor with intact membranes: microbiology of the amniotic fluid and lower genital tract and its relation with maternal and neonatal outcome]. Rev Med Chil. 2000;128(9):985–95. [PubMed] [Google Scholar]
- 109.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185(5):1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 110.Digiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romero R, Kadar N, Miranda J, Korzeniewski SJ, Schwartz AG, Chaemsaithong P, et al. The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med. 2014;27(8):757–69. doi: 10.3109/14767058.2013.844123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125, e1–e15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 113.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med. 2015:1–11. doi: 10.3109/14767058.2015.1006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Combs CA, Garite TJ, Lapidus JA, Lapointe JP, Gravett M, Rael J, et al. Detection of microbial invasion of the amniotic cavity by analysis of cervicovaginal proteins in women with preterm labor and intact membranes. Am J Obstet Gynecol. 2015;212(4):482, e1–e12. doi: 10.1016/j.ajog.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 115.Garite TJ, Freeman RK, Linzey EM, Braly P. The use of amniocentesis in patients with premature rupture of membranes. Obstet Gynecol. 1979;54(2):226–30. [PubMed] [Google Scholar]
- 116.Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol. 1982;59(5):539–45. [PubMed] [Google Scholar]
- 117.Cotton DB, Hill LM, Strassner HT, Platt LD, Ledger WJ. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol. 1984;63(1):38–43. [PubMed] [Google Scholar]
- 118.Zlatnik FJ, Cruikshank DP, Petzold CR, Galask RP. Amniocentesis in the identification of inapparent infection in preterm patients with premature rupture of the membranes. J Reprod Med. 1984;29(9):656–60. [PubMed] [Google Scholar]
- 119.Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66(3):316–21. [PubMed] [Google Scholar]
- 120.Feinstein SJ, Vintzileos AM, Lodeiro JG, Campbell WA, Weinbaum PJ, Nochimson DJ. Amniocentesis with premature rupture of membranes. Obstet Gynecol. 1986;68(2):147–52. [PubMed] [Google Scholar]
- 121.Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ, Escoto DT, Mirochnick MH. Qualitative amniotic fluid volume versus amniocentesis in predicting infection in preterm premature rupture of the membranes. Obstet Gynecol. 1986;67(4):579–83. [PubMed] [Google Scholar]
- 122.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159(3):661–6. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 123.Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol. 1992;167(4 Pt 1):1092–5. doi: 10.1016/s0002-9378(12)80044-5. [DOI] [PubMed] [Google Scholar]
- 124.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169(4):839–51. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 125.Averbuch B, Mazor M, Shoham-Vardi I, Chaim W, Vardi H, Horowitz S, et al. Intrauterine infection in women with preterm premature rupture of membranes: maternal and neonatal characteristics. Eur J Obstet Gynecol Reprod Biol. 1995;62(1):25–9. doi: 10.1016/0301-2115(95)02176-8. [DOI] [PubMed] [Google Scholar]
- 126.Carroll SG, Papaioannou S, Ntumazah IL, Philpott-Howard J, Nicolaides KH. Lower genital tract swabs in the prediction of intrauterine infection in preterm prelabour rupture of the membranes. Br J Obstet Gynaecol. 1996;103(1):54–9. doi: 10.1111/j.1471-0528.1996.tb09515.x. [DOI] [PubMed] [Google Scholar]
- 127.Digiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64(1):38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kacerovsky M, Musilova I, Khatibi A, Skogstrand K, Hougaard DM, Tambor V, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2012;25(10):2014–9. doi: 10.3109/14767058.2012.671873. [DOI] [PubMed] [Google Scholar]
- 129.Kacerovsky M, Musilova I, Andrys C, Hornychova H, Pliskova L, Kostal M, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. 2014;210(4):325, e1–e10. doi: 10.1016/j.ajog.2013.10.882. [DOI] [PubMed] [Google Scholar]
- 130.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2015:1–8. doi: 10.3109/14767058.2015.1006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167(4 Pt 1):1086–91. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 132.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95(5):652–5. doi: 10.1016/s0029-7844(99)00633-x. [DOI] [PubMed] [Google Scholar]
- 133.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198(6):633, e1–8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 134.Bujold E, Morency AM, Rallu F, Ferland S, Tetu A, Duperron L, et al. Bacteriology of amniotic fluid in women with suspected cervical insufficiency. J Obstet Gynaecol Can. 2008;30(10):882–7. doi: 10.1016/S1701-2163(16)32967-X. [DOI] [PubMed] [Google Scholar]
- 135.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38(3):261–8. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gomez R, Romero R, Nien JK, Chaiworapongsa T, Medina L, Kim YM, et al. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2005;192(3):678–89. doi: 10.1016/j.ajog.2004.10.624. [DOI] [PubMed] [Google Scholar]
- 137.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34(1):13–9. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202(5):433, e1–8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18(1):31–7. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 140.Madan I, Romero R, Kusanovic JP, Mittal P, Chaiworapongsa T, Dong Z, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38(3):275–9. doi: 10.1515/JPM.2010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Galask RP, Varner MW, Petzold CR, Wilbur SL. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol. 1984;148(7):915–28. doi: 10.1016/0002-9378(84)90534-9. [DOI] [PubMed] [Google Scholar]
- 142.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 143.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Romero R, Garite TJ. Twenty percent of very preterm neonates (23-32 weeks of gestation) are born with bacteremia caused by genital Mycoplasmas. Am J Obstet Gynecol. 2008;198(1):1–3. doi: 10.1016/j.ajog.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 145.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203(3):211, e1–8. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Digiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. 2012;17(1):2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 147.Allen-Daniels MJ, Serrano MG, Pflugner LP, Fettweis JM, Prestosa MA, Koparde VN, et al. Identification of a gene in Mycoplasma hominis associated with preterm birth and microbial burden in intraamniotic infection. Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gravett MG, Eschenbach DA. Possible role of Ureaplasma urealyticum in preterm premature rupture of the fetal membranes. Pediatr Infect Dis. 1986;5(6 Suppl):S253–7. doi: 10.1097/00006454-198611010-00010. [DOI] [PubMed] [Google Scholar]
