Abstract
Aim:
This work aimed to study the role played by dogs in transmitting zoonotic enteric parasites to humans in Egypt and to analyze the risk factors associated with the occurrence of such infection in dogs. Serodiagnosis of anti-Toxocara immunoglobulin G (IgG) antibodies among human beings as well as analyzing risk factors predispose to Toxocara canis infection in human beings are another objectives of this study.
Materials and Methods:
From June to December 2013, a total of 130 fecal samples from 4 dog populations (Military, nomadic and domiciled dogs from rural and high standard districts) and 150 stool samples of 6 occupational groups were examined for the presence of enteric parasitic infection. Moreover, 150 serum samples were collected from humans from whom stool samples were collected and examined for the presence of anti-T. canis antibodies.
Results:
Enteric parasites were detected in 30% of fecal samples from 4 dog populations in Egypt. High infectivity had been reported in nomadic dogs (63.33%) (Crude odds ratios [COR]=67.36, 95% confidence interval [CI]=8.09-560.8, p<0.000), followed by domiciled dogs from rural areas (40%) (COR=26, 95% CI=3.14-215.54, p=0.003), domiciled dogs from high standard areas (23.33%) (COR=11.87, 95% CI=1.37-102.69, p=0.025) and military dogs (2.5%). Twelve species of enteric parasites were identified, Ancylostomatidae (6.15%), T. canis and Cryptosporidium spp. (5.38%, each), Heterophyes spp. (3.85%), Toxocara leonina and Blastocystis spp. (3.07%), Taenidae eggs (2.31%), Hymenolepis diminuta (1.54%) and Entamoeba canis, Cyclospora cayetanensis, and Paragonimus spp. (0.77%, each). Univariate logestic regression revealed significant association of age (COR=4.73, 95% CI=2.13-10.53, p<0.000), gender (COR=2.63, 95% CI=1.22-5.68, p<0.014), housing system (COR=5.10, 95% CI=2.04-12.75), p<0.000) with enteric parasitic infection in dogs. However, breeds (COR=6.91, 95% CI=0.88-54.52, p=0.067) and type of feeding (COR ranged from 3.5 to 7.62, p>0.05) did not seem to have a significant association among the examined dogs. Enteric parasitic infection was reported in 31/150 human stools (20.67%). Students were the most affected groups (37.14%), followed by nomadic people (24%), house wives (20%), house guarders and military workers (12%, each), and employees (10%). The identified parasites were Cryptosporidium spp. (9.33%), Ascaris lumbercoides (3.33%), Heterophyes spp. and Ancylostoma spp. (2.66%, each) and Paragonimus spp. and Hymenolepis nana (1.33%, each). Toxocara IgG antibodies were detected in 36/150 (24%) serum samples investigated. Toxocara IgG antibodies were more prevalent in males (26.66%) than females (20%). Seroprevalence was highest (17/35, 48.57%) in 7-15 years old (COR=6.93, 95% CI=1.75-27.43, p=0.006). Seroprevalence values for T. canis antibodies were higher in those; raising dogs (29.85%), eating raw vegetables (25.21%) and not washing hands before meals (25.45%). T. canis antibodies were detected in 25% of those contacted with soil compared to 30% of those did not. Students were mostly affected (34.29%), followed by nomadic people (32%), house guarders (28%), housewives (20%), military workers (13%), and employees (10%).
Conclusion:
Detection of enteric parasites in dogs and humans in Egypt substantiates the role posed by dogs in transmitting zoonotic parasites to humans and knock an alarm for common sources of infection for humans and dogs. Common sources may be infected fish or contaminated vegetables that are consumed by dogs or humans or even infected rodents that may contaminate their feed. This pilot study necessitate the need for similar studies and tracing such infection in fish, vegetables, rodent that may be responsible for infecting humans and dogs in order to understand the epidemiology of zoonotic parasitic infection transmitted from dogs to humans.
Keywords: dogs, enteric parasites, humans, risk factors, zoonoses
Introduction
“Dogs are the most successful canids, adapted to human habitation worldwide.” They provide their owners, particularly children with physical, social and emotional benefits [1]. However, in spite of these benefits, close contact of dogs with humans remain a major threat to public health as dogs are main reservoirs of many infective stages of parasites that can be transmitted to man and other domestic animals [1,2]. Dogs act as definitive or reservoir hosts for more than 60 zoonotic parasites, such as Toxocara canis, Echinococcus spp., Taenia spp., Dipylidium caninum, Ancylostoma spp., Giardia spp., as well as Cryptosporidium spp. [3].
In Egypt, like other developing countries, the risk of zoonotic infection related to domiciled, as well as stray dogs is high due to keeping of livestock and pets inside houses in most rural areas [4], less restrictive obligation placed on dog owners [5] and absence of public education about the risk of zoonotic diseases transmitted from dogs, as well as non-existence of a control strategy for stray dogs [6].
Toxocariasis is a zoonotic disease caused by T. canis. Human toxocariasis constitutes one of the most common parasitic infections worldwide, which is more prevalent in developing countries and poor communities. It is caused by ingesting the eggs which were shed in the feces of the definite dog host [7]. Human infection is mostly asymptomatic. Larvae liberate from the eggs in the intestines, penetrate the mucosa, escape to the portal circulation and disseminated into various organs where could possibly become encysted. T. canis cannot mature in humans due to inability to come back to the intestines where they normally go to in dogs, to lay eggs.
Demonstrating the presence of T. canis through traditional diagnostic methods is hard and has remained unsatisfactory as the parasite does not develop nor reproduce in man. Measuring anti-Toxocara immunoglobulin G (IgG) antibodies to excretory–secretory antigens of the larval stage of T. canis using Enzyme linked immunosorbent assays (ELISA) are the best laboratory option for diagnosis [7].
Zoonoses involving dog parasites are both common and important, with some causing serious diseases. Understanding the epidemiology of zoonotic parasitic infections is important to minimize the risk of human infection. The aim of this work was to study the role played by dogs in transmitting zoonotic enteric parasites to humans and to analyze the risk factors associated with occurrence of such infection in dogs. Information on the epidemiology of human toxocariasis in Egypt is scarce so serodiagnosis of anti-Toxocara IgG antibodies among human beings, as well as analyzing risk factors predispose to T. canis infection in human beings are another objectives of this study.
Materials and Methods
From June to December 2013, a total of 130 fecal samples from 4 dog populations (Military, nomadic and domiciled dogs from rural and high standard districts) and 150 stool samples of 6 occupational groups were examined for the presence of enteric parasitic infection. Moreover, 150 serum samples were collected from humans from whom stool samples were collected and examined for the presence of anti-T. canis antibodies.
Ethical approval
Ethical approval from dogs’ owner and assurance of anonymity, witnessed by a veterinarian from the Egyptian Veterinary Medicine Authority was obtained. Stool swabs and blood samples were also collected from human populations who gave an oral consent from the adult participants and parents of school children to participate in the present work.
Sampling
Fecal samples of dogs
Samples from 60 domiciled dogs were collected by the authors during morning visits to 40 randomly chosen domiciles situated in a high class district at Al-Obour city, Qalyubia province and a rural district at Meet El-Ezz village, Fakous city, Sharkia province (30 dogs, from 20 home in each district). All these were semi-restricted dogs, housed indoors or in the yard, reared without free access to the street. Samples from nomadic dogs (n=30) were obtained by their owners at different localities at Sharkia and Qalyubia provinces. These dogs were free roaming, housed outdoors, fed on garbage or were allowed to scavenge on rodents and dead animals and chickens. However, samples from military dogs (n=40) were collected by their care takers. These dogs were highly confined and received high level of care and veterinary attention. All animals did not receive any kind of medicine 4 weeks before collection of samples. Fresh fecal samples were collected from each dog population by the responsible person mentioned above from the ground in the morning after voiding by dogs and individually labeled in plastic container. Number of each dog population was illustrated in Table-1. Each sample was preserved in formalin (10%) until examined.
Table-1.
Frequency of enteric parasites in four dog populations from different localities in Egypt.
| Parasite spp. | Populations of dogs (positive (%)) | Total (n=130) | |||
|---|---|---|---|---|---|
| Military dogs (n=40) | Domiciled dogs from | Nomadic (n=30) | |||
| High standard area (n=30) | Rural area (n=30) | ||||
| Ancylostoma spp. | 1 (2.5) | 1 (3.33) | 2 (6.66) | 4 (13.3) | 8 (6.15) |
| T. canis | - | 1 (3.33) | 2 (6.66) | 4 (13.3) | 7 (5.38) |
| T. leonine | - | - | 1 (3.33) | 3 (10) | 4 (3.07) |
| D. caninum | - | - | - | 2 (6.66) | 2 (1.54) |
| Cryptosporidium spp. | - | 1 (3.33) | 2 (6.66) | 4 (13.33) | 7 (5.38) |
| Blastocystis spp. | - | 2 (6.66) | 2 (6.66) | - | 4 (3.07) |
| E. canis | - | 1 (3.33) | - | - | 1 (0.77) |
| C. caytanensis | - | - | - | 1 (3.33) | 1 (0.77) |
| Paragonimus spp. | - | - | 1 (3.33) | - | 1 (0.77) |
| Heterophyes spp. | - | - | 4 (13.33) | 1 (3.33) | 5 (3.85) |
| H. diminuta | - | - | - | 2 (6.66) | 2 (1.54) |
| Taenidae eggs | - | 2 (6.66) | - | 1 (3.33) | 3 (2.31) |
| Mono-infection | 1 (2.5) | 6 (20) | 10 (33.33) | 16 (53.33) | 33 (25.38) |
| Mixed infection | - | 1 (3.33)a | 2 (6.66)b | 3 (10)c | 6 (4.6) |
| Total parasite infection | 1 (2.5) | 7 (23.33) | 12 (40) | 19 (63.33) | 39 (30) |
Mixed infection with Cryptosporidium spp. + Blastocystis spp.
