Abstract
Triclosan (TCS) is a broad-spectrum antimicrobial agent that has been added to personal care products, including hand soaps and cosmetics, and impregnated in numerous different materials ranging from athletic clothing to food packaging. The constant disposal of TCS into the sewage system is creating a major environmental and public health hazard. Owing to its chemical properties of bioaccumulation and resistance to degradation, TCS is widely detected in various environmental compartments in concentrations ranging from nanograms to micrograms per liter. Epidemiology studies indicate that significant levels of TCS are detected in body fluids in all human age groups. We document here the emerging evidence—from in vitro and in vivo animal studies and environmental toxicology studies—demonstrating that TCS exerts adverse effects on different biological systems through various modes of action. Considering the fact that humans are simultaneously exposed to TCS and many TCS-like chemicals, we speculate that TCS-induced adverse effects may be relevant to human health.
Keywords: bioaccumulation, antibacterial, environmental hazard, hormone homeostasis, antimicrobial resistance, liver pathogenesis, precautionary principle
INTRODUCTION
First introduced in the early 1970s to the health care industry, 5-chloro-2-(2,4-dichlorophenoxy) phenol, commonly known as triclosan (TCS), is a synthetic, lipid-soluble antimicrobial agent that has been used in the United States and globally for more than 40 years as an antiseptic, disinfectant, or preservative in clinical settings (surgical scrubs), personal care products (e.g., hand soaps, shampoos, deodorants, laundry detergents, cosmetics), household items (e.g., cutting boards, kitchenware, textiles, packaging materials), and medical devices (e.g., surgical sutures, catheters, ureteral stents) (1–3). In hospitals, TCS has been employed in surgical scrubs and used in hand washing prior to surgery to eradicate microorganisms such as methicillin-resistant Staphylococcus aureus (MRSA) (4); however, the necessity and effectiveness of TCS-containing products in household and other non-health-care-related settings are the subject of an ongoing scientific and public debate, given the associated risks (5).
TCS is bacteriostatic at low concentrations, as it inhibits fatty acid biosynthesis through inhibition of the enoyl-acyl carrier protein reductase (FabI) enzyme by forming a noncovalent complex with NAD+ in the FabI active site (6, 7). As FabI is essential for normal cellular division, TCS-mediated FabI inhibition effectively suppresses the growth of numerous gram-negative and gram-positive bacteria, whereas at higher concentrations it induces K+ leakage, leading to membrane destabilization and a rapid bactericidal effect (8, 9). As a chlorinated biphenyl ethyl, TCS is structurally similar to polychlorinated biphenyls, bisphenol A, dioxins, and thyroid hormones (10). The aromatic nature of TCS and its high chlorine content make it resistant to degradation and persistent in the environment.
TCS is regulated in the United States by both the Food and Drug Administration (FDA) as an over-the-counter drug (e.g., an additive in hand soaps and deodorants) and the Environmental Protection Agency (EPA) as an antimicrobial agent (e.g., plastic films for packaging). In 1997, the FDA approved the use of TCS (0.3%) in Colgate Total toothpaste to prevent gingivitis and cavities. The use of TCS has been generally considered well-tolerated and safe. For this reason, manufacturers have been adding it to their consumer formulas for the past few decades in the hopes of providing the user with long-lasting antibacterial protection. As a result, the widespread use of TCS allows the chemical to enter the environment through many pathways. The majority of TCS is disposed of in municipal sewer systems, receives treatment in local wastewater treatment plants (WWTPs), and undergoes biodegradation and sorption, resulting in different levels of TCS reaching the surface water through effluents (11). TCS and its derivatives have been detected in the effluent of WWTPs across the globe as well as in their receiving waters and surrounding environment. Within aquatic habitats, TCS likely accumulates in sediments, as it is a lipophilic compound with low aqueous solubility. It is evident now that TCS is one of the most commonly encountered contaminants in solid and water compartments and has been detected in levels from nanograms to several micrograms per liter in sediments, WWTPs, rivers, lakes, and even drinking water sources (11–15). In fact, TCS is listed among the seven most frequently detected compounds in streams across the United States (16). Consequently, TCS imposes a significant impact on aquatic ecosystems and many aquatic species, with algal species being among the most sensitive to TCS toxicity (17).
Based on the mounting evidence of TCS detection in human body fluids, humans are unequivocally exposed to significant and potentially unsafe levels of TCS. TCS is not acutely toxic to mammals, but it can modulate phase I, II, and III drug-process genes by interacting with the nuclear receptors pregnane X receptor (PXR) and constitutive androstane receptor (CAR) (18–21). In animal models, many lines of evidence have suggested that TCS has adverse effects on endocrine function, thyroid hormone homeostasis, and antibiotic resistance. The carcinogenicity of TCS has been studied in rats, mice, and hamsters, and the results summarized by Rodricks et al. (2) indicate that TCS can cause liver pathogenesis—particularly tumor formation—in mice. More recent studies have provided new evidence linking TCS to tumorigenesis in animal models. In this review, in addition to discussing epidemiology studies and environmental impacts of TCS, we focus on potential health issues surrounding the use of TCS by providing a data collection that spans a wide range of in vitro and in vivo experimental models.
FATE AND EFFECTS OF TCS IN THE ENVIRONMENT
The proliferation of TCS use in daily care products coincides with a plethora of evidence of its bioaccumulation and persistence in the environment. Up to 96% of TCS in consumer products is rinsed down the drain, leading to the concentration of TCS, ranging from 1 to 10 mg/L, in WWTP influent (11). During the wastewater treatment process, TCS may convert to other derivatives: It can be biologically methylated into methyltriclosan (22) and/or be transformed during the disinfection of wastewater with free chlorine into chlorinated TCS derivatives, which possess a higher degree of environmental persistence than their parent compound because of their lipophilicity and resistance to biodegradation. Additionally, researchers have reported that the chlorinated TCS derivatives are more toxic than TCS itself, and their median lethal dose (LD50) value decreases as the number of chlorine substitutions in them increases (23). When discharged into surface waters through WWTP effluents, TCS and the chlorinated derivatives may undergo direct photolysis and be photochemically transformed to 2,4-dichlorophenol and polychlorodibenzo-p-dioxins (PCDDs), including 1,2,8-trichlorodibenzo-p-dioxin (1,2,8-TriCDD), 1,2,3,8-tetrachlorodibenzo-p-dioxin (1,2,3,8-TCDD), 2,3,7-trichlorodibenzo-p-dioxin (2,3,7-TriCDD), and 2,8-dichlorodibenzo-p-dioxin (2,8-DCDD) (24, 25) (Figure 1). Notably, PCDDs are generally highly persistent in the environment, and some are associated with carcinogenic activities (26).
Figure 1.