- 149.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179(5):1254–60. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 150.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183(5):1130–7. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 151.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189(4):919–24. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 152.Kim M, Kim G, Romero R, Shim SS, Kim EC, Yoon BH. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med. 2003;31(2):146–52. doi: 10.1515/JPM.2003.020. [DOI] [PubMed] [Google Scholar]
- 153.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187(3):518–21. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 154.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191(4):1382–6. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 155.Jacobsson B, Aaltonen R, Rantakokko-Jalava K, Morken NH, Alanen A. Quantification of Ureaplasma urealyticum DNA in the amniotic fluid from patients in PTL and pPROM and its relation to inflammatory cytokine levels. Acta Obstet Gynecol Scand. 2009;88(1):63–70. doi: 10.1080/00016340802572646. [DOI] [PubMed] [Google Scholar]
- 156.Lewis JF, Johnson P, Miller P. Evaluation of amniotic fluid for aerobic and anaerobic bacteria. Am J Clin Pathol. 1976;65(1):58–63. doi: 10.1093/ajcp/65.1.58. [DOI] [PubMed] [Google Scholar]
- 157.Martius J, Eschenbach DA. The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity--a review. Arch Gynecol Obstet. 1990;247(1):1–13. doi: 10.1007/BF02390649. [DOI] [PubMed] [Google Scholar]
- 158.Hillier SL, Krohn MA, Cassen E, Easterling TR, Rabe LK, Eschenbach DA. The role of bacterial vaginosis and vaginal bacteria in amniotic fluid infection in women in preterm labor with intact fetal membranes. Clin Infect Dis. 1995;20(Suppl 2):S276–8. doi: 10.1093/clinids/20.supplement_2.s276. [DOI] [PubMed] [Google Scholar]
- 159.Romero R, Reece EA, Duff GW, Coultrip L, Hobbins JC. Prenatal diagnosis of Candida albicans chorioamnionitis. Am J Perinatol. 1985;2(2):121–2. doi: 10.1055/s-2007-999928. [DOI] [PubMed] [Google Scholar]
- 160.Bruner JP, Elliott JP, Kilbride HW, Garite TJ, Knox GE. Candida chorioamnionitis diagnosed by amniocentesis with subsequent fetal infection. Am J Perinatol. 1986;3(3):213–8. doi: 10.1055/s-2007-999870. [DOI] [PubMed] [Google Scholar]
- 161.Smith CV, Horenstein J, Platt LD. Intraamniotic infection with Candida albicans associated with a retained intrauterine contraceptive device: a case report. Am J Obstet Gynecol. 1988;159(1):123–4. doi: 10.1016/0002-9378(88)90505-4. [DOI] [PubMed] [Google Scholar]
- 162.Chaim W, Mazor M, Wiznitzer A. The prevalence and clinical significance of intraamniotic infection with Candida species in women with preterm labor. Arch Gynecol Obstet. 1992;251(1):9–15. doi: 10.1007/BF02718273. [DOI] [PubMed] [Google Scholar]
- 163.Chaim W, Mazor M. Pregnancy with an intrauterine device in situ and preterm delivery. Arch Gynecol Obstet. 1992;252(1):21–4. doi: 10.1007/BF02389602. [DOI] [PubMed] [Google Scholar]
- 164.Berry DL, Olson GL, Wen TS, Belfort MA, Moise KJ., Jr Candida chorioamnionitis: a report of two cases. J Matern Fetal Med. 1997;6(3):151–4. doi: 10.1002/(SICI)1520-6661(199705/06)6:3<151::AID-MFM6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 165.Qureshi F, Jacques SM, Bendon RW, Faye-Peterson OM, Heifetz SA, Redline R, et al. Candida funisitis: A clinicopathologic study of 32 cases. Pediatr Dev Pathol. 1998;1(2):118–24. doi: 10.1007/s100249900014. [DOI] [PubMed] [Google Scholar]
- 166.Barth T, Broscheit J, Bussen S, Dietl J. Maternal sepsis and intrauterine fetal death resulting from Candida tropicalis chorioamnionitis in a woman with a retained intrauterine contraceptive device. Acta Obstet Gynecol Scand. 2002;81(10):981–2. doi: 10.1034/j.1600-0412.2002.811014.x. [DOI] [PubMed] [Google Scholar]
- 167.Crawford JT, Pereira L, Buckmaster J, Gravett MG, Tolosa JE. Amniocentesis results and novel proteomic analysis in a case of occult candidal chorioamnionitis. J Matern Fetal Neonatal Med. 2006;19(10):667–70. doi: 10.1080/14767050600738289. [DOI] [PubMed] [Google Scholar]
- 168.Kim SK, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinat Med. 2010;38(1):45–53. doi: 10.1515/JPM.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jalava J, Mantymaa ML, Ekblad U, Toivanen P, Skurnik M, Lassila O, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103(7):664–9. doi: 10.1111/j.1471-0528.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 170.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr Rev. 2002;60(5 Pt 2):S19–25. doi: 10.1301/00296640260130696. [DOI] [PubMed] [Google Scholar]
- 171.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57(3):404–11. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 172.Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest. 2009;89(8):924–36. doi: 10.1038/labinvest.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173(2):606–12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 174.Grigsby PL, Novy MJ, Adams Waldorf KM, Sadowsky DW, Gravett MG. Choriodecidual inflammation: a harbinger of the preterm labor syndrome. Reprod Sci. 2010;17(1):85–94. doi: 10.1177/1933719109348025. [DOI] [PubMed] [Google Scholar]
- 175.Espinoza J, Goncalves LF, Romero R, Nien JK, Stites S, Kim YM, et al. The prevalence and clinical significance of amniotic fluid ‘sludge’ in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25(4):346–52. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 176.Bujold E, Pasquier JC, Simoneau J, Arpin MH, Duperron L, Morency AM, et al. Intra-amniotic sludge, short cervix, and risk of preterm delivery. J Obstet Gynaecol Can. 2006;28(3):198–202. doi: 10.1016/S1701-2163(16)32108-9. [DOI] [PubMed] [Google Scholar]