Mixed infection with Cryptosporidium spp. + Blastocystis spp. and Heterophyes spp. + Blastocystis spp.
Mixed infection with Cryptosporidium spp. + Ancylostoma spp., Cryptosporidium spp. + T. leonina and Cryptosporidium spp. + Cyclospora caytanensis
Human stool samples
A total of 150 stool samples from 6 occupational groups were collected by distributing clean cups on them a day before sampling and informing them to collect morning stool. Then the author visited them to pick up the collected stools. Number of each occupational group was illustrated in Table 2.
Table-2.
Frequency of enteric parasites in 6 occupational groups at different localities in Egypt.
| Enteric parasite spp. | Occupational groups (positive (%)) | Total (n=150) | |||||
|---|---|---|---|---|---|---|---|
| Military workers (n=25) | Nomadic people (n=25) | House guarders (n=25) | Employees (n=20) | House wives (n=20) | Students (n=35) | ||
| Heterophyes spp. | - | 1 (4) | - | - | 1 (5) | 2 (5.71) | 4 (2.67) |
| Paragonimus spp. | - | 1 (4) | - | 1 (5) | - | - | 2 (1.33) |
| Cryptosoridium spp. | 2 (8) | 1 (4) | 2 (8) | 1 (5) | 1 (5) | 7 (20) | 14 (9.33) |
| Ascaris lumbercoides | 1 (4) | 2 (8) | - | - | 1 (5) | 1 (2.86) | 5 (3.33) |
| Ancylostoma spp. | - | 1 (4) | 1 (4) | - | 1 (5) | 1 (2.86) | 4 (2.67) |
| Hymenolepis nana | - | - | - | - | - | 2 (5.71) | 2 (1.33) |
| Total | 3 (12) | 6 (24) | 3 (12) | 2 (10) | 4 (20) | 13 (37.14) | 31 (20.67) |
Parasitological examination
Each fecal sample was examined macroscopically for adult nematodes and tapeworm proglottids. Each sample was subjected for examination by centrifugal fecal floatation technique using zinc sulphate solution [8]. Furthermore, formol ether sedimentation technique [9] was applied for each sample. Iodine solution was used to facilitate protozoan and cyst identification. The modified Ziehl-Neelsen staining technique is used to detect oocysts in the feces. Parasites were identified on the basis of eggs, oocysts or cysts color, shape, and contents [8].
Serodiagnosis of anti-T. canis antibodies in human sera
A total of 150 serum samples were collected from participant from whom stool samples were collected and subjected to ELISA for detection of anti-T. canis antibodies using RIDASCREEN® Toxocara IgG Kits (K7421) as the followings:
Microwell plates coated with T. canis antigen were inoculated with 100 μl diluted sera in buffer diluent (1:50) and ready-to use negative and positive controls and incubated at 20-25°C for 15 min. The wells were then emptied into a waste container containing any disinfectant. After that, the plates were knocked out onto absorbent paper in order to remove the residual moisture. The plates were washed 5 times using 300 μl of diluted washing buffer (1 part wash buffer “phosphate-buffered NaCl solution” concentrate is mixed with 19 parts distilled water) each time. The wells were emptied completely by knocking them out on absorbent paper after each wash. One hundred microliter of the conjugate was added to each well. The plates were then incubated at 20-25°C for 15 min and then washed 5 times. One drop or 50 μl of each of the substrate (urea peroxide) and chromogen (tetramethylbenzidine) were placed in each well and then incubated at 20-25°C for 15 min. The reaction was stopped by adding 50 μl (or 1 drop) stop reagent (0.5 M sulphuric acid) to each well. After mixing carefully (by lightly tapping the side of the plate), the absorbance was measured at 450 nm in a plate photometer. The results were then evaluated and interpreted as the followings:
-
Calculating the sample index:
- The average absorbance is calculated for the negative control.
- The cut-off for the test was calculated by adding 0.150 to the average absorbance of the negative control.
- The sample index is obtained by dividing the absorbance for the sample by the cut-off.
-
Interpreting the test as the followings
- Sample index <0.9 is considered negative, 0.9−1.1 is equivocal and >1.1 is positive.
Risk factors assessment
The potential risk factors substantiate the occurrence and maintenance of enteric parasitic infection among the examined dog populations were studied through a questionnaire designed to obtain information about number, age, gender, breed, feeding, and housing system of each population (Table-3). Moreover, age and gender distribution of anti-T. canis antibodies as well as the influence of some risk factors on seroprevalence of T. canis infection in human populations were studied through a questionnaire included age, gender, raising of dogs, contact with soil, eating raw vegetables, washing hands before eating, as well as the occupation of the examined human populations (Table-4).
Table-3.
Risk factors for enteric parasitic infection in the investigated dog populations.
| Risk factors | Total examined | Enteric parasitic infection (n (%)) | COR | p value | |
|---|---|---|---|---|---|
| −ve | +ve | ||||
| Age | |||||
| >1 year | 83 | 68 (81.90) | 15 (18.10) | 1 | <0.000 |
| <1 year | 47 | 23 (48.90) | 24 (51.10) | 4.73 (2.13-10.53) | |
| Gender | |||||
| Male | 78 | 61 (78.20) | 17 (21.80) | 1 | 0.014 |
| Female | 52 | 30 (57.70) | 22 (42.30) | 2.63 (1.22-5.68) | |
| Breed | |||||
| Exotic | 15 | 14 (93.30) | 1 (6.70) | 1 | 0.067 |
| Local | 115 | 77 (67.00) | 38 (33.00) | 6.91 (0.88-54.52) | |
| Housing | |||||
| Individual | 55 | 48 (87.30) | 7 (12.70) | 1 | <0.000 |
| Communal | 75 | 43 (57.30) | 32 (42.70) | 5.10 (2.04-12.75) | |
| Dog population | |||||
| Military dogs | 40 | 39 (97.50) | 1 (2.50) | 1 | <0.000 |
| High standard area domiciled dogs | 30 | 23 (76.70) | 7 (23.30) | 11.87 (1.37-102.69) | 0.025 |
| Rural areas domiciled dogs | 30 | 18 (60.00) | 12 (40.00) | 26.00 (3.14-215.54) | 0.003 |
| Nomadic dogs | 30 | 11 (36.70) | 19 (63.30) | 67.36 (8.09-560.81) | <0.000 |
| Feeding | |||||
| Dry feed | 15 | 14 (93.30) | 1 (6.70) | 1 | 0.108 |
| Uncooked | 105 | 68 (64.80) | 36 (34.28) | 7.62 (0.96-60.24) | 0.054 |
| Cooked | 10 | 8 (80.00) | 2 (20.00) | 3.50 (0.27-44.95) | 0.336 |
COR=Crude odd ratio
Table-4.
Demographic characteristics of the seroprevalence of T. canis IgG antibodies among various human populations in Egypt.
| Risk factor | Groups | Number of examined | Number of positive (%) | COR (95% CI) | p value |
|---|---|---|---|---|---|
| Gender | Male | 90 | 24 (26.66) | 1.46 (0.66-3.19) | 0.35 |
| Female | 60 | 12 (20) | 1 | ||
| Age in years | 7-15 | 35 | 17 (48.57) | 6.93 (1.75-27.43) | 0.006* |
| 16-25 | 46 | 8 (17.39) | 1.54 (0.37-6.43) | 0.551 | |
| 26-35 | 44 | 8 (18.18) | 1.63 (0.39-6.80) | 0.503 | |
| 36-45 | 25 | 3 (12) | 1 | 0.003* | |
| Raising dogs | Yes | 67 | 20 (29.85) | 1.78 (0.84-3.79) | 0.134 |
| No | 83 | 16 (19.27) | 1 | ||
| Contact with soil | Yes | 120 | 30 (25) | 1.33 (0.50-3.57) | 0.567 |
| No | 30 | 6 (30) | 1 | ||
| Eating raw vegetables | Yes | 115 | 29 (25.21) | 1.35 (0.53-3.42) | 0.528 |
| No | 35 | 7 (20) | 1 | ||
| Washing hands before eating | Yes | 40 | 8 (20) | 0.73 (0.30-1.78) | 0.49 |
| No | 110 | 28 (25.45) | 1 | ||
| Occupation | Military workers | 25 | 3 (13) | 1 | 0.231 |
| Nomadic people | 25 | 8 (32) | 3.45 (0.79-15.01) | 0.099 | |
| House guarders | 25 | 7 (28) | 2.85 (0.64-12.64) | 0.168 | |
| Employees | 20 | 2 (10) | 0.82 (0.12-5.42) | 0.832 | |
| House wives | 20 | 4 (20) | 1.83 (0.36-9.35) | 0.466 | |
| Student | 35 | 12 (34.29) | 3.83 (0.95-15.42) | 0.059 |
COR=Crude odd ratio, CI=Confidence interval, IgG=Immunoglobulin G, T. canis=Toxocara canis,
=Significant association
Statistical analysis
Univariate logistic regression models were fitted to determine risk factors associated with prevalence of enteric parasite spp. in 4 populations of dogs from different localities in Egypt and to study the association of some risk factors with seropositivity of T. canis antibodies among 6 occupational groups in Egypt. The statistical software packages (SPSS for Windows 21.0, Inc., Chicago, IL, USA) was used for data analysis and results are expressed as numbers and percentages in brackets along with crude odds ratios (COR) and their 95% confidence interval (95% CI).