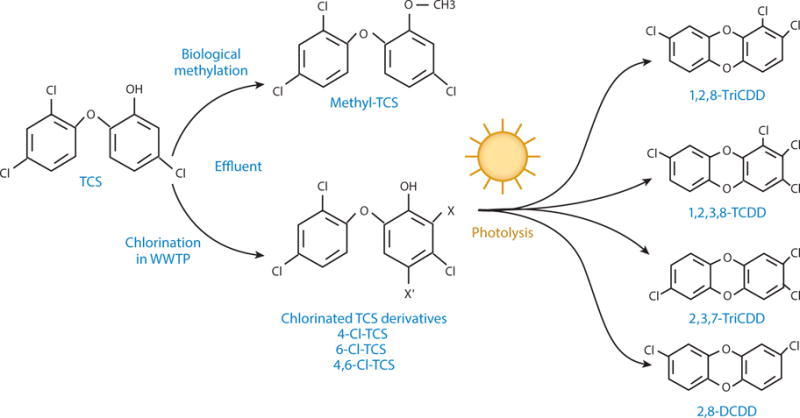
Chlorinated TCS derivatives and their dioxin photoproducts transformed from TCS in the environment. During the disinfection of wastewater with free chlorine, TCS is chemically transformed into chlorinated derivatives, including 4-Cl-TCS, 6-Cl-TCS, and 4,6-Cl-TCS. Through subsequent photolysis, TCS and its chlorinated derivatives are photochemically transformed to various PCDDs, generally with 1,2,8-TriCDD, 1,2,3,8-TCDD, 2,3,7-TriCDD, and 2,8-DCDD being most abundant in the environment. In addition, a small percentage of methyl-TCS is produced during the normal biodegradation process (25). Abbreviations: 1,2,3,8-TCDD, 1,2,3,8-tetrachlorodibenzo-p-dioxin; 1,2,8-TriCDD, 1,2,8-trichlorodibenzo-p-dioxin; 2,3,7-TriCDD, 2,3,7-trichlorodibenzo-p-dioxin; 2,8-DCDD, 2,8-dichlorodibenzo-p-dioxin; PCDD, polychlorodibenzo-p-dioxin; TCS, triclosan; WWTP, wastewater treatment plant.
Depending on the operation of the WWTP, a wide range of TCS concentrations can be released into the environment through receiving waters. A study in which TCS concentrations were measured in US wastewater effluent (27) documented that they ranged from 200 to 2,700 ng/L. Total annual loading of TCS into US surface waters has been estimated at 5,200–18,824 kg/year, with approximately 50% coming from WWTP effluents (28). A recent study of water systems in North America indicated that higher concentrations of TCS, its chlorinated derivatives, and their derivative dioxins in small-scale water systems can be directly attributed to increased TCS use (29). Once in the environment, TCS tends to accumulate and persist in biosolids and can enter the terrestrial environment during the application of sewage sludge to agricultural land (30, 31). TCS has been detected not only in surface water and estuarine sediment but also in freshwater at concentrations of up to 800 ng/kg (31).
Consequently, researchers have detected TCS contamination in both aquatic and terrestrial environments and have observed its bioaccumulation in aquatic biota, such as snails, algae (32), fish (33), and marine mammals (34). TCS also adsorbs to microbial biomass owing to its hydrophobic nature—as shown by its log octanol-water partition coefficient (Kow) of 4.8 (30). A study conducted by Wilson et al. (35) showed that TCS may influence the structure and function of algal communities in water ecosystems that received WWTP effluent. In fact, the toxicity of TCS has been studied using several types of environmentally sensitive species—including microalgae and fish—which have very low median effective concentration (EC50) values approaching the amount of TCS detected in the natural aquatic environment. Algal species appeared to be vulnerable to the toxic effects of TCS, with a 96-h EC50 of 1.4 μg/L and a 96-h no-observed-effect concentration (NOEC) of 0.69 μg/L (17). In the developmental stage, rainbow trout (Oncorhynchus mykiss) was sensitive to TCS toxicity, with significant effects on the survival rate under the 0.071 mg/L concentration. At concentrations above 0.7 mg/L, TCS exhibited teratogenic responses, hatching delay, and mortality in the embryos and larvae of zebrafish, with a 96-h median lethal concentration (LC50) of 0.42 mg/L. When researchers combined results of genetic, developmental, and enzymatic biomarker studies, they estimated that TCS concentrations of no less than 0.3 mg/L pose a hazard to aquatic ecosystems (36). A study measuring the growth-inhibiting effect of 12 different antibacterial agents indicated that TCS is one the most toxic antibacterial compounds for the freshwater microalga Pseudokirchneriella subcapitata, with a NOEC of 200 ng/L (37).
TCS is biodegradable and photo-unstable and continues to break down following its release into the aquatic environment (12–14). TCS has a half-life of approximately 11 days in surface water (38) and is degraded in aerobic soil with a half-life of 18 days. By contrast, it persists in anaerobic soil and sterile aerobic conditions (39). As TCS coexists with microplastic [i.e., polyvinyl chloride (PVC)] in the environment, a recent study uncovered that when lugworms (Arenicola marina) were exposed to PVC that was presorbed with TCS, uptake of TCS from PVC not only diminished their ability to engineer sediments but also raised their mortality (40).
TCS METABOLISM AND HUMAN EXPOSURE
TCS Absorption, Distribution, Metabolism, and Elimination
The most likely routes of exposure to TCS in humans are ingestion and skin absorption (41). When men applied cream containing 2% TCS on their skin in a clinical study, the absorption of TCS, calculated from urinary excretion, was estimated to be less than 10% in all individuals (42). A similar study by Lin (43) determined that the TCS retention rate was 7.33% from mouthwash containing 0.03% TCS. Following absorption, TCS is metabolized primarily through conjugation reactions to glucuronide and sulfate conjugates that are eliminated in feces and urine (44–46). Following the application of 1% TCS in a soap formulation to the skin in rats and guinea pigs, TCS glucuronide was detected as the major urinary metabolite (44). In another TCS metabolism study in which researchers administered a single topical dose of TCS to rats, unchanged TCS and TCS glucuronide were identified in urine and feces, with a small amount of TCS sulfate in urine (45). Wang et al. (20) compared TCS glucuronidation and sulfonation activities in human liver microsomes, and pharmacokinetic studies suggested that TCS glucuronide and sulfate may be formed in the liver at approximately equal rates at the environmentally relevant concentration (1 to 5 μM). Sulfonation is expected to be the major metabolic pathway for TCS elimination at concentrations below 1 μM owing to the fact that TCS sulfonation has a lower Michaelis-Menten constant (Km) than glucuronidation, whereas glucuronidation that exhibits a higher maximal velocity (Vmax) compared to sulfonation would be the predominant route for TCS clearance at higher concentrations (20). Using the cDNA-based cell expression system in COS cells, we found that several of the human UDP-glucuronosyltransferases (UGTs) are capable of glucuronidating TCS, with UGT1A1, 1A3, 1A4, 1A6, and 1A7 exhibiting the highest activities (Figure 2). In reports regarding human exposure, TCS levels range from undetectable or very little to 38% unconjugated after oral ingestion of TCS (3, 43, 47), indicating possibly large discrepancies in individual glucuronidation or sulfonation capacities. We suspect that when certain conditions occur—long-term TCS exposure, presence of concomitant substrates (e.g., clinical drugs and other environmental pollutants that are UGT1A substrates), or pathological liver status associated with lower expression levels of UGTs (e.g., age, alcoholic liver disease, steatosis)—TCS exposure may exceed the metabolic capacity that the body provides (48–50). Thus, people who have impaired or reduced glucuronidation conjugation capacity would be at a higher risk of adverse TCS effects.