- 177.Romero R, Kusanovic JP, Espinoza J, Gotsch F, Nhan-Chang CL, Erez O, et al. What is amniotic fluid ‘sludge’? Ultrasound Obstet Gynecol. 2007;30(5):793–8. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30(5):706–14. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198(1):135, e1–5. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hatanaka AR, Mattar R, Kawanami TE, Franca MS, Rolo LC, Nomura RM, et al. Amniotic fluid “sludge” is an independent risk factor for preterm delivery. J Matern Fetal Neonatal Med. 2014:1–6. doi: 10.3109/14767058.2014.989202. [DOI] [PubMed] [Google Scholar]
- 181.Fuchs F, Boucoiran I, Picard A, Dube J, Wavrant S, Bujold E, et al. Impact of amniotic fluid “sludge” on the risk of preterm delivery. J Matern Fetal Neonatal Med. 2014:1–5. doi: 10.3109/14767058.2014.947575. [DOI] [PubMed] [Google Scholar]
- 182.Boyer A, Cameron L, Munoz-Maldonado Y, Bronsteen R, Comstock CH, Lee W, et al. Clinical significance of amniotic fluid sludge in twin pregnancies with a short cervical length. Am J Obstet Gynecol. 2014;211(5):506, e1–9. doi: 10.1016/j.ajog.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 183.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 184.Lee JH, Romero R, Kim SM, Chaemsaithong P, Park CW, Park JS, et al. A new antibiotic regimen treats and prevents intra-amniotic infection/inflammation in patients with preterm PROM. Am J Obstet Gynecol (Submitted) 2015 [Google Scholar]
- 185.Lee JH, Romero R, Kim SM, Chaemsaithong P, Park CW, Park JS, et al. A new antimicrobial combination prolongs the latency period, reduces acute histologic chorioamnionitis, and funisitis, and improves neonatal outcomes in preterm PROM. J matern Fetal Neonatal Med (Accepted) 2015 doi: 10.3109/14767058.2015.1020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81(6):941–8. [PubMed] [Google Scholar]
- 187.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22(2):281–342. [PubMed] [Google Scholar]
- 188.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177(4):825–30. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 189.Figueroa R, Garry D, Elimian A, Patel K, Sehgal PB, Tejani N. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2005;18(4):241–7. doi: 10.1080/13506120500223241. [DOI] [PubMed] [Google Scholar]
- 190.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195(6):1578–89. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 191.Marconi C, De Andrade Ramos BR, Peracoli JC, Donders GG, Da Silva MG. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am J Reprod Immunol. 2011;65(6):549–56. doi: 10.1111/j.1600-0897.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 192.Puchner K, Iavazzo C, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D, et al. Mid-trimester amniotic fluid interleukins (IL-1beta, IL-10 and IL-18) as possible predictors of preterm delivery. In Vivo. 2011;25(1):141–8. [PubMed] [Google Scholar]
- 193.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161(2):336–41. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 194.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166(5):1576–87. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 195.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177(1):19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 196.Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2015 doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–20. doi: 10.1002/9780470514269.ch13. discussion 20-3. [DOI] [PubMed] [Google Scholar]
- 198.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30(2-3):167–83. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 199.Arntzen KJ, Kjollesdal AM, Halgunset J, Vatten L, Austgulen R. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med. 1998;26(1):17–26. doi: 10.1515/jpme.1998.26.1.17. [DOI] [PubMed] [Google Scholar]
- 200.Hsu CD, Meaddough E, Aversa K, Hong SF, Lu LC, Jones DC, et al. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol. 1998;179(5):1267–70. doi: 10.1016/s0002-9378(98)70144-9. [DOI] [PubMed] [Google Scholar]
- 201.Yoon BH, Romero R, Moon J, Chaiworapongsa T, Espinoza J, Kim YM, et al. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J Matern Fetal Neonatal Med. 2003;13(1):32–8. doi: 10.1080/jmf.13.1.32.38. [DOI] [PubMed] [Google Scholar]
- 202.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Wennerholm UB, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82(2):120–8. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 203.Holst RM, Mattsby-Baltzer I, Wennerholm UB, Hagberg H, Jacobsson B. Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):551–7. doi: 10.1111/j.0001-6349.2005.00708.x. [DOI] [PubMed] [Google Scholar]
- 204.Menon R, Camargo MC, Thorsen P, Lombardi SJ, Fortunato SJ. Amniotic fluid interleukin-6 increase is an indicator of spontaneous preterm birth in white but not black Americans. Am J Obstet Gynecol. 2008;198(1):77, e1–7. doi: 10.1016/j.ajog.2007.06.071. [DOI] [PubMed] [Google Scholar]
- 205.Kacerovsky M, Musilova I, Hornychova H, Kutova R, Pliskova L, Kostal M, et al. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol. 2014;211(4):385, e1–9. doi: 10.1016/j.ajog.2014.03.069. [DOI] [PubMed] [Google Scholar]
- 206.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169(5):1299–303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 207.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78(1):5–10. doi: 10.1016/s0301-2115(97)00236-4. [DOI] [PubMed] [Google Scholar]
- 208.Hsu CD, Meaddough E, Aversa K, Copel JA. The role of amniotic fluid L-selectin, GRO-alpha, and interleukin-8 in the pathogenesis of intraamniotic infection. Am J Obstet Gynecol. 1998;178(3):428–32. doi: 10.1016/s0002-9378(98)70414-4. [DOI] [PubMed] [Google Scholar]
- 209.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Nikolaitchouk N, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82(5):423–31. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 210.Witt A, Berger A, Gruber CJ, Petricevic L, Apfalter P, Husslein P. IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat Med. 2005;33(1):22–6. doi: 10.1515/JPM.2005.003. [DOI] [PubMed] [Google Scholar]
- 211.Cobo T, Kacerovsky M, Palacio M, Hornychova H, Hougaard DM, Skogstrand K, et al. Intra-amniotic inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. PLoS One. 2012;7(8):e43677. doi: 10.1371/journal.pone.0043677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mittal P, Romero R, Kusanovic JP, Edwin SS, Gotsch F, Mazaki-Tovi S, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol. 2008;60(3):246–57. doi: 10.1111/j.1600-0897.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 214.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32(10):452–60. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 216.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124(5):710–9. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 218.Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet Gynecol. 1989;73(1):31–4. [PubMed] [Google Scholar]