Results
Occurrence of enteric parasitic infection in dogs
The overall frequency of enteric parasitic infection of dogs was 30% (39/130), Comparing the prevalence of infection among the four dog populations examined, high infectivity had been reported in nomadic dogs (63.33%), followed by domiciled dogs from rural areas (40%), domiciled dogs from high standard areas (23.33%), however, military dogs had the lowest prevalence (2.5%) of parasitic infection (Table-1). Twelve species of intestinal enteric parasites were detected, in particular Ancylostomatidae (6.15%), T. canis and Cryptosporidium spp. (5.38%, each), Heterophyes spp. (3.85%), Toxocara leonina and Blastocystis spp. (3.07%, each), Taenidae eggs (2.31%), Hymenolepis diminuta and Dipylidium caninum (1.54%, each) and Entamoeba canis, Cyclospora caytanensis, and Paragonimus spp. (0.77%, each). The majority of dogs were infected only with one species parasite (25.38%). Mixed infection caused by two species was recorded in 4.6% of all examined dogs (3.33% in domiciled dogs from high standard areas, 6.66% in domiciled dogs from rural areas and 10% of nomadic dogs) (Table-1, Figure-1 and Figure-2).
Figure-1.
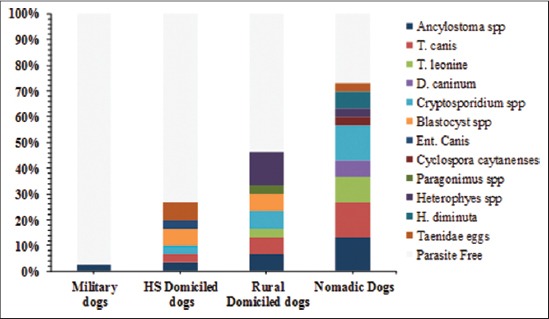
Frequency of enteric parasites in four dog populations from different localities in Egypt. HS=High standard domiciled dogs.
Figure-2.
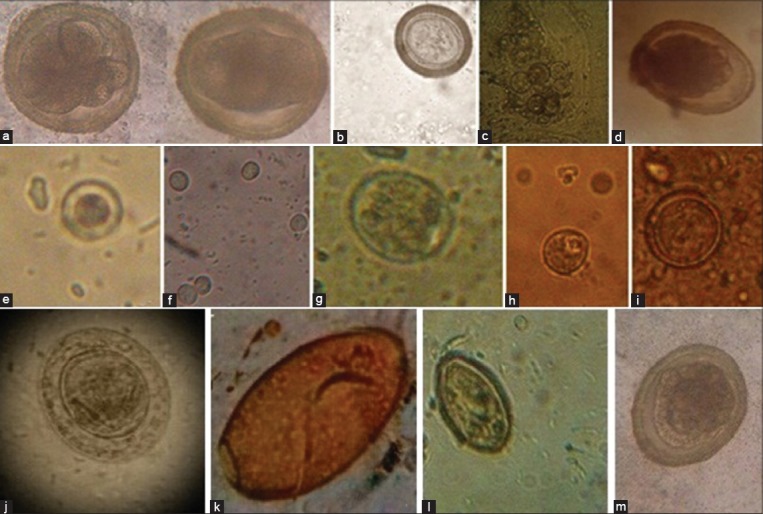
The identified enteric parasites. (a) Ancylostoma eggs, (b) Taenia eggs, (c) Dipylidium caninum eggs, (d) Ascaris lumbercoides eggs, (e) Blastocystis hominis, (f) Cryptosporidium oocyst, (g) Cyclospora caytanesis, (h) Entamobea canis, (i) Toxocara leonina, (j) Hymenolepis nana, (k) Paragonimus westermani, (l) Heterophyes eggs, (m) Toxocara canis eggs.
Risk factors assessment for maintenance of enteric parasitic infections among the four dog populations
Risk factors assessment for maintenance of enteric parasitic infections among the examined dogs using univariate logistic regression models revealed significant association of age (COR=4.73, 95% CI=2.13-10.53, p<0.000), gender (COR=2.63, 95% CI=1.22-5.68, p<0.014), housing system (COR=5.10, 95% CI=2.04-12.75), p<0.000) with the frequency of enteric parasitic infection among the examined dogs. However, breeds (COR=6.91, 95% CI=0.88-54.52, p=0.067) and type of feeding (COR ranged from 3.5 to 7.62, p>0.05) did not seem to have a significant association. Univariate logistic regression revealed that nomadic dogs were 67.36% more likely to be infected with enteric parasites than military dogs (95% CI=8.09-560.8, p<0.000) followed in descending order by dogs from rural areas (COR=26, 95% CI=3.14-215.54, p=0.003) and dogs from high standard areas (COR=11.87, 95% CI=1.37-102.69, p=0.025) (Table-3).
Occurrence of enteric parasitic infection in humans
Of 150 stool samples collected from 6 occupational groups and examined for the presence of enteric parasitic infection, 31 samples (20.67%) were positive. Students were the most affected groups (37.14%), followed by nomadic people (24%), house wives (20%), house guarders and military workers (12%, each), and employees (10%). Six parasite species were detected. Cryptosporidium spp. was the most frequently detected species (9.33%), followed by Ascaris lumbercoides (3.33%), Heterophyes spp. and Ancylostoma spp. (2.66%, each) and Paragonimus spp. and Hymenolepis nana (1.33%, each) (Table-2 and Figure-2).
Occurrence of Toxocara IgG antibodies in humans and risk factor assessment
Of 150 serum samples investigated, 36 (24%) were positive for Toxocara IgG antibodies as determined by RIDASCREEN® Toxocara IgG ELISA. Toxocara IgG antibodies were more frequent in males (26.66%) than females (20%). Seroprevalence was highest (17/35, 48.57%) in 7-15 years old, followed in sequence by 18.18% (8/44) in 26-35 years old, 17.39% (8/46) in 16-26 years old and 12% (3/25) in 35-50 years old. Seroprevalence values for T. canis antibodies were higher in those; raising dogs (29.85%, 20/67), eating raw vegetables (25.21%, 29/115) and not washing hands before meals (25.45%, 28/110). T. canis antibodies were detected in 25% of those contacted with soil compared to 30% of those did not. Students were mostly affected (34.29%), followed by nomadic people (32%), house guarders (28%), housewives (20%), military workers (13%), and employees (10%) (Table-4). Univariate logistic regression analysis revealed only a significant association of age of the examined humans with the seropositivity for T. canis antibodies (COR=6.93, 95% CI=1.75-27.43, p=0.006) among 7-15 years old age group (Table-4).
Discussion
Occurrence of enteric parasitic infection in dogs
“In many parts of the world, the intestinal parasites of dogs receive considerable attention because dogs serve as reservoirs, carriers and transmitters of several pathogens, including parasites, which are considered zoonotic and a number of them are of significant public health concern” [10]. The overall frequency of enteric parasitic infection of dogs in this study revealed a high level of infection (39/130, 30%) compared to the finding (33/180, 18.3%) from recent study carried out in northern part of Egypt [11]. The difference between both studies in spite of conducting at the same country may be related to difference in locality and nature of the examined dogs. They collected their samples from Alexandria province that has higher socio-economic level than Sharkia and Qalyubia provinces from which our samples have been collected. Moreover, their study was confined to 120 police dogs and 60 house dogs that receive high level of care (hygienic measures and veterinary attention) than nomadic and rural dogs included in the current study. Higher prevalence of enteric parasitic infection among the examined dogs; 76% in South Africa [12], 85% in Mexico [13], 71% in Spain [14], 68.4% in Nigeria [15], 39.2% in Japan [16] and 46.7% in Nepal [3] were previously recorded. “The wide range of endoparasite prevalence may be related to geographical location, status of animal ownership, sampling protocols, demographic factors, anthelmintic usage, and diagnostic techniques” [17].