Figure 2.
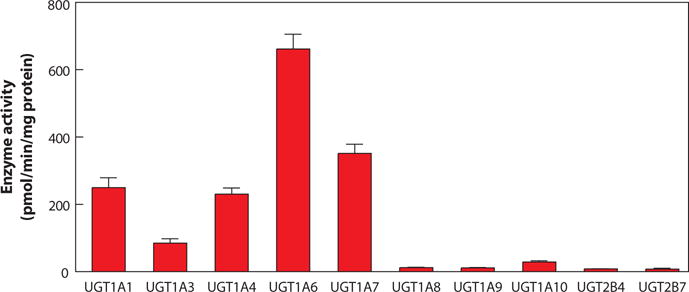
TCS glucuronidation. UGT specificity for the metabolism of TCS was examined using transfected COS cells with individual UGTs. Each of the full-length UGT1As, UGT2B4, and UGT2B7 cDNAs was subcloned to the expression vector. The recombinant plasmids were transfected into COS cells, and TCS glucuronidation activities were measured using a mixture containing cell lysates, TCS, and 14C-UDPGlcA as described previously (119). Abbreviations: TCS, triclosan; UGT, UDP-glucuronosyltransferase.
A human pharmacokinetic study demonstrated that TCS can be rapidly absorbed, metabolized, and eliminated following a single oral dose. The maximum plasma concentration was reached within 1–3 h, and the estimated terminal plasma half-life was 21 h, with baseline levels reached within 8 days after exposure (47). Regardless of the route of administration, the primary elimination route in humans is urinary, with a median excretion half-life of 11 h after oral intake of TCS (47), whereas fecal elimination prevails in rodents (1), which have a half-life of elimination ranging from 8 to 15 h. Following the skin application of 14C-labeled TCS in mice, maximum absorption was obtained approximately 12 h after dosing, and radioactivity appeared in all organ tissues examined, with higher levels in the gall bladder, gastrointestinal tract, liver, and lung (46). Of the radioactivity detected in the feces, the majority of TCS was in the free form, suggesting the hydrolysis of TCS conjugates by gut microflora (44).
TCS-Mediated Regulation of Drug-Processing Genes
Complex metabolic pathways in mammals, orchestrated by multiple families of drug-processing genes, are responsible for the detoxification of potentially harmful endogenous metabolic products and xenobiotics and are tightly regulated by various nuclear receptors—namely CAR, PXR, and peroxisome proliferator activating receptor α (PPARα) (51–53)—in responding to a wide range of structurally diverse xenobiotics, providing an adaptive response to environmental challenges. Many in vivo and in vitro experiments have shown that TCS can interact with nuclear receptors and regulate the corresponding downstream drug-processing genes. In a PXR reporter assay in which investigators transfected cells with the human PXR and a reporter plasmid containing the PXR response element in the CYP3A4 promoter region, TCS moderately activated human PXR (19), indicating a potential regulation of CYP3A4 gene expression. By employing probe substrate-specific enzyme assays, researchers have shown that TCS treatment increased protein and enzyme activities of rat Cyp2b1/2 and Cyp3a1—target genes of CAR and PXR—with hepatic microsomes or in hepatocytes (18, 54).
To understand the underlying mechanism through which TCS modulates gene expression, we monitored the activities of a series of mouse xenobiotic receptors (XenoRs) in response to TCS treatment, including PXR, CAR, liver X receptor α, farnesoid X receptor, vitamin D receptor, PPARα, PPARβ, PPARγ, estrogen receptor α (ERα), ERβ, and glucocorticoid receptor. Of the 11 XenoRs screened with TCS (10 μM), only CAR was activated by TCS, with a moderate induction of luciferase activity; all other nuclear receptors produced statistically insignificant induction (21). By conducting a ligand binding assay, we further demonstrated that TCS acted as a CAR activator but not an agonist ligand. TCS-mediated CAR activation elicited a significant induction of hepatic CYP2b10 in mice, and this induction was nearly completely abolished in livers of CAR−/− mice, indicating that TCS-induced Cyp2b10 gene induction requires CAR activation. Another study using various reporter assays consisting of the nuclear receptors PXR and CAR across species—human, mouse, and rat—showed that TCS is an agonist for both human PXR (hPXR) and hCAR. These in vitro results put forward the estimation that the lowest observable effect level for TCS activation of hPXR is approximately 15 mg/kg/day. The authors concluded that TCS is not likely to mediate adverse outcomes resulting from induction of drug-processing genes because current human exposures to TCS are insufficient to activate hPXR and hCAR, judging from the estimated human oral exposure to TCS of 0.13 mg/kg/day (55).
The concept of TCS upregulating enzymes that are responsible for thyroid hormone clearance is supported by a study conducted by Paul et al. (56) in which oral exposure to TCS in rats produced hypothyroxinemia with a significant reduction in serum thyroxine (T4) levels. These results indicated that—possibly through activation of the nuclear receptors PXR and CAR—TCS induces hepatic drug-processing genes that are responsible for T4 clearance, thus decreasing serum T4. The presence of TCS in the biological system may also interfere with the metabolism of coexisting xenobiotics or endogenous compounds. Pollock et al. (57) investigated the interaction of TCS with bisphenol A and found that TCS enhanced the presence of bisphenol A in specific tissues of adult female and male mice. Following combined TCS and bisphenol A administration, levels of bisphenol A were elevated in the lung, heart, muscle, uterus, ovaries, and serum of female mice and the epididymis and serum of male mice. These experiments implied that TCS may interfere with hepatic conjugating enzymes and inhibit the metabolism of bisphenol A (57). In summary, in a subset of people who have poor TCS metabolism, are exposed to higher amounts of TCS and TCS-like compounds, or both, TCS—by acting on activation of inducible enzymatic pathways through interactions with PXR and CAR—may have a significant impact on many aspects of xenobiotic and endobiotic metabolism and disposition, potentially affecting the toxicity of drugs and chemical pollutants as well as endocrine homeostasis.
Human Exposure to TCS
Numerous epidemiology studies that documented TCS detection in urine, blood, and breast milk in different regions of the world suggest that the general population is exposed to TCS. A study examining the body burden of phenolic halogenated compounds in Sweden identified TCS as one of many such compounds present in the plasma of the tested population (58). A study of TCS detection in urine samples among the US general population revealed that a wide range of TCS concentrations (2.4–3,790 μg/L) was present in 74.6% of 2,517 participants, with the highest levels occurring in young adults in their twenties and those in higher socioeconomic positions (41).