- 219.Bry K, Lappalainen U, Hallman M. Interleukin-1 binding and prostaglandin E2 synthesis by amnion cells in culture: regulation by tumor necrosis factor-alpha, transforming growth factor-beta, and interleukin-1 receptor antagonist. Biochim Biophys Acta. 1993;1181(1):31–6. doi: 10.1016/0925-4439(93)90086-g. [DOI] [PubMed] [Google Scholar]
- 220.Fidel PL, Jr., Romero R, Ramirez M, Cutright J, Edwin SS, Lamarche S, et al. Interleukin-1 receptor antagonist (IL-1ra) production by human amnion, chorion, and decidua. Am J Reprod Immunol. 1994;32(1):1–7. doi: 10.1111/j.1600-0897.1994.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 221.Romero R, Mazor M, Manogue K, Oyarzun E, Cerami A. Human decidua: a source of cachectin-tumor necrosis factor. Eur J Obstet Gynecol Reprod Biol. 1991;41(2):123–7. doi: 10.1016/0028-2243(91)90089-4. [DOI] [PubMed] [Google Scholar]
- 222.Vince G, Shorter S, Starkey P, Humphreys J, Clover L, Wilkins T, et al. Localization of tumour necrosis factor production in cells at the materno/fetal interface in human pregnancy. Clin Exp Immunol. 1992;88(1):174–80. doi: 10.1111/j.1365-2249.1992.tb03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Trautman MS, Dudley DJ, Edwin SS, Collmer D, Mitchell MD. Amnion cell biosynthesis of interleukin-8: regulation by inflammatory cytokines. J Cell Physiol. 1992;153(1):38–43. doi: 10.1002/jcp.1041530107. [DOI] [PubMed] [Google Scholar]
- 224.Keelan JA, Sato T, Mitchell MD. Interleukin (IL)-6 and IL-8 production by human amnion: regulation by cytokines, growth factors, glucocorticoids, phorbol esters, and bacterial lipopolysaccharide. Biol Reprod. 1997;57(6):1438–44. doi: 10.1095/biolreprod57.6.1438. [DOI] [PubMed] [Google Scholar]
- 225.Laham N, Brennecke SP, Rice GE. Interleukin-8 release from human gestational tissue explants: the effects of lipopolysaccharide and cytokines. Biol Reprod. 1997;57(3):616–20. doi: 10.1095/biolreprod57.3.616. [DOI] [PubMed] [Google Scholar]
- 226.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171(6):1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 227.Witkin SS, Gravett MG, Haluska GJ, Novy MJ. Induction of interleukin-1 receptor antagonist in rhesus monkeys after intraamniotic infection with group B streptococci or interleukin-1 infusion. Am J Obstet Gynecol. 1994;171(6):1668–72. doi: 10.1016/0002-9378(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 228.Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3(3):121–6. doi: 10.1177/107155769600300304. [DOI] [PubMed] [Google Scholar]
- 229.Gravett MG, Novy MJ. Endocrine-immune interactions in pregnant non-human primates with intrauterine infection. Infect Dis Obstet Gynecol. 1997;5(2):142–53. doi: 10.1155/S1064744997000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188(1):252–63. doi: 10.1067/mob.2003.70. [DOI] [PubMed] [Google Scholar]
- 231.Gravett MG, Adams KM, Sadowsky DW, Grosvenor AR, Witkin SS, Axthelm MK, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol. 2007;197(5):518, e1–8. doi: 10.1016/j.ajog.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16(1):56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 233.Chang J, Jain S, Carl DJ, Paolella L, Darveau RP, Gravett MG, et al. Differential host response to LPS variants in amniochorion and the TLR4/MD-2 system in Macaca nemestrina. Placenta. 2010;31(9):811–7. doi: 10.1016/j.placenta.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG. 2011;118(2):136–44. doi: 10.1111/j.1471-0528.2010.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Adams Waldorf KM, Gravett MG, Mcadams RM, Paolella LJ, Gough GM, Carl DJ, et al. Choriodecidual group B streptococcal inoculation induces fetal lung injury without intra-amniotic infection and preterm labor in Macaca nemestrina. PLoS One. 2011;6(12):e28972. doi: 10.1371/journal.pone.0028972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Vanderhoeven JP, Bierle CJ, Kapur RP, Mcadams RM, Beyer RP, Bammler TK, et al. Group B streptococcal infection of the choriodecidua induces dysfunction of the cytokeratin network in amniotic epithelium: a pathway to membrane weakening. PLoS Pathog. 2014;10(3):e1003920. doi: 10.1371/journal.ppat.1003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA, et al. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod. 2015;92(2):23. doi: 10.1095/biolreprod.114.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kallapur SG, Nitsos I, Moss TJ, Polglase GR, Pillow JJ, Cheah FC, et al. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med. 2009;179(10):955–61. doi: 10.1164/rccm.200811-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Collins JJ, Kallapur SG, Knox CL, Nitsos I, Polglase GR, Pillow JJ, et al. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol. 2010;299(6):8. doi: 10.1152/ajplung.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Berry CA, Nitsos I, Hillman NH, Pillow JJ, Polglase GR, Kramer BW, et al. Interleukin-1 in lipopolysaccharide induced chorioamnionitis in the fetal sheep. Reprod Sci. 2011;18(11):1092–102. doi: 10.1177/1933719111404609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Snyder CC, Wolfe KB, Gisslen T, Knox CL, Kemp MW, Kramer BW, et al. Modulation of lipopolysaccharide-induced chorioamnionitis by Ureaplasma parvum in sheep. Am J Obstet Gynecol. 2013;208(5):11. doi: 10.1016/j.ajog.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal immune response to chorioamnionitis. Semin Reprod Med. 2014;32(1):56–67. doi: 10.1055/s-0033-1361823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kemp MW, Miura Y, Payne MS, Watts R, Megharaj S, Jobe AH, et al. Repeated maternal intramuscular or intraamniotic erythromycin incompletely resolves intrauterine Ureaplasma parvum infection in a sheep model of pregnancy. Am J Obstet Gynecol. 2014;211(2):28. doi: 10.1016/j.ajog.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 244.Payne MS, Kemp MW, Kallapur SG, Kannan PS, Saito M, Miura Y, et al. Intrauterine Candida albicans infection elicits severe inflammation in fetal sheep. Pediatr Res. 2014;75(6):716–22. doi: 10.1038/pr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wolfs TG, Kramer BW, Thuijls G, Kemp MW, Saito M, Willems MG, et al. Chorioamnionitis-induced fetal gut injury is mediated by direct gut exposure of inflammatory mediators or by lung inflammation. Am J Physiol Gastrointest Liver Physiol. 2014;306(5):23. doi: 10.1152/ajpgi.00260.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Dombroski RA, Woodard DS, Harper MJ, Gibbs RS. A rabbit model for bacteria-induced preterm pregnancy loss. Am J Obstet Gynecol. 1990;163(6 Pt 1):1938–43. doi: 10.1016/0002-9378(90)90777-5. [DOI] [PubMed] [Google Scholar]
