Abstract
Aim:
The aim was to detect the glmM gene of Helicobacter pylori (H. pylori) in cow’s milk from different dairy farms in Khartoum State using Nested polymerase chain reaction (PCR).
Materials and Methods:
A total of 50 milk samples were collected from different dairy farms in Khartoum State (13 from Khartoum, 24 Khartoum North, and 13 from Omdurman Provinces).
Results:
The generated results showed that 11/50 (22%) were harboring the investigated H. pylori glmM gene in Khartoum State (1/13 [7.7%] Khartoum, 9/24 [37.5%] Khartoum North, and 1/13 [7.7%] Omdurman provinces, respectively).
Conclusion:
To the best of our knowledge, this was the first report on the detection of H. pylori glmM gene in cattle milk in Khartoum State. Nonetheless, the high percentages of H. pylori DNA detection in milk opened new avenues toward exploring the risk of human infection with H. pylori through the consumption of raw milk.
Keywords: bovine, Helicobacter pylori, Khartoum, milk, Nested polymerase chain reaction, glmM gene
Introduction
Helicobacter pylori (H. pylori) infection constitutes a public health concern in developed and developing countries [1,2], since it was associated with chronic gastritis, gastric and duodenal ulcers, and gastric adenocarcinoma in humans, as well as mucosa-associated lymphoid tissue lymphoma [3-6]. In the year 1994, the International Agency for Research on Cancer of WHO classified H. pylori as a Type I, or definite carcinogen to humans and reconfirmed this classification in 2009 [7].
Cow’s milk is usually consumed as human food, especially by children. Therefore, one of the suggested theories is the transmission of H. pylori through milk from animals to human beings. Some epidemiologic studies have reported the presence and survival of H. pylori in raw and pasteurized milk and milk products of cow, sheep, and goat directly or artificially inoculated was investigated either using Nested or semi-Nested PCR or by culturing methods or by the two techniques together [8-12]. Furthermore, epidemiologic data have shown higher prevalence in shepherds and their families than in the general population [13,14].
Nested PCR was already employed for the detection of H. pylori glmM gene from water and was reported to be as a highly sensitive and specific method for the rapid detection and screening of sheep, goat and cow milk [11-15].
The ureC gene of H. pylori encodes for the phosphoglucosamine mutase catalyzing the interconversion of GlcN-6-phosphate (GlcN-6-P) and GlcN-1-P isomers required for the biosynthesis of lipopolysaccharides and peptidoglycan. This gene has been shown to play an essential and unique role for H. pylori growth and survival [16-19]. It is presently named as glmM rather than ureC since it’s unrelated to urease production [17]. The aim of this study was to detect the glmM gene of H. pylori in cow’s milk from different dairy farms in Khartoum State using Nested PCR.
Materials and Methods
Ethical approval
The milk samples were collected aseptically with adequate precautionary measures to minimize pain and/or discomfort to the animals and carried out in accordance with the Sudan animal welfare laws. Furthermore, the human biopsies were taken under high precautionary measures and include approval of the ethical committee and informed consent was obtained from all of study patients to use these biopsies in this research.
Study area
Khartoum State is the capital of Sudan and includes “Khartoum, Khartoum North, and Omdurman Localities,” comprising the semi-arid zone between the latitude 15.08°N to 16.39°N and longitude 31.36°E to 34.25°E. This study was carried out in the Department of Molecular Biology, Veterinary Research Institute, Khartoum, Sudan.
Specimen collection
A total of 50 fresh raw cow’s milk samples were collected from different dairy farms in Khartoum State, Sudan. The samples were put into sterile ice containers and promptly delivered to the laboratory. Furthermore, four biopsies were taken from four human patients (two biopsies each) who were undergoing upper gastroduodenal endoscopy and tested by rapid urease test (RUT). After test, the human’s biopsies were checked for the presence of glmM gene using Nested PCR, and used later as a positive control for the collected milk samples.