Pregnant women and their fetuses are uniquely vulnerable to endocrine disruptors, potentially including TCS. Researchers focusing on this special population detected TCS in human milk at concentrations ranging from 100 to 2,100 μg/kg lipid in 51 out of 62 samples from the Breast Milk Banks in California and Texas (59). In a recent survey of pregnant Canadian women, 99% had detectable levels of TCS glucuronides and 80% had the unconjugated, free TCS form in their urine. Urinary TCS concentrations appeared to have increased with age and higher socioeconomic status (60). Geens et al. (61) reported that a large portion of the free TCS present in the human body is localized within the liver. A pilot study focusing on childhood exposures across major cities in the United States showed that two-thirds of 90 girls, aged 6–8 years old, exhibited detectable urinary TCS, ranging from 1.6 to 956.0 μg/L, indicating the prevalence of TCS exposure among youths (62). A risk assessment study conducted in a population of Swedish women reported that higher concentrations of TCS in milk and serum were correlated with the use of TCS-containing daily care products (63). By contrast, no significant plasma TCS concentrations were detected between a control group and a group exposed to TCS-containing personal care products in a study of 12 adult humans (64). In an epidemiology study exploring potential health effects of prenatal exposure to TCS on birth size, researchers reported no significant association (65).
By incorporating in vitro data on metabolic clearance and plasma protein binding activities, Rotroff et al. (66) established a population-based in vitro–to–in vivo extrapolation model to estimate the daily human oral dose (oral equivalent dose) of an array of environmental chemicals, including TCS. This dose can predict a steady-state concentration that is equivalent to in vitro AC50 (concentration at 50% of maximum activity) and the lowest effective concentration obtained from a wide range of high-throughput toxicity screening assays across multiple cellular pathways developed in the EPA ToxCast program (67). Among 35 chemicals screened, the highest estimated human oral exposures were generally well below the estimated oral equivalent doses. However, TCS was one of only two chemicals that had an estimated human oral exposure level (0.13 mg/kg/day) greater than an oral equivalent dose of 0.0117 mg/kg/day. These data challenge the safe use of TCS in humans and support the concept that the level of human TCS exposure is within the range at which significant in vitro bioactivity occurs. Figure 3 depicts the fate and effects of TCS in the environment and the potential routes of human exposure to TCS.
Figure 3.
Fate and effects of TCS in the environment. Abbreviations: TCS, triclosan; WWTP, wastewater treatment plant.
BIOLOGICAL AND PHYSIOLOGICAL EFFECTS OF TCS IN EXPERIMENTAL ANIMAL MODELS
Mutagenicity and Genotoxicity
Many independent studies have assessed the mutagenic potential of TCS and indicated that TCS is neither genotoxic nor mutagenic (68). A recent study reported that 0.5 mg/L TCS inhibited the vegetative growth of the unicellular alga Closterium ehrenbergii and produced DNA damage at 0.25 mg/L in the Comet assay (69). More recently, Binelli et al. (70) employed a battery of biomarkers to assess the genotoxicity and cytotoxicity of TCS in hemocytes of the freshwater zebra mussel. In both the single-cell gel electrophoresis assay and the micronucleus test, TCS induced significant DNA genetic damage at all tested concentrations (1, 2, and 3 nM) in a concentration-dependent fashion. These results suggest that although TCS is not genotoxic in most animal models, aquatic organisms are more susceptible to its genotoxic and mutagenic effects.
Liver Disease and Carcinogenesis
When evaluated in chronic carcinogenesis studies in mice, rats, and hamsters, TCS treatment–related tumors were found in the liver of male and female mice with signs of hepatocyte hypertrophy and vacuolization (2). Researchers have proposed a few hypotheses linking TCS exposure to liver tumor development. Mice may be sensitive to TCS-activated peroxisome proliferator–type effects in the liver, although peroxisome proliferator actions are not considered a risk to human health. Another conjecture involves the hypothesis that in chlorine-treated tap water, TCS enhances the production of chloroform (24), which the EPA classifies as a probable human carcinogen. A link of TCS to dioxins—a family of compounds with widely ranging toxicities, including carcinogenesis and weakening of the immune system and reproductive function (71)—has been also suggested. TCS chlorinated by-products can produce 2,8-DCDD and 2,4-dichlorophenol following photochemical degradation by sunlight exposure, although one study argued that low concentrations of dioxin compounds would be formed owing to the low efficiency of the direct photolysis of TCS (72). These hypotheses remain speculative without concrete experimental evidence.
Through a long-term feeding study in mice, we recently discovered that TCS substantially accelerates hepatocellular carcinoma development, acting as a liver tumor promoter. Following diethylnitrosamine initiation, TCS-treated mice exhibited a large increase in tumor multiplicity, size, and incidence compared to control mice (21). By conducting a nuclear reporter activation assay using a series of mouse nuclear receptors, we showed that mouse PPARα displayed insignificant activation in response to TCS, and we debated whether TCS exerts its hepatic proliferation independent from PPARα activation, contrary to the previous suggestion (2). Through in vivo and in vitro experiments with a variety of biomarkers, we demonstrated that TCS enhances hepatocyte proliferation, induces fibrogenesis, produces oxidative stress, and promotes inflammatory responses (21). These results suggest that these modes of action that precede liver tumorigenesis may constitute the primary tumor-promoting mechanism through which TCS functions as a liver tumor promoter. In addition to the study conducted by this laboratory, a few recent studies, detailed below, addressed the potential mechanisms underlying TCS-mediated carcinogenesis.
Oxidative stress
Following TCS treatment in the diet, we found that increased levels of superoxide have been observed in the livers of TCS-treated mice. In addition, TCS-treated livers exhibited a marked increase in expression of oxidative stress responsive genes, including heme oxygenase-1 (Ho-1), NADPH hydrogenase quinone 1 (Nqo-1), and glutathione S-transferase a1 (Gsta1), indicating occurrence of oxidative stress (21, 73). In another similar study, TCS treatment in human hepatoma HepG2 cells led to the accumulation of 8-hydroxy-2-deoxyguanosime (8-OHdG), supporting the notion that TCS exposure contributes to the generation of oxidative stress (74). In the lysosomal membrane stability assay, Binelli et al. (70) demonstrated that severe TCS-induced DNA injuries in mussel hemocytes were linked to reactive oxygen species generation and oxidative stress. The concept that TCS exposure produces oxidative stress has also gained support from a recent study using a quantitative toxicogenomic-based toxicity assessment (75) in which toxicity changes during the degradation of TCS were evaluated by a Fenton-based process. The results showed that TCS caused severe oxidative stress as well as DNA stress. The authors indicated that the sustained TCS toxicity associated with oxidative stress was likely attributed, at least partially, to the production of 2,4-dichlorophenol—a chlorinated TCS by-product. When assessing the risk imposed by TCS in terrestrial organisms, studies of earthworms (Eisenia fetida) and snails (Achatina fulica) showed that the adverse effects of TCS on these organisms are associated with oxidative stress, as evidenced by the induction of oxidative stress responsive genes and increased content of malondialdehyde (76, 77). TCS dose-dependent DNA damage was also observed in earthworms, implying that TCS genotoxicity in this organism may be caused by oxidative stress (78).