Mono-infection was the rule during this study in all dog populations; however, mixed infection was reported in a domiciled dog from high standard areas, 2 domiciled dog from rural areas and 3 nomadic dogs. These results agreed with those reported by Katagiri and Oliveira-Sequeira [17] who stated that dogs harboring one parasite were more common (31.4%) than those harboring two (18.5%), three (3.2%) or four (1.2%) and Ugbomoiko et al. [15] who reported single infection in 49.4% of examined dogs compared to 13.1%, 4.5% and 1.3% for those harboring two, three and four or more parasites, respectively. On the other hand, mixed infection with more than one species was more common than mono-infection as reported by Dalimi et al. [18], (75.68% for polyinfection vs. 24.32% for mono-infection) and Eslami et al. [19] who found that 80% of the examined dogs were polyparasitised.
Twelve species of enteric parasites were detected in fecal samples of dogs, in particular Ancylostomatidae (6.15%), T. canis and Cryptosporidium spp. (5.38%, each), Heterophyes spp. (3.85%), T. leonina and Blastocystis spp. (3.07%), Taenidae eggs (2.31%), H. diminuta and D. caninum (1.54%, each) and Entamoeba canis, Cyclospora cayetanensis, and Paragonimus spp. (0.77%, each). The same parasite species were recorded in dogs all over the world. Ancylostoma spp., were previously recorded in dogs with percentages of 37.8 in Brazil [17], 16.9 in Nigeria [15] and 1.9 in Japan [16].
T. canis were frequently recorded in dogs either, stray, domiciled, police or military dogs. Ahmed et al. [11] in Egypt detected T. canis in 0.8% of police dogs and 5% of house dogs. In Iran, Eslami et al. [19], Mirzaei and Fooladi [20] and Adinezadeh et al. [21] detected T. canis in 22%, 7% and 4.3% of the examined stray dogs, respectively. Moreover, T. canis infections were recorded in dogs with values of, 1.4% of domiciled dogs in UK [22], 13.9% of stray dogs and 3.2% of domiciled dogs in Brazil [17], 41.7% of domiciled dogs in Nigeria [15], 13.3% of military dogs in Turkey [23] and 25% of stray dogs in Japan [16].
T. leonina was previously detected in house dogs (1.7%) but not in police dogs in Alexandria, Egypt [11]. Dalimi et al. [18], Adinezadeh et al. [21] and Kimura et al. [16] detected T. leonina in 32.53%, 53% and 0.5% of the examined stray dogs, respectively.
Similar frequency of Cryptosporidium spp. in faeces of dogs (5%) to those recorded in this study (5.38%) was previously reported by Fatahi-Burani [24]. Lower prevalence of Cryptosporidium spp. was previously recorded by Katagiri and Oliveira-Sequeira [17] in Brazil (3.1%) and Ahmed et al. [11] in Egypt (1.7%). Detection of Cryptosporidium spp. oocyst in the examined dogs may be attributed to the fact that dogs eat faces of dogs, as well as faces of many other species as well. “This brings the possibility that Cryptosporidium was from ingested faces of other species and may just have been passing through the dog, not an actual infection.” The lack of these infections in military dogs which were well managed and not allowed to roam would support this.
Nearly similar frequency of D. caninum to those recorded in this study were reported by Katagiri and Oliveira-Sequeira [17] and Eslami et al. [19] whose results were 4.6% and 4%, respectively. However, Adinezadeh et al. [21] detected D. caninum in 46% of the examined dogs. In Giza, Egypt [25] detected D. caninum in 13.33% of puppies and 66.67% of adult dogs immediately after hunting and in 53.33% of puppies and 66.6% of adult dogs after 3 months in captivity.
Blastocystis spp. was detected in 3.07% of the examined dogs in this study. Recently, a special concern has been paid to Blastocystis spp. as agent of human intestinal disease. A high prevalence of Blastocystis hominis has been reported in Egypt among 22.4% of asymptomatic patients [26] and 12.1% of patients with diarrhea and immunosuppressed children [27]. Blastocysis spp., were previously isolated from 10% of the examined cats in Sharkia province, Egypt [28]. Blastocystis spp. causes a variety of nonspecific symptoms including intense abdominal disorders, together with pain; diarrhea and constipation were reported in most cases [29].
H. diminuta was detected in 1.54% of the examined dogs in this study. El Shazly et al. [26] detected H. diminuta in 1.4% of human stools in Dakahlia province, Egypt. Moreover, Abd El-Wahed et al. [30] stated that rodents are considered the main reservoir of this parasite in Egypt that may infect animals or humans.
Risk factors assessment for maintenance of enteric parasitic infections among the 4 dog populations
Potential risk factors for occurrence and maintenance of parasitic infection among various dog populations at different localities in Egypt were studied during this work (Table-3). This study showed a significantly higher enteric parasitic infection (p>0.000) in dogs under the age of 1 year (51.10%) compared with those above 1 year old (18.10%). Higher prevalence of enteric parasitic infection in puppies than adults were previously recorded by Eslami et al. [19] in Iran, Abere et al. [31] in Ethiopia and Ahmed et al. [11] in Egypt. They attributed this finding to the age acquired specific immunity against parasites or probably as a result of multiple re-infections. Moreover, Oliveira-Sequeira et al. [6] related the highest occurrence in puppies to transplacental or transmammary infection during the first few days of life.
Regarding gender as a risk factor for acquiring enteric parasitic infection, female dogs were 2.63 times at risk of acquiring enteric parasitic infection than male dogs (p=0.014). These results agreed with Davoust et al. [32] in Gabon and Ahmed et al. [11] in Egypt. On contrary, Zelalem and Mekonnen [33] in Ethiopia reported high prevalence of enteric parasitic infection in male dogs (79.2%) than female ones (76.8%). However, Mirzaei and Fooladi [20] and Gharekhani [34] reported non-significant difference in the overall prevalence between males and females.
Breeds were found to be a non-significant risk factor for occurrence of enteric parasitic infection among dog populations in Egypt (33% in local breeds versus 6.7% in exotic breeds, COR=6.91, 95% CI=0.88-54.52, p=0.067). Zelalem and Mekonnen [33] reported higher prevalence of helminthes in exotic breeds (81.3%) than local breed dogs (76.5%). However, Swai et al. [35] in Tanzania and Ahmed et al. [11] in Egypt stated that all breeds equally susceptible to infection if exposed to infected material. The higher occurrence of enteric parasitic infection among local than exotic breeds examined during this study may be related to the fact that Egyptians deal with exotic breeds with great care, vaccinate and deworm them, not let them move freely out doors and seek a veterinarian support if they acquired any illness due to their high prices.
Parasitic infection was found to be more frequent in dogs fed uncooked feeds (34.28%) than those fed cooked (20%) and dry (6.7%) feeds. Univariate regression analysis clarified that; type of feeding did not consider a significant risk factor for maintenance of parasitic infection among dogs (p>0.05) and that undercooked feed was 7.62 times carrying the risk of enteric parasitic infection than cooked and dry feed (Table-3). These results were in accordance with those reported by Zelalem and Mekonnen [33] and Ahmed et al. [11]. Uncooked feed that were introduced to dog in Egypt mainly included viscera of fish and chicken that may carry many parasitic infection, while cooking of feed can kill or inactivate infective eggs or cysts of gastrointestinal helminthes which could be transferred to dogs via feed [33].
Communal housing of dogs was found to be a significant risk factor for acquiring enteric parasitic infection than individual housing (42.7% vs. 12.7%, COR=5.10, 95% CI=2.04-12.75, p<0.000). This may be related to direct spread of infection through feeds contaminated with faces of dogs infected with various parasitic infections.
Univariate logistic regression revealed that nomadic dogs were 67.36% more likely to be infected with enteric parasites than military dogs (95% CI=8.09-560.8, p<0.000) followed in descending order by dogs from rural areas (COR=26, 95% CI=3.14-215.54, p=0.003) and dogs from high standard areas (COR=11.87, 95% CI=1.37-102.69, p=0.025). Mateus et al. [36] reported differences in the prevalence of enteric parasites according to nature of the examined dogs (59.8% in environmental dogs, 57.44% in farm dogs and 81.18% in hunting dogs) in Portugal. Higher frequency of enteric parasitic infection in nomadic dogs may be related to the free roaming nature of these dogs that categorize them as stray dogs. These dogs roam freely and mostly live outdoors, eat garbage, scavenge rodents and other dead animals that may be a potential source for their infection with enteric parasites. Furthermore, high infection rate in domiciled dogs from rural areas than high standard areas may be related to lower hygienic measures, communal nature of living, less or no veterinary attention in contrast to better hygienic and veterinary care and keeping majority of dogs in flats in urban areas [37]. Ahmed et al. [11] in Egypt recorded parasitic infection in 7.5% of police dogs compared to 40% in house dogs. In Turkey, Senlik et al. [23] found that 30.4% of Turkish military dogs frequently harbor intestinal nematodes. They concluded that, however, the hygienic measures, regular deworming, high quality feeding of police and house dogs, different parasites were recorded.