Bacterial culture
A total of 50 fresh milk samples were cultured immediately after collection into broth medium firstly and then transferred to solid media. Collected milk samples were cultivated bacteriologically for the isolation of H. pylori using the following protocol: 3 ml of each sample were added to 10 ml of Brain Heart Infusion Broth supplemented with 5% of horse serum and Campylobacter Selective Supplement, Skirrow powder (Cat. No. CSS80004, Biolab, Hungary) and amphotericin B solution (250 µg/mL, 1 ml/L) (Cat. No. A2942, Sigma). This supplement contain polymyxin B (0,2 mg/vial), Trimethoprim (2.5 mg/vial), and vancomycin (5 mg/vial), reconstituted with 4 ml of sterile distilled water and added aseptically to 500 ml sterile broth at 50°C, mixed well before pouring and incubated at 37°C under microaerophilic conditions. After 3-5 days incubation in 0.1 ml of the selective enrichment broth, the culture was plated on brain heart infusion agar, blood agar base (No. 2) and egg yolk emulsion agar supplemented with 5% defibrinated sheep blood, campylobacter selective supplement, skirrow powder (Cat. No. CSS80004, Biolab, Hungary), and amphotericin B solution (250 µg/mL, 1 ml/L) (Cat. No. A2942, Sigma) were added aseptically to 500 ml sterile broth at 50°C, mixed well before pouring and incubated for 3-5 days at 37°C under microaerophilic conditions according to Poms and Tatini [20].
DNA extraction
Using 1 ml from each milk sample (50 samples), DNA was extracted using QIAamp Blood Mini Kit (Cat. No. 51104, QIAGEN), with slightly modifying the supplier’s instructions according to Quaglia et al. [11].
Oligonucleotide primers
Oligonucleotide primers (Cat. No. 011101853 Tib Molbiol Syntaborhesel GmbH, Berlin, Germany) as published by Quaglia et al. [11] were used throughout this investigation. Outer oligonucleotide primers Hp 1 and Hp 2 were used to amplify H. pylori glmM gene at 294 bp region:
Hp 1: 5’-AAGCTTTTAGGGGTGTTAGGGGTTT-3′
Hp 2: 5’-AAGCTTACTTTCTAACACTAAACGC-3′
Internal primers Hp 3 and Hp 4 were used to amplify a 252 bp region located 21 base pairs:
Hp 3: 5’-CTTTCTTCTCAAGCGGTTGTC-3′
Hp 4: 5’-CAAGCCATCGCCGGTTTTAGC-3′
Nested PCR protocol
A volume of 5 µl of each extracted DNA were amplified in 50 µl reaction mixture containing ×10 Taq buffer BD (0.8 M Tris-HCl, 0.2 M (NH4)2 SO4), 25 mM MgCl2), 200 µM dNTPs mix, 0.5 µM of each primer, and 5 U/µl FIREPol® Taq DNA polymerase (Cat. No. 01-01-00500, Solis BioDyne, Riia).
PCR amplification was performed according to the Quaglia et al. [11] protocol as follows: 95°C for 3 min and 35 cycles at 93°C for 1 min, 58°C for 1 min, 72°C for 2 min followed by 72°C for 7 min. After the amplification, 2 µl of the final product were transferred to the second PCR run. The reaction mixture containing the PCR product of the first run was re-amplified for 30 cycles using primers Hp 3 and Hp 4 under the following conditions: 95°C for 2 min and 30 cycles at 94°C for 1 min, 60°C for 2 min, 72°C for 3 min followed by 72°C for 7 min.
Nested-PCR products were visualized under UV transillumination following electrophoresis on 1.5% agarose gel stained with ethidium bromide 0.5 mg/ml using Gene Ruler VC 100-bp DNA Ladder as a reference standard (Cat. No. NL 1401, Vivantis Company, Malaysia).
Positive control
The positive control was prepared by extraction of DNA and conducting glmM Nested PCR for three positive a RUT human patients’ biopsies (two biopsies each).
Results
Nested PCR results
The test revealed that 11/50 (22%) raw cow’s milk samples from different dairy farms in Khartoum State were positive. The positive samples were distributed in the State as follows; Khartoum 1/13 (7.7%), Khartoum North 9/24 (37.5%), and 1/13 (7.7%) Omdurman, respectively (Figure-1). Moreover, ¾ of the human gastric biopsies were positive for H. pylori while the 4th one was negative. These positive biopsies were used as positive controls in Nested PCR for the detection of H. pylori glmM (ureC) gene. The results of the gastric biopsies on Nested PCR were identical to those of the RUT (Figure-2).
Figure-1.