Epigenetic factors and epithelial-mesenchymal transition
Ma et al. (74) demonstrated that TCS significantly reduced the level of global DNA methylation in human HepG2 cells and inhibited DNA methyltransferase 1 activity, implying that TCS may exert its tumorigenesis promotion ability by altering DNA methylation status, as global DNA hypomethylation is considered to be a biomarker of cancer progression (79). Using anoikis resistant human H460 lung cancer cells that reflect cancer aggressiveness as an experimental model, Winitthana et al. (80) discovered that TCS exposure predisposes lung cancer cells to undergo epithelial-mesenchymal transition (EMT), manifesting the mesenchymal phenotype. When cancer cells were treated with TCS at physiologically relevant concentrations, these cells exhibited decreased cell-to-cell adhesion and increased levels of biomarkers associated with EMT, inducing N-cadherin, Vimentin, Snail, and Slug, implying that TCS may promote EMT and increase the cells’ migration, invasion, survival, and metastasis abilities (80).
Cell proliferation and fibrogenesis
Following 8-month TCS exposure at 800 ppm in the diet, we found that mice exhibited an increased liver to body weight ratio without affecting body weight, accompanied by elevated expression of gene markers associated with DNA synthesis and cell proliferation, including Ki-67, c-Myc, and Cyclin D1 (21). The TCS-induced proliferative response was associated with increased expression of fibrogenic genes—Collagen 1a1, smooth muscle alphaactin (α-Sma), and tissue inhibitor of metalloproteinase 1 (Timp1)—in livers as well as elevated levels of apoptosis. These results suggest that TCS causes chronic liver damage and hepatocyte apoptosis in mice, and surviving hepatocytes undergo compensatory proliferation and fibrogenesis with the regenerative capacity that hepatocytes possess (21). In a xenograft mouse model with injection of MCF-7 human breast cancer cells, exposure to TCS appeared to trigger the growth of breast cancer cells, leading to a significant increase in the development of breast tumor masses. MCF-7 cell proliferation following TCS treatment was accompanied by increased expression of Cyclin D1 and decreased expression of p21, suggesting that TCS exposure is associated with the control of the G1/S transition of the cell cycle during cell proliferation in carcinogenesis (81). A similar study using ER-positive BG-1 ovarian cancer cells reported that TCS stimulated cell proliferation at a concentration of 1 μM through an ER-dependent pathway (82). Treatment of BG-1 cells with TCS promoted cell cycle progression, as evidenced by upregulation of Cyclin D1, and suppressed apoptosis, as shown by a reduction in p21 and Bax transcription and protein levels.
Disturbance of immune function
Scientists have long recognized a relationship between inflammation and cancer development (83). In the TCS-feeding experiments, TCS-treated mice exhibited an increase in liver inflammation, as shown by significantly higher expression levels of the proinflammatory cytokines tumor necrosis factor-α (Tnf-α) and Il-6 (21). A recent epidemiology study investigated the ability of TCS to affect the immune system by using immune parameters in combination with a national cross-sectional survey conducted by the US Centers for Disease Control and Prevention in 2003–2006 (84). The study results showed that higher concentrations of urinary TCS were associated with a greater probability of having been diagnosed with allergies or hay fever in the <18-years-old age group. A recent study examined the effects of TCS on intracellular zinc concentrations, as zinc plays a critical role in proper immune function. Using a flow cytometer with appropriate probes, the researchers determined the correlation between elevated levels of intracellular zinc following TCS treatment and decreased levels of the thiol content in rat thymocytes. The results suggest that TCS at a dose of 1–3 μM produced oxidative stress that depletes cellular thiol contents, leading to the disturbance of cellular Zn2+ homeostasis (85). A study of TCS exposure using in vitro natural killer (NK) cells showed that TCS, at concentrations as low as 1 μM with prolonged exposure (6 days), diminished the ability of human NK cells to lyse tumor cells, an essential function for inhibiting infected cells and tumors (86).
Currently, mechanism-based studies in humans are lacking in both number and scope. Our mouse model in the tumorigenesis study strongly suggests that adverse health effects—particularly enhanced liver fibrogenesis and tumor promotion—are associated with long-term TCS exposure. In addition, the aforementioned data in different experimental models collectively provide potential underlying mechanisms—oxidative stress, cell proliferation and fibrogenesis, epigenetic modification, and immune function disturbance—through which TCS exerts its effect on liver pathogenesis, carcinogenesis, or both. Although many of these animal studies used higher chemical concentrations than are predicted for human exposure, these mechanism-based studies are important, and their relevance to humans should be closely evaluated.
Endocrine Disruption
The potential of TCS to act as an endocrine disruptor has been examined in different organisms, and many studies have reported reproductive and developmental toxicity and endocrine-disrupting effects of TCS in both in vitro and in vivo models. TCS has been shown to possess weak androgenic effects in fish (87) and antiandrogenic effects in rats (88). In vitro reporter assays using human breast cancer MCF-7 cells transfected with estrogen response element–containing plasmids revealed that TCS possessed antiestrogenic and antiandrogenic activities through interaction with ERα, ERβ, and the androgen receptor (AR). Acting as an antagonist, TCS inhibited the activity of these receptors when administered concomitantly with their endogenous ligands (89). Researchers further studied the endocrine disrupting potential of TCS by using in vitro cell-based assays consisting of nuclear receptor response elements to detect activities of the aryl hydrocarbon (Ah) receptor, ER, AR, and ryanodine receptors. Acting as both an Ah receptor agonist and antagonist, TCS not only induced luciferase expression to 40% of that of 2,3,7,8-TCDD induction but also inhibited 2,3,7,8-TCDD-induced luciferase expression by 30%. The authors also concluded that TCS antagonistically regulates ER and AR and is a potent disruptor of Ca2+ regulation (90). In a study that analyzed TCS for its action on placental secretion of progesterone, estradiol, and β-human chorionic gonadotropin (β-hCG) in human choriocarcinoma-derived placental JEG-3 cells, TCS altered main placental hormone production by stimulating estradiol and progesterone secretion and reducing β-hCG at environmentally relevant doses in these cells (91).
Providing a comparison with the above in vitro studies, several in vivo studies determined that TCS functions as an ER agonist and exhibits estrogenic activity (92–94). TCS is reported to have estrogenic activity, as it increased the vitellogenin levels in male fish (92). Furthermore, TCS exposure led to an earlier onset of vaginal opening and an earlier age of the first estrus in female Wistar rats (93). Recent work by Jung et al. (94) tested the estrogenic activity of TCS by in vivo uterotrophic assays, and the results showed that uterine weight was significantly increased by TCS in the uteri of immature rats at doses as low as 7.5 mg/kg. In addition, expression of uterine CaPB-9k—a common biomarker regulated by estrogen in the uterus—is elevated following TCS treatment, indicating that TCS elicits estrogenic effects in rat uteri (94). Aside from involving the ER-dependent pathway, TCS can negatively modulate estrogen sulfotransferase, through which TCS exerts its estrogenic effects by inhibiting the metabolism of estrone and 17β-estradiol into their biologically inactive forms (95). Collectively, these results combine in vitro and in vivo data to put forward the idea that TCS possesses (anti)estrogenic and (anti)androgenic properties depending on species, tissues, and cell types.