Occurrence of enteric parasitic infection in humans
In developing countries, Cryptosporidium spp. infections have been frequently incriminated in diarrheal disease in humans, particularly in children younger than 5 years. Cryptosporidium parvum (human and cattle genotype) and Cryptosporidium hominis have been identified in a majority of human patients. Other species (Cryptosporidium meleagridis and Cryptosporidium felis) and genotypes (C. parvum dog genotype) were diagnosed in a proportion of immunocompetent children [38]. In this study, Cryptosporidium spp. was the most frequently detected parasites in human stool samples (9.33%). In Egypt, studies on Cryptosporidium spp. among individuals with diarrhea attending inpatient and outpatient clinics reported prevalence ranges from 0% to 49% [26,39-41]. Sargent et al. [42] detected Cryptosporidium spp. in dogs and cats and concluded that these animals may represent potential sources of infection for human. Moreover, the possibility of Cryptosporidium transmission among human and dogs has been reported by Xiao et al. [43] who diagnosed Ctenocephalides canis infection in 2 children and their dog during the same period.
A. lumbercoides was the second most frequently detected enteric parasites in human stool (3.33%). Similar prevalence of A. lumbercoides in stool samples (4.9%) was previously recorded in Egypt by Esteban et al. [44]. Higher frequencies were recorded by El Sahn et al. [45] in Egypt, Dreyer et al. [46] in Brazil and Agi [47] in Nigeria. Other studies showed lower prevalence that were 1.4% [48] and 1.8% [26]. Shalaby et al. [49] detected A. lumbricoides in 8% of examined dogs and clarified the role played by dogs as a reservoir for and an environmental contaminator with A. lumbricoides which in turn increasing the risk of humans infection.
Ancylostoma spp. and Heterophyes spp. were the third detected enteric parasites in human stools (2.67%, each). Ancylostoma spp. has been considered one of the most frequent intestinal parasites of dogs [6]. Not only A. caninum but also different Ancylostoma species are involved in human infection mainly cutaneous larva migrans [50]. Eosinophilic enteritis and unexplained abdominal pain with peripheral eosinophilia are other manifestation of A. caninum infection in humans. Bahgat et al. [51] detected IgG antibodies to A. caninum in 11 out of 95 (11.6%) patients with obscure acute or recurrent abdominal pain.
Heterophyiasis is a highly endemic disease in Egypt. Fishermen (33.8%) [52] and local residents in northern Egypt (13.3%) [53] are the highest susceptible to infection. Detection of Heterophyes spp. in fecal samples of dogs (3.85%) and stool of humans (2.67%) in this study substantiates the evidence that they were infected through a common source of infection that may be fish. Heterophyid infection has been reported in 22% of brackish water fish and 42% for fresh water fish with an overall prevalence of 32% [53]. However, Ibrahim and Soliman [54] detected heterophyid metacercaria in 95.4% of fresh water fish examined in Ismailia province. Moreover, H. heterophyes was detected in 3% of stray cats in Kafr Elsheikh province in the northern region of the Delta [55] which may be scavenged by dogs or contaminates human food especially green vegetables and in turn transmit the infection to dogs and man.
H. nana and Paragonimus westermani were the fourth prevalent enteric parasites in human stools (1.33%, each). H. nana was recorded by Merwad [56], Esteban et al. [44], El Shazly et al. [26], and Bakr et al. [57] in stool samples in Egypt with percentages of 6.7, 4.9, 3.9, and 3, respectively. Moreover, H. nana frequency varied from country to country; 7.2% in Morocco [58], 14.29% in Venezuela [59], 0.1% in Libya [60] and 11.3% in Ecuador [61]. H. nana infection may be either asymptomatic or symptomatic and mainly affect preschool and primary schoolchildren. Symptomatic manifestations include; abdominal pain, nausea, vomiting, and diarrhea, anorexia, itching, irritability, sleeplessness and enuresis [62], as well as anemia due to reducing the intestinal absorption of vitamin B12 and folic acid [63].
“Paragonimiasis is a zoonotic disease in which humans may act as definitive hosts. The prevalence of paragonimiasis throughout the world is difficult to ascertain. It was estimated 293 million people are at risk, whereas several million are actually infected with paragonimiasis” [64]. The acute phase (invasion and migration) of paragonimiasis is manifested by diarrhea, abdominal pain, fever, cough, urticaria, hepatosplenomegaly, pulmonary abnormalities, and eosinophilia. Pulmonary manifestations include cough, expectoration of discolored sputum, hemoptysis, and chest radiographic abnormalities are the main characteristics of the chronic phase. More severe manifestations occur as a result of extrapulmonary localization of the adult worms, especially when the brain is involved [65]. Detection of Paragonimus spp. eggs in 0.77% of dogs and 1.33% of human in this study may be related to a common source of infection. This source may be raw or undercooked crustaceans.
In human, examining stool for parasite and eggs using the direct parasitological diagnosis is not useful and extremely difficult because T. canis cannot mature to the adult stage, thus serological methods are the diagnostic method of choice. Serological diagnosis of toxocariasis can be done using ELISA for detection of anti-T. canis antibodies against larval excretory secretory antigen [66].
The frequency of anti-T. canis antibodies among human populations in Egypt in this study (24%, 36/150) were higher than those recorded in developed countries; 0.7% in New Zealand, 1.6% in Japan, 2.4% in Denmark, 3.9% in Canada, 7.5% in Australia, 14% in USA, 15% in Poland [66-68]. By contrast, higher seroprevalence have been recorded in less developed countries in; Asia (81% in Nepal, 63.2% in Indonesia and 58% in Malaysia), Africa (30% in Nigeria, 45% in Swaziland and 93% in La Reunion), and South America (36% in Brazil and 37% in Peru) [69-71].
Occurrence of Toxocara IgG antibodies in humans and risk factor assessment
The frequency of anti-T. canis antibodies among the examined males (26.66%) was higher than females (20%). These results agreed with Won et al. [72], Roldan et al. [69] and Sariego et al. [7]. “High frequency in males than females may be attributed to the observation that males tend to spend more time outdoors than females and are generally less particular about personal hygiene, thus being at risk of contact with parasites” [67]. On the other hand, Alonso et al. [73] and Alderete et al. [74] reported that the association between male gender and seropositivity to toxocara infection is not consistently present. Regarding gender as a risk factors for acquiring enteric parasitic infection, univariate regression analysis verified that gender did not seem to be an important risk factor attributed to T. canis infection among humans in Egypt (COR=1.46, 95% CI=0.66-3.19, p=0.35). This is in accordance with the results reported by Fan et al. [75] in Taiwan, Ajayi et al. [76] in Nigeria, and Sadjjadi et al. [77] in Spain.
Moreover, age group (7-15 years old) was found to be significantly infected with enteric parasitic infection more than other age groups (COR=6.93, 95% CI=1.75-27.43, p=0.006). Fan et al. [75] stated that the age was not an important factor related to T. canis infection.
On the other hand, a non-significant association was observed between raising of dogs and toxocara infection in humans in Egypt. A recent study indicated that dogs infected with T. canis might infect people by direct contact because of the high density of embryonated eggs in their fur [78]. Nevertheless, high seropositive rates of 29.85% in dog owners and 19.27% in non-owners of dogs in the present study suggest that these two groups are equally at risk of being infected. The results are in line with those of Fan et al. [75] who reported a similar frequency of anti-T. canis antibodies among dog owners (79.4%) and non-owners (67.9%). Moreover, Woodruff et al. [79] observed that 50% of patients with clinical toxocariasis had never owned a dog or had close contact with pets.
A non-significant association were found between playing with soil (COR=1.33, 95% CI=0.50-3.57, p=0.567), eating of raw vegetables (COR=1.35, 95% CI=0.53-3.42, p=0.528) and lacking of hand washing before meals (COR=0.73, 59% CI=0.30-1.78, p=0.49) with the frequency of anti-T. canis antibodies in the examined human sera. Fu et al. [80] also recorded insignificant association between contact with soil or consumption of raw vegetables with the frequency of anti-T. canis antibodies in human sera. In spite of insignificant association between eating of raw vegetables and the seropositivity to T. canis infection, raw vegetable should be considered as a potential source of T. canis since its detection in different types of vegetables all over the world Kozan et al. [81] in Turkey, Abougraina et al. [82] in Libya and Doaa [83] in Alexandria, Egypt. Doaa [83] detected T. canis eggs in rocket (25%), lettuce (31.7%), parsley (20%), leek (8.3%) and green onion (10%) with a total contamination of 19%.
Although there was no significant association between contact with soil and seropositivity to T. canis infection, contact with soil should be done with precaution and proper personal hygiene must be carried out after contact with soil since the reported existence of Toxocara eggs in soil samples in previous studies; Rinaldi et al. [84] in Italy, Martinez-Moreno et al. [14] in Spain, Mizgajska et al. [85] in Poland and de Castro et al. [86] in Brazil.