- 247.Mcduffie RS, Jr., Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol. 1992;167(6):1583–8. doi: 10.1016/0002-9378(92)91745-v. [DOI] [PubMed] [Google Scholar]
- 248.Bry K, Hallman M. Transforming growth factor-beta 2 prevents preterm delivery induced by interleukin-1 alpha and tumor necrosis factor-alpha in the rabbit. Am J Obstet Gynecol. 1993;168(4):1318–22. doi: 10.1016/0002-9378(93)90388-y. [DOI] [PubMed] [Google Scholar]
- 249.Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177(4):797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- 250.Fidel PI, Jr., Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Med. 1998;7(5):222–6. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<222::AID-MFM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 251.Leslie KK, Lee SL, Woodcock SM, Davies JK, Mcduffie RS, Jr., Hirsch E, et al. Acute intrauterine infection results in an imbalance between pro- and anti-inflammatory cytokines in the pregnant rabbit. Am J Reprod Immunol. 2000;43(5):305–11. doi: 10.1111/j.8755-8920.2000.430510.x. [DOI] [PubMed] [Google Scholar]
- 252.Fidel P, Ghezzi F, Romero R, Chaiworapongsa T, Espinoza J, Cutright J, et al. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern Fetal Neonatal Med. 2003;14(1):57–64. doi: 10.1080/jmf.14.1.57.64. [DOI] [PubMed] [Google Scholar]
- 253.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165(4 Pt 1):969–71. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 254.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167(4 Pt 1):1041–5. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 255.Dudley DJ, Chen CL, Branch DW, Hammond E, Mitchell MD. A murine model of preterm labor: inflammatory mediators regulate the production of prostaglandin E2 and interleukin-6 by murine decidua. Biol Reprod. 1993;48(1):33–9. doi: 10.1095/biolreprod48.1.33. [DOI] [PubMed] [Google Scholar]
- 256.Fidel PL, Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170(5 Pt 1):1467–75. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 257.Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol. 1995;172(5):1598–603. doi: 10.1016/0002-9378(95)90503-0. [DOI] [PubMed] [Google Scholar]
- 258.Fidel PL, Jr., Romero R, Cutright J, Wolf N, Gomez R, Araneda H, et al. Treatment with the interleukin-I receptor antagonist and soluble tumor necrosis factor receptor Fc fusion protein does not prevent endotoxin-induced preterm parturition in mice. J Soc Gynecol Investig. 1997;4(1):22–6. doi: 10.1177/107155769700400104. [DOI] [PubMed] [Google Scholar]
- 259.Hirsch E, Muhle RA, Mussalli GM, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin-1 signaling. Am J Obstet Gynecol. 2002;186(3):523–30. doi: 10.1067/mob.2002.120278. [DOI] [PubMed] [Google Scholar]
- 260.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12(3):145–55. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 261.Yoshimura K, Hirsch E. Effect of stimulation and antagonism of interleukin-1 signaling on preterm delivery in mice. J Soc Gynecol Investig. 2005;12(7):533–8. doi: 10.1016/j.jsgi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 262.Kallapur SG, Jobe AH, Ball MK, Nitsos I, Moss TJ, Hillman NH, et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol. 2007;179(12):8491–9. doi: 10.4049/jimmunol.179.12.8491. [DOI] [PubMed] [Google Scholar]
- 263.Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15(2):101–7. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- 264.Kuypers E, Wolfs TG, Collins JJ, Jellema RK, Newnham JP, Kemp MW, et al. Intraamniotic lipopolysaccharide exposure changes cell populations and structure of the ovine fetal thymus. Reprod Sci. 2013;20(8):946–56. doi: 10.1177/1933719112472742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kuypers E, Willems MG, Jellema RK, Kemp MW, Newnham JP, Delhaas T, et al. Responses of the spleen to intraamniotic lipopolysaccharide exposure in fetal sheep. Pediatr Res. 2015;77(1-1):29–35. doi: 10.1038/pr.2014.152. [DOI] [PubMed] [Google Scholar]
- 266.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292(4):462–9. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 267.Romero R, Kusanovic JP, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23(4):261–80. doi: 10.3109/14767050903067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183(1):94–9. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 269.Moon JB, Kim JC, Yoon BH, Romero R, Kim G, Oh SY, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med. 2002;30(4):301–6. doi: 10.1515/JPM.2002.044. [DOI] [PubMed] [Google Scholar]
- 270.El-Bastawissi AY, Williams MA, Riley DE, Hitti J, Krieger JN. Amniotic fluid interleukin-6 and preterm delivery: a review. Obstet Gynecol. 2000;95(6 Pt 2):1056–64. [PubMed] [Google Scholar]
- 271.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116(2 Pt 1):393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 272.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG. 2011;118(9):1042–54. doi: 10.1111/j.1471-0528.2011.02923.x. [DOI] [PubMed] [Google Scholar]
- 273.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197(3):294, e1–6. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 274.Cobo T, Kacerovsky M, Jacobsson B. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;211(6):708. doi: 10.1016/j.ajog.2014.06.060. [DOI] [PubMed] [Google Scholar]
- 275.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191(4):1339–45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 276.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, Dubard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178(3):546–50. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 277.Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intra-amniotic infection with Ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand. 1999;78(5):379–82. [PubMed] [Google Scholar]