Animal studies have made it evident that TCS acts as a thyroid-disrupting chemical. Recent work by Veldhoen et al. (96) examined the effects of TCS on the development of tadpoles of the North American bullfrog (Rana catesbeiana). TCS disrupted thyroid hormone–mediated action in the context of metamorphosis in tadpoles. Premetamorphic tadpoles displayed changes in growth and disruption of thyroid hormone–dependent gene expression following exposure to TCS concentrations as low as 0.15 μg/L. Using in vitro Xenopus laevis XTC-2 cells, the researchers showed that exposure to environmentally relevant concentrations of TCS alters thyroid hormone–associated gene expression and disrupts developmental processes of R. catesbeiana and other anuran species. TCS-induced thyroid hormone alteration has also been demonstrated in X. laevis (97). A series of experiments with rats showed that TCS interfered with thyroid hormone by decreasing T4 levels in juvenile rats and that short-term oral TCS exposure caused hypothyroxinemia in weaning rats (10, 55, 98).
Using pregnant rats as an animal model, researchers found that TCS exhibited adverse effects on both thyroid homeostasis and reproductive function: TCS decreased serum triiodothyronine and T4 in pregnant rats, disrupted sex ratio balance, lowered pup body weights, and delayed vaginal opening in offspring (99). TCS markedly lowered maternal T4 levels in rat dams during gestation and lactation as well as in neonatal rats following perinatal exposures (100, 101). Based on its thyroid-disrupting properties, TCS is thought to be a potential developmental neurotoxicant because maternal hypothyroxinemia has been linked to impaired cognitive and motor function in children. TCS-induced hypothyroxinemia has been proposed to have these effects on children, as increased catabolism of thyroid hormone results from activation of xenobiotic nuclear receptors and subsequent upregulation of phase II conjugation enzymes (56). Although a direct linkage between nuclear receptor activation by TCS and increased levels of thyroid catabolism remains to be established in experimental studies, the murine knockout model has demonstrated previously that PXR and CAR are required for the downstream effects of pregnenolone-16α-carbonitrile and phenobarbital on thyroid hormone elimination through glucuronidation (102). Researchers have conducted short-term (14 days) and long-term (4 years) studies to investigate possible adverse effects of 0.3% TCS in toothpaste on thyroid function in humans (64, 103). The results showed that TCS toothpaste had no detectable effect on thyroid function.
Antimicrobials and TCS Resistance
Investigators have carried out many studies to identify a possible association between the increased use of TCS and the emergence of resistant bacterial strains. In 1991, Cookson et al. (104) documented cross-resistance to TCS and mupirocin in MRSA. As the substrate for the AcrAB efflux pump in members of Enterobacteriaceae, TCS can be actively effluxed from the bacterial cell, which is believed to be one of the underlying mechanisms for TCS bacterial resistance (105). Recent work by Beier et al. (106) evaluated the antibiotic and antiseptic susceptibilities of vancomycin-resistant Enterococcus faecium (VRE). They reported no correlation between antibiotic resistance and antiseptic susceptibility; however, the majority of the VRE isolates examined had a substantially increased tolerance to TCS and were resistant to 14 antibiotics. Other researchers have detected an S. aureus strain tolerant of TCS with increased resistance to penicillin and gentamicin (107, 108). A study with mutants of serovar Typhimurium (Salmonella enterica) indicated that TCS at subinhibitory concentrations helps to retain certain antibiotic-resistant bacterial strains, although it does not increase the mutation frequency (109). By contrast, surveys evaluating TCS and antibiotic sensitivities found no relationship between TCS usage and antibiotic resistance (110, 111).
Although different bacterial strains have produced variants with reduced susceptibility to both TCS and antibiotics in laboratory settings (112, 113), no comprehensive environmental surveys have shown a causal relationship between TCS usage and antibiotic resistance. Recently, however, researchers demonstrated a significant correlation between sediment TCS concentrations and the proportion of cultivable benthic bacteria that were resistant to TCS in the environment (114). After testing rivers in the Chicago metropolitan region, Drury et al. (114) reported that urbanization is directly correlated with higher TCS levels and that the levels of TCS present in these streams affected the native bacterial communities. In another experimental setting with artificial streams, TCS caused a significant decrease in sediment bacterial diversity and modified the taxonomic composition of bacterial communities, with a great increase in relative abundance of cyanobacterial sequences and massive die-offs of algae. This work provides a direct link between TCS exposure and an increase in TCS resistance in bacterial communities. More research that monitors specific bacterial strains with reduced susceptibility to TCS and to antibiotics is necessary, and it is worth investigating whether ingested TCS would change the microbial composition and disrupt the homeostasis of gut flora in humans.
Other Health Effects
A recent study conducted in primary mouse myotubes and myofibers showed that TCS adversely affects hemodynamic functions and cardiac and skeletal muscle contractility by interfering with signaling between the dihydropyridine and ryanodine receptors (115). Consistent with these results, when fathead minnows were used as a model for aquatic toxicity, researchers observed a negative impact on predator-avoidance performance in larvae and decreased activities in behavioral aggression assays (116).
The fibroproliferative property of TCS is not restricted to the liver. In a long-term (8 months) TCS feeding experiment in mice, we discovered that 8.3% of mice exposed to TCS developed renal hypertrophy, resulting in a marked change in the kidney structure with increased fibrous tissue contents, as evidenced by the accumulation of collagen in both the glomerulus and tubulointerstitium (Figure 4a,b) (21). Comparisons of gene expression also support the notion that TCS exposure promotes cell proliferation and fibrosis, indicated by elevated expression of the Ki-67 gene and Timp, α-Sma, collagen 1a1, and Cd11b in the kidneys of TCS-treated mice (Figure 4c). These changes were accompanied by inflammatory responses, assessed by alteration in proinflammatory cytokines. We found that expression of the cytokine genes Tnf-α and Il-6 were greater in TCS-treated mice compared with control mice. Increased levels of inflammatory cell recruitment, characterized by both immunohistochemistry and gene expression of Cd45, were detected by both real-time polymerase chain reaction (PCR) and immunostaining, further confirming the activation state of inflammatory responses (Figure 4e,f). Simultaneously, the fibrotic kidney induced by TCS exposure exhibited an increased number of apoptotic cells as detected by the TUNEL assay (Figure 4g). These results, together with the compelling evidence of liver fibrogenesis induced by TCS, indicate that TCS has a profound effect on organ fibrogenesis and proliferation.
Figure 4.