In Egypt, there is no information about whether the frequency of anti-T. canis antibodies in various occupational groups either exposed or not to dogs. The present study was performed to investigate whether the frequency of anti-T. canis antibodies was associated with certain occupation. Students were the most frequently infected groups (34.29%), followed by nomadic people (32%), house guarders (28%), and house wives (20%), however, military workers (13%) and employees (10) were the least infected groups. Univariate regression analysis revealed no significant association between occupation of the examined persons and the frequency of anti-T. canis antibodies. In Egypt, Sabry and Lotfy [25] detected anti-T. canis antibodies in 3/20 (15%) of workers with no history of contact with dogs and 0% in those contacted with dogs. Sadjjadi et al. [77] in Iran detected IgG against T. canis in 25.6% of the examined children. Anti-T. canis antibodies were detected in 1.8% of gardeners and 13% of waste pickers in Mexico [87,88]. Fan et al. [68] stated that young children up to the age of 12 years appear to be the primary population susceptible to T. canis infection because of dirt pica, poor hygiene or frequent contact with dirt.
Conclusion
Detection of enteric parasites in dogs and human in Egypt substantiate the role posed by dogs in transmitting zoonotic parasites to humans and knock an alarm for common sources of infection for humans and dogs. Common sources may be infected fish or contaminated vegetables that are consumed by dogs or humans or even infected rodents that may contaminate their feed. This pilot study necessitate the need for similar studies and tracing such infection in fish, vegetables, rodent that may be responsible for infecting humans and dog in order to understand the epidemiology of zoonotic parasitic infection transmitted from dogs to human.
Authors’ Contributions
MAIA and LMAS: Planned the study design, collected and examined samples, drafted and revised the manuscript. Both authors read and approved the final manuscript.
Acknowledgments
The authors are thankful to Dr. Abdoraboh E. M. Hammad, Colonel Surgeon, Egyptian Military Forces for collection of blood samples of humans and bringing fecal samples from military dogs, Prof. Dr. Mohamad S. M. Nada, Professor of Parasitology, Faculty of Veterinary Medicine, Zagazig University for identification of parasites, Mohamad Afifi, demonstrator of Statistics, Faculty of Veterinary Medicine, Zagazig University for statistical analysis. This work was done on authors’ expense without funding from any organization. Necessary facilities of department of Zoonoses, Faculty of Veterinary Medicine, Zagazig University were used.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Robertson I.D, Irwin P.J, Lymbery A.J, Thompson R.C.A. The role of companion animals in the emergence of parasitic disease. Int. J. Parasitol. 2000;30:1369–1377. doi: 10.1016/s0020-7519(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux D.H. ‘Neglected’ diseases but unrecognized successes – Challenges and opportunities for infectious disease control. Lancet. 2004;364:380–383. doi: 10.1016/S0140-6736(04)16728-7. [DOI] [PubMed] [Google Scholar]
- 3.Satyal R.C, Manandhar S, Dhakal S, Mahato B.R, Chaulagain S, Ghimire L, Pandeya Y.R. Prevalence of gastrointestinal zoonotic helminthes in dogs of Kathmandu, Nepal. Int. J. Infect. Microbiol. 2013;2:91–94. [Google Scholar]
- 4.Youssef A.I, Uga S. Review of parasitic zoonoses in Egypt. Trop. Med. Health. 2014;42(1):3–14. doi: 10.2149/tmh.2013-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macpherson C.N.L. Human behavior and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005;35:1319–1331. doi: 10.1016/j.ijpara.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira-Sequeira T.C, Amarante A.F, Ferrari T.B, Nunes L.C. Prevalence of intestinal parasites in dogs from Sao Paulo State, Brazil. Vet. Parasitol. 2002;3:19–27. doi: 10.1016/s0304-4017(01)00575-1. [DOI] [PubMed] [Google Scholar]
- 7.Sariego I, Kanobana K, Junco R, Vereecken K, Núñez F.A, Polman K, Bonet M, Rojas L. Frequency of antibodies to Toxocara in Cuban schoolchildren. Trop. Med. Int. Health. 2012;17(6):711–714. doi: 10.1111/j.1365-3156.2012.02996.x. [DOI] [PubMed] [Google Scholar]
- 8.Zajac A.M, Conboy G.A. Veterinary Clinical Parasitology. 8th ed. UK: John Wiley and Sons; 2012. pp. 40–87. [Google Scholar]
- 9.Lee J, Kang S, Kim N, Lee C, Ahn K, Kwon H, Park C, Kim S. Investigation of helminths and protozoans infecting old world monkeys: Captive vervet, cynomolgus, and rhesus monkeys. Korean J. Vet. Res. 2010;50(4):273–277. [Google Scholar]
- 10.Gracenea M, Gomez M.S, Torres J. Prevalence of intestinal parasites in shelter dogs and cats in the metropolitan area of Barcelona (Spain) Acta Parasitol. 2009;54:73–77. [Google Scholar]
- 11.Ahmed W.M, Mousa W.M, Aboelhadid S.M, Tawfik M.M. Prevalence of zoonotic and other gastrointestinal parasites in police and house dogs in Alexandria, Egypt. Vet. World. 2014;7:275–280. [Google Scholar]
- 12.Minnaar W.N, Krecek R.C, Fourie L.J. Helminths in dogs from a peri-urban resource-limited community in free State province, South Africa. Vet. Parasitol. 2002;107:343–349. doi: 10.1016/s0304-4017(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 13.Eguia-Aguilar P, Cruz-Reyes A, Martinez-Maya J.J. Ecological analysis and description of the intestinal helminthes present in dogs in Mexico City. Vet. Parasitol. 2005;127:139–146. doi: 10.1016/j.vetpar.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Moreno F.J, Hernandez S, Lopez-Cobos E, Becerra C, Acosta I, Martinez-Moreno A. Estimation of canine intestinal parasites in Cordoba (Spain) and their risk to public health. Vet. Parasitol. 2007;143:7–13. doi: 10.1016/j.vetpar.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Ugbomoiko U.S, Ariza L, Heukelbach J. Parasites of importance for human health in Nigerian dogs: High prevalence and limited knowledge of pet owners. BMC Vet. Res. 2008;4:49. doi: 10.1186/1746-6148-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura A, Morishima Y, Nagahama S, Horikoshi T, Dagawa A, Kawabuchi-Kurata T, Sugiyama H, Yamasaki H.A. Coprological survey of intestinal helminthes in stray dogs captured in Osaka prefecture, Japan. J. Vet. Med. Sci. 2013;75(10):1409–1411. doi: 10.1292/jvms.12-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katagiri S, Oliveira-Sequeira T.C.G. Prevalence of dog intestinal parasites and risk perception of zoonotic infection by dog owners in Sa˜o Paulo State, Brazil. Zoonoses and Public Health. 2008;55:406–413. doi: 10.1111/j.1863-2378.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 18.Dalimi A, Sattari A, Motamedi G.H. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet. Parasitol. 2006;142:129–133. doi: 10.1016/j.vetpar.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Eslami A, Ranjbar-Bahadori S.H, Meshgi B, Dehghan M, Bokaie S. Helminth infections of stray dogs from garmsar, semnan province, central Iran. Iran. J. Parasitol. 2010;5:37–41. [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzaei M, Fooladi M. Prevalence of intestinal helminthes in owned dogs in Kerman city, Iran. Asian Pac. J. Trop. Med. 2012;5:735–737. doi: 10.1016/S1995-7645(12)60116-3. [DOI] [PubMed] [Google Scholar]
- 21.Adinezadeh A, Kia E.B, Mohebali M, Shojaee S, Rokni M.B, Zarei Z, Mowlavi G. Endoparasites of stray dogs in mashhad, khorasan razavi province, Northeast Iran with special reference to zoonotic parasites. Iran. J. Parasitol. 2013;20(8):459–466. [PMC free article] [PubMed] [Google Scholar]
- 22.Batchelor D.J, Tzannes S, Graham P.A, Wastling J.M, Pinchbeck G.L, German A.J. Detection of enteric parasites with zoonotic potential in dogs with gastrointestinal disease in the UK. Transbound. Emerg. Dis. 2008;55(2):99–104. doi: 10.1111/j.1865-1682.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 23.Senlik B, Cirak V.Y, Karabacak A. Intestinal nematode infections in Turkish military dogs with special reference to Toxocara canis. J. Helminthol. 2006;80(3):299–303. [PubMed] [Google Scholar]
- 24.Fatahi-Burani Z. Garmsar branch: Collage of Veterinary Medicine, Islamic Azad University. Study on the infection to cryptosporidiosis in stray dogs of Garmsar. 2005 [Google Scholar]
- 25.Sabry M.A, Lotfy H.S. Captive dogs as reservoirs of some zoonotic parasites. Res. J. Parasitol. 2009;4(4):115–122. [Google Scholar]
- 26.El Shazly A.M, Awad S.E, Sultan D.M, Sadek G.S, Khalil H.H, Morsy T.A. Intestinal parasites in Dakahlia Governorate, with different techniques in diagnosing protozoa. J. Egypt. Soc. Parasitol. 2006;36:1023–1034. [PubMed] [Google Scholar]