- 278.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185(5):1162–7. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 279.Biggio JR, Jr., Ramsey PS, Cliver SP, Lyon MD, Goldenberg RL, Wenstrom KD. Midtrimester amniotic fluid matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile are a marker for subsequent preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192(1):109–13. doi: 10.1016/j.ajog.2004.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Thomakos N, Daskalakis G, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2010;148(2):147–51. doi: 10.1016/j.ejogrb.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 281.Gervasi MT, Romero R, Bracalente G, Erez O, Dong Z, Hassan SS, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40(4):329–43. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kim A, Lee ES, Shin JC, Kim HY. Identification of biomarkers for preterm delivery in mid-trimester amniotic fluid. Placenta. 2013;34(10):873–8. doi: 10.1016/j.placenta.2013.06.306. [DOI] [PubMed] [Google Scholar]
- 283.Chaemsaithong P, Romero R, Korzeniewski SJ, Dong Z, Yeo L, Hassan SS, et al. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J Matern Fetal Neonatal Med. 2014:1–10. doi: 10.3109/14767058.2014.961417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kacerovsky M, Musilova I, Stepan M, Andrys C, Drahosova M, Jacobsson B. Detection of intraamniotic inflammation in fresh and processed amniotic fluid samples with the interleukin-6 point of care test. Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 285.Nien JK, Yoon BH, Espinoza J, Kusanovic JP, Erez O, Soto E, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol. 2006;195(4):1025–30. doi: 10.1016/j.ajog.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 286.Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197(3):292, e1–5. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 287.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36(6):497–502. doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lee SJ, Won HS, Kim MN, Lee PR, Shim JY, Kim A. Diagnostic value of the matrix metalloproteinase-8 rapid test for detecting microbial invasion of the amniotic cavity. Eur J Clin Microbiol Infect Dis. 2008;27(12):1257–60. doi: 10.1007/s10096-008-0566-7. [DOI] [PubMed] [Google Scholar]
- 289.Kim SM, Lee JH, Park CW, Park JS, Jun JK, Yoon BH. 556: One third of early spontaneous preterm delivery can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. Am J Obstet Gynecol. 2015;212(1):S277. [Google Scholar]
- 290.Park HS, Kim SA. The value of the genedia MMP-8 rapid test for diagnosing intraamniotic infection/inflammation and predicting adverse pregnancy outcomes in women with preterm premature rupture of membranes (Abstract number 322). Am J Obstet Gynecol. 2015;212(1):S174. [Google Scholar]
- 291.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Chaiyasit N, Kim YM, et al. A Comparison of Rapid IL-6, Rapid MMP-8 Point of Care Test and ELISA IL-6 for the Identification of Intra-amniotic Inflammation and Impending Preterm Delivery. J Matern Fetal Neonatal Med (in preparation) 2015 [Google Scholar]
- 292.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Chaiyasit N, Kim YM, et al. A Rapid MMP-8 Point of Care Test had Similar Diagnostic Performance to Rapid IL-6 test in the Identification of Intra-amniotic Inflammation in Patients with Preterm PROM. J Matern Fetal Neonatal Med (in preparation) 2015 [Google Scholar]
- 293.Kim SM, Romero R, Lee J, Chaemsaithong P, Lee M, Lee H, et al. Forty percent of early spontaneous preterm deliveries can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. Journal of Maternal Fetal and Neonatal Medicine (in preparation) 2015 doi: 10.3109/14767058.2015.1094049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182(3):675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 295.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110(Suppl 20):124–7. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 296.Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32(10):461–9. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Keelan JA, Wang K, Chaiworapongsa T, Romero R, Mitchell MD, Sato TA, et al. Macrophage inhibitory cytokine 1 in fetal membranes and amniotic fluid from pregnancies with and without preterm labour and premature rupture of membranes. Mol Hum Reprod. 2003;9(9):535–40. doi: 10.1093/molehr/gag068. [DOI] [PubMed] [Google Scholar]
- 298.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18(6):405–16. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Jacobsson B, Holst RM, Wennerholm UB, Andersson B, Lilja H, Hagberg H. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol. 2003;189(4):1161–7. doi: 10.1067/s0002-9378(03)00594-5. [DOI] [PubMed] [Google Scholar]
- 300.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17(6):365–73. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 301.Holst RM, Laurini R, Jacobsson B, Samuelsson E, Savman K, Doverhag C, et al. Expression of cytokines and chemokines in cervical and amniotic fluid: relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med. 2007;20(12):885–93. doi: 10.1080/14767050701752601. [DOI] [PubMed] [Google Scholar]
- 302.Kacerovsky M, Celec P, Vlkova B, Skogstrand K, Hougaard DM, Cobo T, et al. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One. 2013;8(3):e60399. doi: 10.1371/journal.pone.0060399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jacobsson B, Holst RM, Andersson B, Hagberg H. Monocyte chemotactic protein-2 and -3 in amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation and preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):566–71. doi: 10.1111/j.0001-6349.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- 304.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32(2):108–13. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 305.Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21(11):763–75. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Keelan JA, Yang J, Romero RJ, Chaiworapongsa T, Marvin KW, Sato TA, et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol Reprod. 2004;70(1):253–9. doi: 10.1095/biolreprod.103.016204. [DOI] [PubMed] [Google Scholar]