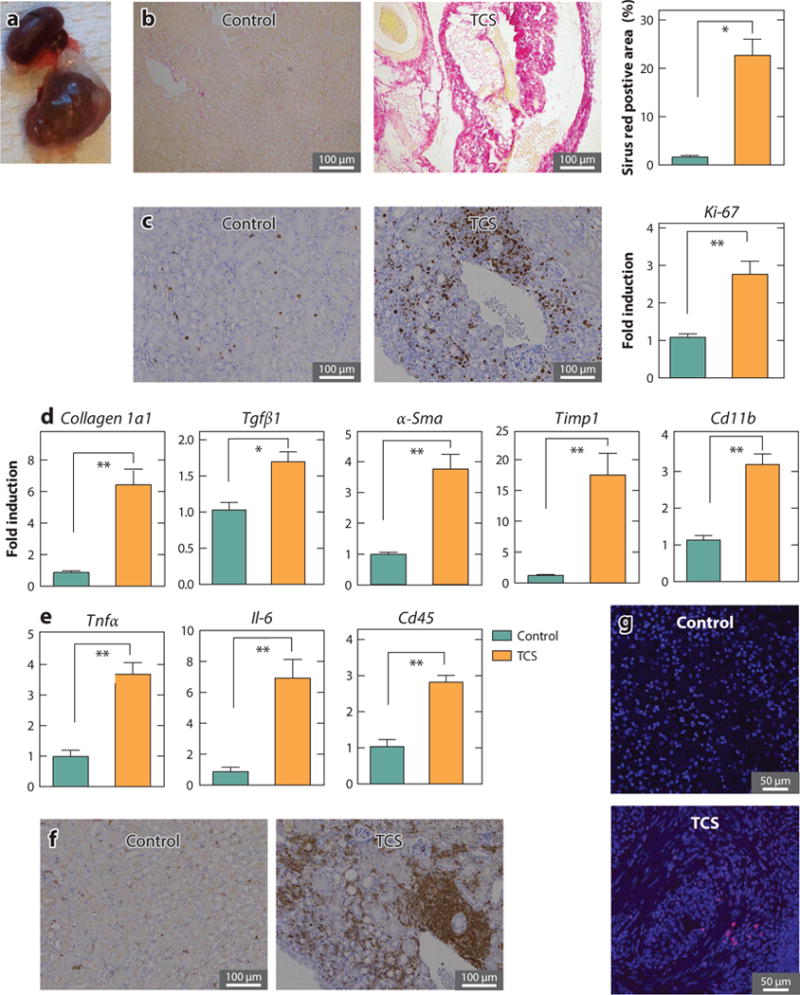
TCS treatment induces kidney fibrosis in mice. Following 8-month treatment with a chow diet containing 0.08% TCS, 8.3% of mice developed kidney fibrosis. Comparisons were made between fibrotic (n = 3) and nondiseased kidneys (n = 3). (a) A normal (top) and enlarged fibrotic (bottom) kidney from TCS-treated mice. (b) Collagen deposition was examined by Sirius red staining (left and center) and its quantification (right). (c) Expression of Ki-67 was determined by immunohistochemistry (left and center) and real-time PCR (right). (d) Expression of genes relevant to renal fibrosis, including Collagen 1a1, Tgfβ1, α-Sma, Timp1, and Cd11b, was detected by real-time PCR. (e) Expression of inflammatory genes, including Tnfα and Il-6, was assessed by real-time PCR. In addition, expression of CD45 was shown by immunostaining with the anti-Cd45 antibody in liver sections (f) and quantitated by real-time PCR (e). (g) Liver cell apoptosis was determined by TUNEL staining. Throughout the figure, P values < 0.05 were considered statistically significant; one asterisk indicates a statistically significant difference of P < 0.005, and two asterisks indicate P < 0.0005. Abbreviations: PCR, polymerase chain reaction; TCS, triclosan. Parts of this figure adapted from Reference 21.
FINAL REMARKS
Washed down the drain, TCS amasses in sewage, trickles into the environment, and is potentially creating an environmental and public health hazard (Figure 5). Despite increasing research on the effects of TCS on human health, controversy surrounds the issue of what concentrations—if any—of TCS are safe for human use. The fact that significant levels of TCS are detected in urine, plasma, and breast milk in populations across the globe indicates the potential for humans in all age groups to receive lifetime exposures to TCS. Exposure to TCS can lead to a host of negative consequences: impaired thyroid function, endocrine disruption, developmental disorders, oxidative stress, liver carcinogenesis, and hindrance of muscle strength, among others. In mice, investigators have demonstrated conclusively that TCS exerts carcinogenic properties, potentially by promoting hepatocyte apoptosis, compensatory cell proliferation, and fibrogenesis. In addition, research has recently shown that TCS in the environment exerts selective pressure on exposed microorganisms, thereby altering the composition of the bacterial community (114). Although the causal relationship between TCS exposure and disturbance on physiological function and biological signaling pathways has been established in experimental animals, critics have questioned the relevance of these studies in predicting human TCS toxicity, partially owing to the higher-than-environmentally-relevant concentrations used in some of the aforementioned animal studies. Considering that we are exposed to hundreds of synthetic chemicals simultaneously, and TCS and many TCS-like chemicals (e.g., chlorinated hydrocarbons) coexist in the environment, we probably underestimate TCS toxicity by neglecting to consider the formation of TCS chlorinated derivative compounds and dioxins that may be more harmful and the potential synergistic effects manifested from TCS and TCS-like compounds. These facts, together with the bioaccumulative nature of TCS, strongly suggest that the health implications of long-term TCS exposure should be of concern and carefully evaluated.
Figure 5.
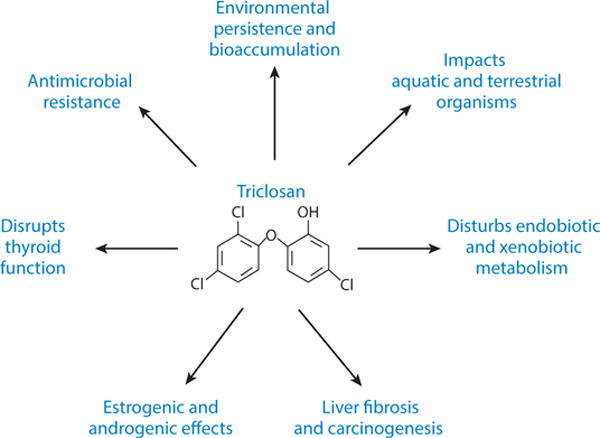
Environmental impacts and health issues surrounding triclosan.
Researchers recognize that biocides—including TCS—have an important role to play in disinfection, antisepsis, and preservation when used appropriately. In clinical settings, TCS has been employed to effectively eradicate microorganisms (4); however, the necessity of the pervasive use of TCS in many household consumer products is questionable. Researchers found that household soaps with less than 1% TCS were not significantly more effective than plain soaps when the efficacy was determined by overall bacterial counts (5). Another application of TCS as a biocide in consumer products is Microban, which is registered with the EPA to inhibit bacterial growth in plastic products, such as polyethylene films as packaging materials, enabling TCS to be incorporated into virtually any type of plastic materials used by the food packaging industry. In fact, the EPA has acted to prevent manufacturers from claiming that the use of TCS in such products provides protection against disease (117). For both hand soaps and food packaging materials, the risk associated with their long-term, daily use may not justify the benefit that manufacturers intended to accomplish, underscoring the fact that TCS is not subject to stringent government regulation.