- 27.Abdel-Hafeez E.H, Ahmad A.K, Ali B.A, Moslam F.A. Opportunistic parasites among immunosuppressed children in Minia District, Egypt. Korean J. Parasitol. 2012;50:57–62. doi: 10.3347/kjp.2012.50.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maysa A.I.A. Endoparasites of zoonotic importance. Glob. Vet. 2010;5(6):348–355. [Google Scholar]
- 29.Vassalos C.M, Papadopoulou C, Vakalis N.C. Blastocystosis: Emerging or reemerging potential zoonoses. Vet. Ital. 2008;44:679–684. [PubMed] [Google Scholar]
- 30.Abd El-Wahed M.M, Salem G.H, El-Assaly T.M. The role of wild rats as a reservoir of some internal parasites in Qalyubia Governorate. J. Egypt. Soc. Parasitol. 1999;29:495–503. [PubMed] [Google Scholar]
- 31.Abere T, Bogale B, Melaku A. Gastrointestinal helminth parasites of pet and stray dogs as a potential risk for human health in Bahir Dar town, north-western Ethiopia. Vet. World. 2013;6(7):388–392. [Google Scholar]
- 32.Davoust B, Normand T, Bourry O, Dang H, Leroy E, Bourdoiseau G. Epidemiological survey on gastrointestinal and blood-borne helminths of dogs in north east Gabon. Onderstepoort J. Vet. Res. 2008;75:359–364. doi: 10.4102/ojvr.v75i4.112. [DOI] [PubMed] [Google Scholar]
- 33.Zelalem G, Mekonnen A. Prevalence of gastrointestinal helminthes among dogs in Bahir Dar town, Ethiopia. World Appl. Sci. J. 2012;19(5):595–601. [Google Scholar]
- 34.Gharekhani J. Study on gastrointestinal zoonotic parasites in pet dogs in western Iran. Turk. Parazitol. Derg. 2014;38:172–176. doi: 10.5152/tpd.2014.3546. [DOI] [PubMed] [Google Scholar]
- 35.Swai E.S, Kaaya E.J, Mshanga D.A, Mbise E.W. A survey on gastro-intestinal parasites of nondescript dogs in and around Arusha Municipality, Tanzania. Int. J. Anim. Vet. Adv. 2010;3(2):63–67. [Google Scholar]
- 36.Mateus T.L, Castro A, Ribeiro J.N, Vieira-Pinto M. Multiple zoonotic parasites identified in dog feces collected in Ponte de lima, Portugal—A potential threat to human health. Int. J. Environ. Res. Public Health. 2014;11:9050–9067. doi: 10.3390/ijerph110909050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szabová E, Juriš P, Miterpáková M, Antolová D, Papajová I, Šefčíková H. Prevalence of important zoonotic parasites in dog populations from the Slovak Republic. Helminthol. 2014;44(4):170–176. [Google Scholar]
- 38.Xiao L, Fayer R, Ryan U, Upton S.J. Cryptosporidium taxonomy: Recent advances and implications for public health. Clin. Microbiol. Rev. 2004;17:72–97. doi: 10.1128/CMR.17.1.72-97.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youssef F.G, Adib I, Riddle M.S, Schlett C.D. A review of cryptosporidiosis in Egypt. J. Egypt. Soc. Parasitol. 2008;38:9–28. [PubMed] [Google Scholar]
- 40.Mousa K.M, Abdel-Tawab A.H, Khalil H.H, El-Hussieny N.A. Diarrhea due to parasites particularly Cryptosporidium parvum in great Cairo, Egypt. J. Egypt. Soc. Parasitol. 2010;40:439–450. [PubMed] [Google Scholar]
- 41.Helmy Y.A, Krucken J, Nockler K, von Samson-Himmelstjerna G, Zessin K.H. Molecular epidemiology of Cryptosporidium in livestock animals and humans in the Ismailia province of Egypt. Vet. Parasitol. 2013;193(1-3):15–24. doi: 10.1016/j.vetpar.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Sargent K.D, Morgan U.M, Elliot A.D, Thompson R.C.A. Morphological and genetic characterization of Cryptosporidium oocysts from domestic cats. Vet. Parasitol. 1998;77:221–227. doi: 10.1016/s0304-4017(98)00122-8. [DOI] [PubMed] [Google Scholar]
- 43.Xiao L, Cama V.A, Cabrera L, Ortega Y, Pearson J, Gilman R.H. Possible transmission of Cryptosporidium canis among children and a dog in a household. J. Clin. Microbiol. 2007;45:2014–2016. doi: 10.1128/JCM.00503-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esteban J.G, Gonzales C, Curtale F, Antoli-Muytoz C, Valero M.A, Bargues M.D, El-Sayed M, El-Wakeel A, Abdel-Wahab Y, Montresor A, Engles D, Savioli L, Mas-Comas S. Hyper endemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am. J. Trop. Med. Hyg. 2003;69:924–437. [PubMed] [Google Scholar]
- 45.El Sahn F.F, Deghedi B.M, Mahdy N.H, El Sahn A. The impact of intestinal parasitic infections on the nutritional status of primary school children in Alexandria, Egypt. J. Egypt. Public Health Assoc. 1997;72:113–151. [PubMed] [Google Scholar]
- 46.Dreyer G, Fernandes-Silva E, Alves S, Rocha A, Albuquerque R, Addis D. Patterns of detection of Strongyloides stercoralis in stool specimens: Implication for diagnosis and clinical trials. J. Clin. Microbiol. 1996;34(10):2569–2571. doi: 10.1128/jcm.34.10.2569-2571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agi P.I. Comparative helminth infections of man in two rural communities of the Niger Delta, Nigeria. West Afr. J. Med. 1997;16(7):232–236. [PubMed] [Google Scholar]
- 48.El-Kadi M.A, Dorrah A.O, Shoukry N.M. Patients with gastrointestinal complains due to enteric parasites, with reference to Entamoeba histolytica/dispar as detected by ELISA E. histolytica adhesion in stool. J. Egypt. Soc. Parasitol. 2006;36:53–64. [PubMed] [Google Scholar]
- 49.Shalaby H.A, Abdel-Shafy S, Derbala A.A. The role of dogs in transmission of Ascaris lumbricoides for humans. Parasitol. Res. 2010;106:1021–1026. doi: 10.1007/s00436-010-1755-8. [DOI] [PubMed] [Google Scholar]
- 50.Velho P.E.N, Faria A.V, Cintra M.L, Souza E.M, Moraes A.M. Larva migrans: A case report and review. Rev. Inst. Med. Trop. Sao Paulo. 2003;45:167–171. doi: 10.1590/s0036-46652003000300010. [DOI] [PubMed] [Google Scholar]
- 51.Bahgat M.A, El Gindy A.E, Mahmoud L.A, Hegab M.H, Shahin A.M. Evaluation of the role of Ancylostoma caninum in humans as a cause of acute and recurrent abdominal pain. J. Egypt. Soc. Parasitol. 1999;29:873–882. [PubMed] [Google Scholar]
- 52.Abou-Basha L.M, Abdel-Fattah M, Orecchia P, Di Cave D, Zaki A. Epidemiological study of heterophyiasis among humans in an area of Egypt. East Mediter. Health J. 2000;6:932–938. [PubMed] [Google Scholar]
- 53.Lobna S.M, Metawea Y.F, Elsheikha H.M. Prevalence of heterophyiosis in Tilapia fish and humans in Northern Egypt. Parasitol. Res. 2010;107:1029–1034. doi: 10.1007/s00436-010-1976-x. [DOI] [PubMed] [Google Scholar]
- 54.Ibrahim M.M, Soliman M.F. Prevalence and site preferences of heterophyid metacercariae in Tilapia zilli from Ismalia fresh water canal, Egypt. Parasite. 2010;17:233–239. doi: 10.1051/parasite/2010173233. [DOI] [PubMed] [Google Scholar]
- 55.Khalafalla R.E. A survey study on gastrointestinal parasites of stray cats in northern region of Nile delta, Egypt. PLoS One. 2011;6:20283. doi: 10.1371/journal.pone.0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merwad A.M.A. Zoonotic studies on helminthes causing diarrhea in man. Egypt: PhD Thesis of Veterinary Medical Science, Zoonoses, Faculty of Veterinary Medicine, Zagazig University; 2009. [Google Scholar]
- 57.Bakr I.M, Arafa N.A, Ahmed M.A, Mostafa M.H, Mohamed M.K. Prevalence of intestinal parasitosis in a rural population in Egypt, and its relation to socio-demographic characteristics. J. Egypt. Soc. Parasitol. 2009;39:371–381. [PubMed] [Google Scholar]
- 58.Habbary K, Tifnouti A, Bitton G, Mandil A. Intestinal parasitosis and environmental pollution: 1343 pediatric cases in Beni-Mellal, Morocco. Tunis Med. 2000;78:109–114. [PubMed] [Google Scholar]
- 59.Iris-Diaz A, Zulbey R.R, Angela M.B, Maria S.C, Ellen A, Marinella L.C, Ricardo T.A. Prevalence of intestinal parasites in children of Yukpa Ethina in Toromo, Zulia State, Venezuela. Rev. Med. Chile. 2006;134:72–78. doi: 10.4067/s0034-98872006000100010. [DOI] [PubMed] [Google Scholar]
- 60.Sadaga G.A, Kassem H.H. Prevalence of intestinal parasites among primary schoolchildren in Derna District, Libya. J. Egypt. Soc. Parasitol. 2007;37:205–215. [PubMed] [Google Scholar]
- 61.Jacobsen K.H, Ribeiro P.S, Quist B.K, Rybdeck B.V. Prevalence of intestinal parasites in young Quichua children in the highlands of rural Ecuador. J. Health Popul. Nutr. 2007;25:399–405. [PMC free article] [PubMed] [Google Scholar]
- 62.Romero Cabello R. Human Microbiology and parasitology 3rd edition Editorial Medica Panamericana. Mexico: 2007. pp. 1405–1412. [Google Scholar]
- 63.Mohammad M.A, Hegazi M.A. Intestinal permeability in Hymenolepis nana as reflected by non -invasive lactulosa/manitol dual permeability test and its impaction on nutritional parameters of patients. J. Egypt. Soc. Parasitol. 2007;37:877–891. [PubMed] [Google Scholar]
- 64.Blair D, Agatsuma T, Wang W. Paragonimiasis. In: Murrell K.D, Fried B, editors. Food-Borne Parasitic Zoonoses: Fish and Plant-Borne Parasites. Vol. 11. New York, NY: Springer; 2008. pp. 117–150. [Google Scholar]
- 65.CDC. Paragonimiasis, Clinical Features. 2010. [Last accessed on 06-09-2014]. https://en.wikipedia.org/wiki/Paragonimiasis .