- 307.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181(4):989–94. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 308.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 309.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–65. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 310.Harris HE, Raucci A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7(8):774–8. doi: 10.1038/sj.embor.7400759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 312.Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36(5):388–98. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21(7):449–61. doi: 10.1080/14767050802054550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Bianchi ME, Manfredi AA. Immunology. Dangers in and out. Science. 2009;323(5922):1683–4. doi: 10.1126/science.1172794. [DOI] [PubMed] [Google Scholar]
- 315.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010:2010. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24(12):1444–55. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nunez G. Intracellular sensors of microbes and danger. Immunol Rev. 2011;243(1):5–8. doi: 10.1111/j.1600-065X.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 319.Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25(6):558–67. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Pankuch GA, Appelbaum PC, Lorenz RP, Botti JJ, Schachter J, Naeye RL. Placental microbiology and histology and the pathogenesis of chorioamnionitis. Obstet Gynecol. 1984;64(6):802–6. [PubMed] [Google Scholar]
- 321.Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012;17(1):20–5. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 322.Martinelli P, Sarno L, Maruotti GM, Paludetto R. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med. 2012;25(Suppl 4):29–31. doi: 10.3109/14767058.2012.714981. [DOI] [PubMed] [Google Scholar]
- 323.Dong Y, St Clair PJ, Ramzy I, Kagan-Hallet KS, Gibbs RS. A microbiologic and clinical study of placental inflammation at term. Obstet Gynecol. 1987;70(2):175–82. [PubMed] [Google Scholar]
- 324.Roberts DJ, Celi AC, Riley LE, Onderdonk AB, Boyd TK, Johnson LC, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS One. 2012;7(3):e31819. doi: 10.1371/journal.pone.0031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47. doi: 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1(2):135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 327.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 328.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179(1):186–93. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 329.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198(1):43, e1–5. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Polin RA, Committee On F. Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129(5):1006–15. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 331.Puopolo KM. Response to the American Academy of Pediatrics, Committee on the Fetus and Newborn statement, “management of neonates with suspected or proven early-onset bacterial sepsis”. Pediatrics. 2012;130(4):e1054–5. doi: 10.1542/peds.2012-2302C. author reply e5-7. [DOI] [PubMed] [Google Scholar]
- 332.Polin RA, Watterberg K, Benitz W, Eichenwald E. The conundrum of early-onset sepsis. Pediatrics. 2014;133(6):1122–3. doi: 10.1542/peds.2014-0360. [DOI] [PubMed] [Google Scholar]
- 333.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181(4):773–9. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 334.Chaiworapongsa T, Romero R, Kim JC, Kim YM, Blackwell SC, Yoon BH, et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol. 2002;186(6):1178–82. doi: 10.1067/mob.2002.124042. [DOI] [PubMed] [Google Scholar]
- 335.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49(5):506–14. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, et al. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med. 2009;37(5):543–52. doi: 10.1515/JPM.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63(1):73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Romero R, Savasan ZA, Chaiworapongsa T, Berry SM, Kusanovic JP, Hassan SS, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med. 2011;40(1):19–32. doi: 10.1515/JPM.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Chaiworapongsa T, Romero R, Berry SM, Hassan SS, Yoon BH, Edwin S, et al. The role of granulocyte colony-stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. J Perinat Med. 2011;39(6):653–66. doi: 10.1515/JPM.2011.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Vaisbuch E, Romero R, Gomez R, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, et al. An elevated fetal interleukin-6 concentration can be observed in fetuses with anemia due to Rh alloimmunization: implications for the understanding of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2011;24(3):391–6. doi: 10.3109/14767058.2010.507294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Romero R, Soto E, Berry SM, Hassan SS, Kusanovic JP, Yoon BH, et al. Blood pH and gases in fetuses in preterm labor with and without systemic inflammatory response syndrome. J Matern Fetal Neonatal Med. 2012;25(7):1160–70. doi: 10.3109/14767058.2011.629247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Savasan ZA, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Xu Y, et al. Interleukin-19 in fetal systemic inflammation. J Matern Fetal Neonatal Med. 2012;25(7):995–1005. doi: 10.3109/14767058.2011.605917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lee J, Romero R, Chaiworapongsa T, Dong Z, Tarca AL, Xu Y, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70(4):265–84. doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 345.Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997;25(11):1789–95. doi: 10.1097/00003246-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 346.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 347.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–74. [PubMed] [Google Scholar]
- 348.Gomez R, Ghezzi F, Romero R, Yoon BH, Mazor M, Berry SM. Two thirds of human fetuses with microbial invasion of the amniotic cavity have a detectable systemic cytokine response before birth. Am J Obstet Gynecol. 1997;176(1):S14. [Google Scholar]
- 349.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 350.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–26. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50(3):652–83. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 352.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9(5):517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 353.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995;173(4):1315–20. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 354.Matsuda Y, Kato H, Imanishi K, Mitani M, Ohta H, Uchiyama T. T cell activation in abnormal perinatal events. Microbiol Immunol. 2010;54(1):38–45. doi: 10.1111/j.1348-0421.2009.00181.x. [DOI] [PubMed] [Google Scholar]