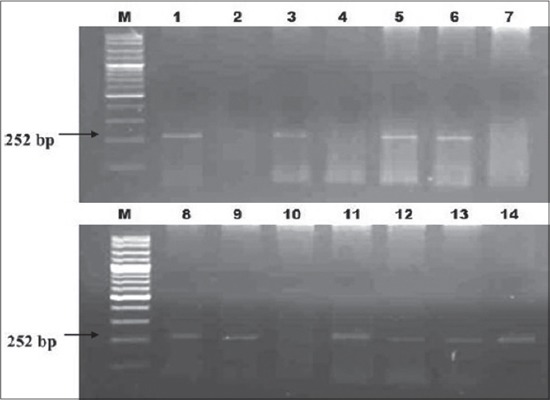
Nested PCR of glmM gene of Helicobacter pylori in raw Cow milk, Lane M = 100 bp ladder, Lane 1 = positive control, Lane 7 = negative control, Lane 3, 5, 6, 8, 9, 11, 12, 13, and 14 = positive milk samples and lane 2, 4, and 10 = negative milk samples.
Figure-2.
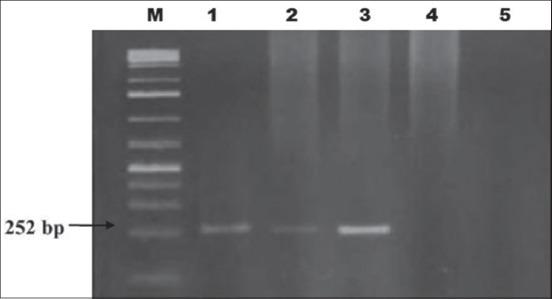
Nested PCR of glmM gene of Helicobacter pylori in four human gastric biopsy specimens, Lane M = 100 bp, Ladder, Lane 1, 2, and 3 = positive gastric biopsies, Lane 4 = negative gastric biopsies, and Lane 5 = negative control.
Discussion
This study was formulated for the detection of H. pylori glmM (ureC) gene by nested PCR. The prevalence rate was 22% from fresh raw cow’s milk in Khartoum State. However, the highest rate (37.5%) was observed on Khartoum North and an equal rate (7.7%) was detected in Khartoum and Omdurman. Whereas scandinavian researchers found H. pylori in 60% of 38 sheep gastric tissue [8]. In the other study which was conducted in Japan, H. pylori was found in 72.2% of cow raw milk specimens [9]. The Italian survey on 400 milk samples by nested PCR assay indicated that the prevalence of this bacterium in cow, sheep and goat populations were 50%, 33% and 25.6% respectively [10]. Furthermore, Rahimi and Kheirabadi declared H. pylori existed in 12.2% of sheep, 8.7% of goat, and 14.1% of cow milk by PCR method [21]. In addition, Hassan et al., [22] was reported that the prevalence of H. pylori in cow’s and sheep’s gastric biopsies was 3% and 16%, respectively, in Iran. Furthermore, Angelidis et al. [23] detected H. pylori in 20% of bovine bulk tank milk by fluorescence in situ hybridization. On the contrary, Bianchini et al. [24], isolated other Helicobacteraceae than H. pylori from raw milk of dairy cattle by cultural methods and confirmed results by Nested PCR with prevalence of 1.8% (3/163 samples), while Mousavi et al., reported a prevalence of isolated H. pylori of 19.8% (16.66%, 35%, 28%, 15%, and 13.33%, from bovine, ovine, caprine, buffalo, and camel raw milk samples, respectively) and 19.2% of dairy products samples based on cultural method, Nested PCR, and antimicrobial susceptibility testing which revealed high presence of antibiotic-resistant isolated strains of H. pylori in milk and dairy products to ampicillin (84.4%), tetracycline (76.6%), erythromycin (70.5%), and metronidazole (70%), [25]. Our finding were in agreement with those of Quaglia, et al. [11]., Ghasemian et al., [26] and Rahimi and Kheirabadi, [21] who reported that glm M (ureC) gene nested PCR is a highly sensitive and specific for detection of H. pylori from cows, goats, sheep, camels, and buffalo milk samples. Therefore, all those animals mentioned and their products may act as a reservoir of H. pylori and transmitted this bacterium to humans.