- 66.Macpherson C.N.L. The epidemiology and public health importance of toxocariasis: A zoonosis of global importance. Int. J. Parasitol. 2013;43:999–1008. doi: 10.1016/j.ijpara.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Jarosz W, Mizgajska-Wiktor H, Kirwan P, Konarski J, Rychlicki W, Wawrzyniak G. Developmental age, physical fitness and Toxocara seroprevalence amongst lower – secondary students living in rural areas contaminated with Toxocara eggs. Parasitol. 2010;137:53–63. doi: 10.1017/S0031182009990874. [DOI] [PubMed] [Google Scholar]
- 68.Fan C.K, Liao C.W, Cheng Y.C. Factors affecting disease manifestation of toxocarosis in humans: Genetics and environment. Vet. Parasitol. 2013;193:342–352. doi: 10.1016/j.vetpar.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 69.Roldan W.H, Espinoza Y.A, Huapaya P.E, Huiza A.F, Sevilla C.R, Jimenez S. Frequency of human toxocariasis in a rural population from Cajamarca, Peru determined by DOT-ELISA test. Rev. Inst. Med. Trop. Sao Paulo. 2009;51:67–71. doi: 10.1590/s0036-46652009000200002. [DOI] [PubMed] [Google Scholar]
- 70.Liao C.W, Sukati H, D’Lamini P, Chou C.M, Liu Y.H, Huang Y.C, Chung M.H, Mtsetfwa J.S, Jonato J, Chiu W.T, Chang P.W.S, Du W.Y, Chan H.C, Chu T.B, Cheng H.C, Su W.W, Tu C.C, Cheng C.Y, Fan C.K. Seroprevalence of Toxocara canis infection among children in the Kingdom of Swaziland, Southern Africa. Ann. Trop. Med. Parasitol. 2010;104:73–80. doi: 10.1179/136485910X12607012373795. [DOI] [PubMed] [Google Scholar]
- 71.Schoenardie E.R, Scaini C.J, Brod C.S, Pepe M.S, Villela M.M, McBride A.J, Borsuk S, Berne M.E. Seroprevalence of Toxocara infection in children from southern Brazil. J. Parasitol. 2013;99:537–539. doi: 10.1645/GE-3182. [DOI] [PubMed] [Google Scholar]
- 72.Won K.Y, Kruszon-Moran D, Schantz P.M, Jones J.L. National seroprevalence and risk factors for zoonotic Toxocara spp. infection. Am. J. Trop. Med. Hyg. 2008;79:552–557. [PubMed] [Google Scholar]
- 73.Alonso J.M, Bojanich M.V, Chamorro M, Gorodner J.O. Toxocara seroprevalence in children from a subtropical city in Argentina. Rev. do Inst. de Med. Trop. de Sao Paulo. 2000;42:235–237. doi: 10.1590/s0036-46652000000400010. [DOI] [PubMed] [Google Scholar]
- 74.Alderete J.M.S, Jacob C.M.A, Pastorino A.C, Elefant G.R, Castro A.P, Fomin A.B, Chieffi P.P. Prevalence of Toxocara infection in schoolchildren from the Butanta region, Sao Paulo, Brazil. Mem. Inst. Oswaldo. Cruz. 2003;98:593–597. doi: 10.1590/s0074-02762003000500002. [DOI] [PubMed] [Google Scholar]
- 75.Fan C.K, Hung C.C, Du W.Y, Liao C.W, Su K.E. Seroepidemiology of Toxocara canis infection among mountain aboriginal schoolchildren living in contaminated districts in eastern Taiwan. Trop. Med. Int. Health. 2004;9:1312–1318. doi: 10.1111/j.1365-3156.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 76.Ajayi O.O, Duhlinska D.D, Agwale S.M, Njoku M. Frequency of human toxocariasis in Jos, lateau State, Nigeria. Mem. Inst. Oswaldo. Cruz. 2000;95:147–149. doi: 10.1590/S0074-02762000000200002. [DOI] [PubMed] [Google Scholar]
- 77.Sadjjadi S.M, Khosravi M, Mehrabani D, Orya A. Seroprevalence of Toxocara infection in schoolchildren in Shiraz, Southern Iran. J. Trop. Pediatr. 2000;46:327–330. doi: 10.1093/tropej/46.6.327. [DOI] [PubMed] [Google Scholar]
- 78.Wolfe A, Wright I.P. Human toxocariasis and direct contact with dogs. Vet. Rec. 2003;152:419–422. doi: 10.1136/vr.152.14.419. [DOI] [PubMed] [Google Scholar]
- 79.Woodruff A.W, de Savigny D.H, Jocobs D.E. Study of toxocaral infection in dog breeders. Br. Med. J. 1978;2:1747–1748. doi: 10.1136/bmj.2.6154.1747-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu C, Chuang T, Lin H, Wu C, Liu Y, Langinlur M.K, Lu M, Hsiao W.W, Fan C. Seroepidemiology of Toxocara canis infection among primary schoolchildren in the capital area of the republic of the Marshall Islands. BMC Infect. Dis. 2014;14:261. doi: 10.1186/1471-2334-14-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kozan E, Sevimli F.K, Köse M, Eser M, Ciçek H. Examination of helminth contaminated wastewaters used for agricultural purposes in Afyonkarahisar. Turk. Parazitol. Derg. 2007;31(3):197–200. [PubMed] [Google Scholar]
- 82.Abougraina A.K, Nahaisi M.H, Madia N.S, Saied M.M, Ghengheshc K.S. Parasitological contamination in salad vegetables in Tripoli – Libya. Iran. Food Control. 2010;21:760–762. [Google Scholar]
- 83.Doaa E.S. Detection of parasites in commonly consumed raw vegetables. Alex. J. Med. 2012;48(4):345–352. [Google Scholar]
- 84.Rinaldi L, Biggeri A, Carbone S, Musella V, Catelan D, Veneziano V, Cringoli G. Canine faecal contamination and parasitic risk in the city of Naples (Southern Italy) BMC Vet. Res. 2006;2:29. doi: 10.1186/1746-6148-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizgajska H, Jarosz W, Rejmenciak A. Distribution of sources of Toxocara spp. infection in urban and rural environments in Poland. Wiad Parazytol. 2001;47(3):399–404. [PubMed] [Google Scholar]
- 86.de Castro J.M, dos Santos S.V, Monteiro N.A. Contamination of public gardens along seafront of Praia Grande City, São Paulo, Brazil, by eggs of Ancylostoma and Toxocara in dogs feces. Rev. Soc. Bras. Med. Trop. 2005;38(2):199–201. doi: 10.1590/s0037-86822005000200017. [DOI] [PubMed] [Google Scholar]
- 87.Alvarado-Esquivel C. Toxocariasis in waste pickers: A case control seroprevalence study. PLoS One. 2013;8(1):54897. doi: 10.1371/journal.pone.0054897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alvarado-Esquivel C, Hernández-Tinoco J, Sánchez-Anguiano L.F. Toxocara infection in gardeners: A case control seroprevalence study. Asian Pac. J. Trop. Med. 2014;7(Suppl 1):S79–S81. doi: 10.1016/S1995-7645(14)60207-8. [DOI] [PubMed] [Google Scholar]