- 355.Luciano AA, Yu H, Jackson LW, Wolfe LA, Bernstein HB. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS One. 2011;6(2):e16698. doi: 10.1371/journal.pone.0016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med (accepted) 2015 doi: 10.1515/jpm-2015-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.De Felice C, Toti P, Santopietro R, Stumpo M, Pecciarini L, Bagnoli F. Small thymus in very low birth weight infants born to mothers with subclinical chorioamnionitis. J Pediatr. 1999;135(3):384–6. doi: 10.1016/s0022-3476(99)70140-x. [DOI] [PubMed] [Google Scholar]
- 358.Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, D'addario V, et al. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol. 2006;194(1):153–9. doi: 10.1016/j.ajog.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 359.Yinon Y, Zalel Y, Weisz B, Mazaki-Tovi S, Sivan E, Schiff E, et al. Fetal thymus size as a predictor of chorioamnionitis in women with preterm premature rupture of membranes. Ultrasound Obstet Gynecol. 2007;29(6):639–43. doi: 10.1002/uog.4022. [DOI] [PubMed] [Google Scholar]
- 360.Musilova I, Hornychova H, Kostal M, Jacobsson B, Kacerovsky M. Ultrasound measurement of the transverse diameter of the fetal thymus in pregnancies complicated by the preterm prelabor rupture of membranes. J Clin Ultrasound. 2013;41(5):283–9. doi: 10.1002/jcu.22027. [DOI] [PubMed] [Google Scholar]
- 361.Sciaky-Tamir Y, Hershkovitz R, Mazor M, Shelef I, Erez O. The use of imaging technology in the assessment of the fetal inflammatory response syndrome-imaging of the fetal thymus. Prenat Diagn. 2015;35(5):413–9. doi: 10.1002/pd.4560. [DOI] [PubMed] [Google Scholar]
- 362.Romero R, Espinoza J, Goncalves LF, Gomez R, Medina L, Silva M, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2004;16(3):146–57. doi: 10.1080/14767050400009279. [DOI] [PubMed] [Google Scholar]
- 363.Yoon BH, Romero R, Jun JK, Maymon E, Gomez R, Mazor M, et al. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol. 1998;179(5):1107–14. doi: 10.1016/s0002-9378(98)70114-0. [DOI] [PubMed] [Google Scholar]
- 364.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174(5):1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 365.Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr. 2000;12(2):99–104. doi: 10.1097/00008480-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 366.Korzeniewski SJ, Romero R, Cortez J, Pappas A, Schwartz AG, Kim CJ, et al. A “multi-hit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J Perinat Med. 2014;42(6):731–43. doi: 10.1515/jpm-2014-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Yoon BH, Kim YA, Romero R, Kim JC, Park KH, Kim MH, et al. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1999;181(4):784–8. doi: 10.1016/s0002-9378(99)70301-7. [DOI] [PubMed] [Google Scholar]
- 368.Gantert M, Been JV, Gavilanes AW, Garnier Y, Zimmermann LJ, Kramer BW. Chorioamnionitis: a multiorgan disease of the fetus? J Perinatol. 2010;30(Suppl):S21–30. doi: 10.1038/jp.2010.96. [DOI] [PubMed] [Google Scholar]
- 369.Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr. 2013;162(2):236–42. e2. doi: 10.1016/j.jpeds.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 370.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166(1 Pt 1):129–33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 371.Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J, et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol. 1990;163(3):757–61. doi: 10.1016/0002-9378(90)91063-i. [DOI] [PubMed] [Google Scholar]
- 372.Mazor M, Hershkovitz R, Ghezzi F, Maymon E, Horowitz S, Leiberman JR. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand. 1996;75(7):624–7. doi: 10.3109/00016349609054686. [DOI] [PubMed] [Google Scholar]
- 373.Yoon BH, Park KH, Koo JN, Kwon JH, Jun JK, Syn HC, et al. Intraamniotic infection of twin pregnancies with preterm labor. Am J Obstet Gynecol. 1997;176(1):S35. [Google Scholar]
- 374.Romero R, Hanaoka S, Mazor M, Athanassiadis AP, Callahan R, Hsu YC, et al. Meconium-stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1991;164(3):859–62. doi: 10.1016/0002-9378(91)90529-z. [DOI] [PubMed] [Google Scholar]
- 375.Romero R, Yoon BH, Chaemsaithong P, Cortez J, Park CW, Gonzalez R, et al. Bacteria and endotoxin in meconium-stained amniotic fluid at term: could intra-amniotic infection cause meconium passage? J Matern Fetal Neonatal Med. 2014;27(8):775–88. doi: 10.3109/14767058.2013.844124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Digiulio DB, Gervasi M, Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med. 2010;38(5):503–13. doi: 10.1515/JPM.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Digiulio DB, Gervasi MT, Romero R, Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med. 2010;38(5):495–502. doi: 10.1515/JPM.2010.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Blackwell S, Romero R, Chaiworapongsa T, Kim YM, Bujold E, Espinoza J, et al. Maternal and fetal inflammatory responses in unexplained fetal death. J Matern Fetal Neonatal Med. 2003;14(3):151–7. doi: 10.1080/jmf.14.3.151.157. [DOI] [PubMed] [Google Scholar]
- 379.Lannaman K, Romero R, Chaemsaithong P, Ahmed A, Yeo L, Hassan S, et al. Fetal death: an extreme form fo maternal anti-fetal rejection. Am J Obstet Gynecol. 2015;212(1 (Supplement)):S251. [Google Scholar]
- 380.Sorokin Y, Romero R, Mele L, Iams JD, Peaceman AM, Leveno KJ, et al. Umbilical cord serum interleukin-6, C-reactive protein, and myeloperoxidase concentrations at birth and association with neonatal morbidities and long-term neurodevelopmental outcomes. Am J Perinatol. 2014;31(8):717–26. doi: 10.1055/s-0033-1359723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181(5 Pt 1):1142–8. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 382.Dudley DJ, Hunter C, Varner MW, Mitchell MD. Elevation of amniotic fluid interleukin-4 concentrations in women with preterm labor and chorioamnionitis. Am J Perinatol. 1996;13(7):443–7. doi: 10.1055/s-2007-994385. [DOI] [PubMed] [Google Scholar]
- 383.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21(8):529–47. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36(3):217–27. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]