The ability of H. pylori to survive in an acid pH environment is urea dependent [27,28] and since urea is present in milk [21], the urea-dependent acid resistance of H. pylori may account for the long-term survival of H. pylori in this foodstuff [29]. This was in line with the finding of Quaglia, et al., [12] who reported that H. pylori was survival for 9 days in pasteurized milk and 12 days in ultrahigh temperature milk stored at 4°C. However, the presence of H. pylori glmM gene in milk in the present study in Khartoum State might be due to the poor hygienic management in dairy farms and ability of this bacterium to survive in milk for a relatively long term. Several investigations showed a number of animals, mostly living in human environment, had H. pylori in their stomach and in their production and, therefore, to be involved in the transmission of this bacterium [11,21,26,30]. Despite the fact that the transmission pathway of H. pylori is unclear, but the poor hygienic management in dairy farms might contribute to spread of bacterium from animal to animal through udder teats from contaminated milking machines or by milkers’ hands and towels. Furthermore, contaminated manure, bedding, soil, and water are might be transferred organism to the udder during the milking process or shortly thereafter. Also milk could become contaminated during production or because of low hygiene after the open of package [25], so insufficient post-processing hygienic management of the milk, can carry the contamination of the matrix by humans [12]. Therefore, we need food safety and quality standards (good dairy farming practices, good manufacturing practices, and the hazard analysis and critical control point system to be applied and performed in consumed milk by a human.
Concerning the glmM (ureC) gene Nested PCR, which was reported to be the most sensitive and specific for the detection of H. pylori in gastric biopsies by Saurabh et al., [16]. The findings of the present investigation supported this statement when we examined positive RUT gastric biopsies using the above-mentioned technique. In reference to this statement, the RUT positive gastric biopsies were used in this study as positive controls. Furthermore, single round PCR may give positive amplification only when more than 70 bacterial cells are present in a given biopsy sample, while Nested PCR is capable of detecting the bacterium as low as 3 cells only. Therefore, Nested PCR may be proposed as the gold standard for detection of H. pylori. [16].
The failure of H. pylori recovery from fresh raw milk samples by cultural methods in this study was supported by the findings of Dore et al., [8]; Fujimura et al.,[9]; Ghasemian et al.,[26]; and Angelidis et al.,[23], who reported that, H. pylori has rarely been isolated from raw and pasteurized milk samples. In addition, other studies failed to isolate H. pylori from raw cow [10], sheep [26], and goat [31] milk samples.
It has been reported that H. pylori change to three different morphological forms under environmental stress. These forms include the viable spiral, viable coccoid, and nonviable degenerative forms. While the viable spiral forms are culturable, virulent, and infectious, and induce inflammation in experimental animals. The viable coccoid forms are nonculturable, less virulent, and less likely to colonize, and induce inflammation in experimental animals. On the other hand, the nonviable degenerative forms are dying forms of H. pylori [32]. Therefore, the present failure in isolating the bacterium can be attributed to the fact that H. pylori can survive for short periods of time in milk and that it may have changed into the other non-cultivable forms during sample transit from field to laboratory. This has been strongly supported by the findings of Quaglia et al. [12]. Moreover, the method employed for H. pylori isolation may lack sufficient sensitivity to recover very low numbers of H. pylori due to the presence of viable nonculturable form (10 CFU per ml of liquid samples to recover at least one colony per plate) [20-23,26-29]. Furthermore, isolation is extremely difficult due to the presence of accompanying microflora and very low numbers of H. pylori in foodstuffs [33].
To the best of our knowledge, the present study was the first report that demonstrated H. pylori DNA in bovine milk in Khartoum State. Nonetheless, the presence of H. pylori DNA of milk analyzed in this work, open new avenues toward exploring the risk of human infection with H. pylori through the consumption of raw milk and bovine may play a role as a natural reservoir of the microorganism.
Conclusion
The present investigation demonstrated the presence of H. pylori glmM (ureC) gene in 11/50 (22%) raw cow’s milk samples from different dairy farms in Khartoum State, Sudan. This study’s outcome provides evidence on the possible transmission of H. pylori from milk to human, through consumption of raw milk.
Authors’ Contributions
AMSE helped in the study design and revised the manuscript. SBM helped EYO to conduct the nested PCR and analyzed the results. AEA collected the human patient’s biopsies using endoscopy and carried out the rapid urease test (RUT). EYO & AMM Collected the bovine milk samples and drafted the manuscript. MBAR helped in the revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to express their sincere thanks and gratitude to authorities of the Veterinary Research Institute, ALAmart (St. 1), Khartoum, Sudan for the financial support of this work as well as giving the permission for publication.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Hunt C.R.H, Xiao S.D, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, Vaz Coelho L.G, Fock K.M, Fedail S, Cohen H, Malfertheiner P, Vakil N, Hamid S, Goh K.L, Wong B.C.Y, Krabshuis J.H. Helicobacter pylori in developing countries. World gastroenterology organisation global guideline. J. Gastrointest. Liver. 2011;20(3):299–304. [PubMed] [Google Scholar]
- 2.Leonardo H, Eusebi L.H, Zagari R.M, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19(1):1–5. doi: 10.1111/hel.12165. [DOI] [PubMed] [Google Scholar]
- 3.Marina de Bernard and Christine Josenhans. Pathogenesis of Helicobacter pylori Infection. Helicobacter. 2014;19(1):11–18. doi: 10.1111/hel.12160. [DOI] [PubMed] [Google Scholar]
- 4.Bauer B, Meyer T.F. The human gastric pathogen Helicobacter pylori and its association with gastric cancer and ulcer disease. Ulcers. 2011:23. 2011:Article ID:340157. [Google Scholar]
- 5.Neumeister P, Troppan K, Raderer M. Management of gastric mucosa-associated lymphoid tissue lymphoma. J. Dig. Dis. 2015;33(1):11–18. doi: 10.1159/000366030. [DOI] [PubMed] [Google Scholar]
- 6.Meine G.C, Rota C, Dietz J, Sekine S, Prolla J.C. Relationship between cagA-positive Helicobacter pylori infection and risk of gastric cancer:A case control study in Porto Alegre, RS, Brazil. Arq Gastroenterol. 2011;48(1):41–45. doi: 10.1590/s0004-28032011000100009. [DOI] [PubMed] [Google Scholar]
- 7.IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports No. 8); 2014. [Last accessed on 2015 January 27]. Available from: http://www.iarc.fr/en/publications/pdfsonline/wrk/wrk8/index.php . [Google Scholar]
- 8.Dore M.P, Sepulveda A.R, El-Zimaity H, Yamaoka Y, Osato M.S, Mototsugu K, Nieddu A.M, Realdi G, Graham D.Y. Isolation of Helicobacter pylori from sheep—implications for transmission to humans. Am. J. Gastroenterol. 2001;96(5):1396–1401. doi: 10.1111/j.1572-0241.2001.03772.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura S, Kawamura T, Kato S, Tateno H, Watanabe A. Detection of Helicobacter pylori in cow’s milk. Lett. Appl. Microbiol. 2002;35:504–507. doi: 10.1046/j.1472-765x.2002.01229.x. [DOI] [PubMed] [Google Scholar]
- 10.Quaglia C.N, Dambrosio A, Normanno G, Parisi A, Patrono R, Ranieri G, Rella A, Celano G.V. High occurrence of Helicobacter pylori in raw goat, sheep and cow milk inferred by glmM gene:A risk of food-borne infection. Int. J. Food Microbiol. 2008;124:43–47. doi: 10.1016/j.ijfoodmicro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Quaglia N.C, Dambrosio A, Normanno G, Celano G.V. Evaluation of a nested-PCR assay based on the phosphoglucosamine mutase gene (glmM) for the detection of Helicobacter pylori from raw milk. Food Control. 2009;20:119–123. [Google Scholar]
- 12.Quaglia N.C, Dambrosio A, Normanno G, Parisi A, Firinu A, Lorusso V. Survival of Helicobacter pylori in artificially contaminated ultrahigh temperature and pasteurized milk. Lancet. 2007;24:296–300. doi: 10.1016/j.fm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Dore M.P, Bilotta M, Vaira D, Manca A, Massarelli G, Leandro G, Atzei A, Pisanu G, Graham D. Y, Realdi G. High prevalence of Helicobacter pylori infection in shepherds. Dig. Dis. Sci. 1999;44:1161–1164. doi: 10.1023/a:1026676223825. [DOI] [PubMed] [Google Scholar]
- 14.Plonka M, Bielanski W, Konturek S.J, Targosz A, Sliwowski Z, Dobrzanska M, Kaminska A, Sito E, Konturek P.C, Brzozowski T. Helicobacter pylori infection and serum gastrin, ghrelin and leptin in children of polish shepherds. Dig. Liver Dis. 2006;38:91–97. doi: 10.1016/j.dld.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Bahrami A.R, Rahimi E, Safaei H.G. Detection of Helicobacter pylori in city water, dental units’ water, and bottled mineral water in Isfahan, Iran. Sci. World J. 2013;2013:5. doi: 10.1155/2013/280510. Article ID:280510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S.K, Mishra G.N, Pratap C.B, Jain A.K, Nath G. Helicobacter pylori is not eradicated after triple therapy:A nested PCR based study. BioMed. Res. Int. 2014;2014:8. doi: 10.1155/2014/483136. Article ID:483136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Reuse H, Labigne A, Mengin-Lecreulx D. The Helicobacter pylori ureC gene codes for a phosphoglucosamine mutase. J. Bacteriol. 1997;179:3488–3493. doi: 10.1128/jb.179.11.3488-3493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J. Bacteriol. 1991;173:1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mengin-Lecreulx D, van Heijenoort J. Characterization of the essential glmM gene encoding phosphoglucosamine mutase in Escherichia coli. J. Biol. Chem. 1996;271:32–39. doi: 10.1074/jbc.271.1.32. [DOI] [PubMed] [Google Scholar]
- 20.Poms R.E, Tatini S.R. Survival of Helicobacter pylori in ready-to-eat foods at 4°C. Int. J. Food Microbiol. 2001;63:281–286. doi: 10.1016/s0168-1605(00)00441-4. [DOI] [PubMed] [Google Scholar]
- 21.Rahimi E, Kheirabad E. K. Detection of Helicobacter pylori in bovine buffalo, camel, ovine, and caprine milk in Iran. Foodborne Pathog. Dis. 2012;9:453–556. doi: 10.1089/fpd.2011.1060. [DOI] [PubMed] [Google Scholar]
- 22.Momtaz H, Dabiri H, Souod N, Gholami M. Study of Helicobacter pylori genotype status in cows, sheep, goats and human beings. BMC Gastroenterol. 2014;14:61. doi: 10.1186/1471-230X-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angelidis A.S, Tirodimos I, Bobos M, Kalamaki M.S, Papageorgiou D.K, Arvanitidou M. Detection of Helicobacter pylori in raw bovine milk by fluorescence in situ hybridization (FISH) Int. J. Food Microbiol. 2011;151(2):252–256. doi: 10.1016/j.ijfoodmicro.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Bianchini V, Recordati C, Borella L, Gualdi V, Scanziani E, Selvatico E, Luini M. Helicobacteraceae in bulk tank milk of dairy herds from Northern Italy. BioMed. Res. Int. 2014;2014:4. doi: 10.1155/2015/639521. Article ID:63952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mousavi S, Dehkordi S.F, Rahimi E. Virulence factors and antibiotic resistance of Helicobacter pylori isolated from raw milk and unpasteurized dairy products in Iran. JVATiTD. 2014;20:51. doi: 10.1186/1678-9199-20-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghasemian Safaei H, Rahimi E, Zandi A, Rashidipourb A. Helicobacter pylori as a zoonotic infection:the detection of Helicobacter pylori antigens in the milk and faeces of cows. J. Res. Med. Sci. 2011;16:184–187. [PMC free article] [PubMed] [Google Scholar]
- 27.Dunne C, Dolan B, Clyne M. Factors that mediate colonization of the human stomach by Helicobacter pylori. World J. Gastroentero. 2014;20(19):5610–5624. doi: 10.3748/wjg.v20.i19.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modolo L.V, de Souza A.X, Horta L.P, Araujo D.P, de Fatima A. An overview on the potential of natural products as ureases inhibitors:A review. J. Adv. Res. 2015;6:35–44. doi: 10.1016/j.jare.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X, Doyle M.P. Effect of environmental and substrate factors on survival and growth of Helicobacter pylori. J. Food Protect. 1998;61:929–933. doi: 10.4315/0362-028x-61.8.929. [DOI] [PubMed] [Google Scholar]
- 30.Kianpour F, Mehdipour S.Z, Mazroee M.A, Kazemeini H.R, Rahimi E, Jafari A. Prevalence of Helicobacter pylori in bufflo milk in Iran. TLS. 2014;3(2):28–33. [Google Scholar]
- 31.Turutoglu H, Mudul S. Investigation of Helicobacter pylori in raw sheep milk samples. J. Vet. Med. B. Infect. Dis. Vet. Pub. Health. 2002;9:308–309. doi: 10.1046/j.1439-0450.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- 32.Oliver J.D. The viable but nonculturable state in bacteria. J. Microbiol. 2005;43:93–100. [PubMed] [Google Scholar]
- 33.Stevenson T.H, Castillo A, Lucia L.M, Acuff G.R. Grow of Helicobacter pylori in various liquid and plating media. Lett. Appl. Microbiol. 2000;30(3):192–196. doi: 10.1046/j.1472-765x.2000.00699.x. [DOI] [PubMed] [Google Scholar]