As we indicate in this review, considerable evidence suggests that exposure to TCS can lead to changes in normal homeostasis in humans. Many years ago, the precautionary principle was invoked by the Danish Environment Agency, leading to restrictions on the use of phthalates as plasticizers in plastic toys for children (reviewed in Reference 118). There were far fewer convincing mechanistic data on phthalates linking exposure to toxicity than currently exist for the potential toxicological implications of TCS exposure. At the Wingspread Conference in 1998 (http://www.sehn.org/wing.html), the precautionary principle was defined. The precautionary principle states that “when an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause and effect relationships are not fully established scientifically” (120, p. 8). In 2014, the Minnesota legislature passed a bill that will restrict the use of TCS in most retail consumer products. The significant findings we report here demonstrate that TCS needs to be considered as a serious environmental toxicant that impacts the biology of many species in the environment and has the potential to negatively affect human health. In closing, the studies and data presented here are part of an effort aimed at raising the awareness of the public as well as alerting regulatory agencies to the adverse effects of TCS.
Acknowledgments
This work has been supported in part by US Public Health Service Grants ES010337, GM086713, and GM100481 (R.H.T.) and R21ES023906 (M.-F.Y.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Mei-Fei Yueh, Email: mfyueh@ucsd.edu.
Robert H. Tukey, Email: rtukey@ucsd.edu.
LITERATURE CITED
- 1.Fang JL, Stingley RL, Beland FA, Harrouk W, Lumpkins DL, Howard P. Occurrence, efficacy, metabolism, and toxicity of triclosan. J Environ Sci Health C: Environ Carcinog Ecotoxicol Rev. 2010;28:147–71. doi: 10.1080/10590501.2010.504978. [DOI] [PubMed] [Google Scholar]
- 2.Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. 2010;40:422–84. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- 3.Dann AB, Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol. 2011;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox MH, Hall J, Pike H, Templeton PA, Fawley WN, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54:196–201. doi: 10.1016/s0195-6701(03)00147-6. [DOI] [PubMed] [Google Scholar]
- 5.Aiello AE, Larson EL, Levy SB. Consumer antibacterial soaps: effective or just risky? Clin Infect Dis. 2007;45(Suppl. 2):S137–47. doi: 10.1086/519255. [DOI] [PubMed] [Google Scholar]
- 6.Heath RJ, Yu YT, Shapiro MA, Olson E, Rock CO. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J Biol Chem. 1998;273:30316–20. doi: 10.1074/jbc.273.46.30316. [DOI] [PubMed] [Google Scholar]
- 7.McMurry LM, Oethinger M, Levy SB. Triclosan targets lipid synthesis. Nature. 1998;394:531–32. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- 8.Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev. 2004;17:863–93. doi: 10.1128/CMR.17.4.863-893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escalada MG, Russell AD, Maillard JY, Ochs D. Triclosan-bacteria interactions: single or multiple target sites? Lett Appl Microbiol. 2005;41:476–81. doi: 10.1111/j.1472-765X.2005.01790.x. [DOI] [PubMed] [Google Scholar]
- 10.Crofton KM, Paul KB, DeVito MJ, Hedge JM. Short-term in vivo exposure to the water contaminant triclosan: evidence for disruption of thyroxine. Environ Toxicol Pharmacol. 2007;24:194–97. doi: 10.1016/j.etap.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.McAvoy DC, Schatowitz B, Jacob M, Hauk A, Eckhoff WS. Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem. 2002;21:1323–29. [PubMed] [Google Scholar]
- 12.Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002;46:1485–89. doi: 10.1016/s0045-6535(01)00255-7. [DOI] [PubMed] [Google Scholar]
- 13.Gómez MJ, Martínez Bueno MJ, Lacorte S, Fernández-Alba AR, Agüera A. Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere. 2007;66:993–1002. doi: 10.1016/j.chemosphere.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ Sci Technol. 2002;36:1202–11. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 15.Bedoux G, Roig B, Thomas O, Dupont V, Le BB. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res Int. 2012;19:1044–65. doi: 10.1007/s11356-011-0632-z. [DOI] [PubMed] [Google Scholar]
- 16.Singer H, Muller S, Tixier C, Pillonel L. Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol. 2002;36:4998–5004. doi: 10.1021/es025750i. [DOI] [PubMed] [Google Scholar]
- 17.Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V. Aquatic toxicity of triclosan. Environ Toxicol Chem. 2002;21:1338–49. [PubMed] [Google Scholar]
- 18.Hanioka N, Jinno H, Nishimura T, Ando M. Effect of 2,4,4′-trichloro-2′-hydroxydiphenyl ether on cytochrome P450 enzymes in the rat liver. Chemosphere. 1997;34:719–30. doi: 10.1016/s0045-6535(97)00464-5. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicol Appl Pharmacol. 2005;209:123–33. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Wang LQ, Falany CN, James MO. Triclosan as a substrate and inhibitor of 3′-phosphoadenosine 5′-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos. 2004;32:1162–69. doi: 10.1124/dmd.104.000273. [DOI] [PubMed] [Google Scholar]
- 21.Yueh MF, Taniguchi K, Chen S, Evans RM, Hammock BD, et al. The commonly used antimicrobial additive triclosan is a liver tumor promoter. PNAS. 2014;111:17200–5. doi: 10.1073/pnas.1419119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balmer ME, Poiger T, Droz C, Romanin K, Bergqvist PA, et al. Occurrence of methyl triclosan, a transformation product of the bactericide triclosan, in fish from various lakes in Switzerland. Environ Sci Technol. 2004;38:390–95. doi: 10.1021/es030068p. [DOI] [PubMed] [Google Scholar]
- 23.Kanetoshi A, Katsura E, Ogawa H, Ohyama T, Kaneshima H, Miura T. Acute toxicity, percutaneous absorption and effects on hepatic mixed function oxidase activities of 2,4,4′-trichloro-2′-hydroxydiphenyl ether (Irgasan DP300) and its chlorinated derivatives. Arch Environ Contam Toxicol. 1992;23:91–98. doi: 10.1007/BF00226000. [DOI] [PubMed] [Google Scholar]
- 24.Fiss EM, Rule KL, Vikesland PJ. Formation of chloroform and other chlorinated byproducts by chlorination of triclosan-containing antibacterial products. Environ Sci Technol. 2007;41:2387–94. doi: 10.1021/es062227l. [DOI] [PubMed] [Google Scholar]
- 25.Buth JM, Grandbois M, Vikesland PJ, McNeill K, Arnold WA. Aquatic photochemistry of chlorinated triclosan derivatives: potential source of polychlorodibenzo-p-dioxins. Environ Toxicol Chem. 2009;28:2555–63. doi: 10.1897/08-490.1. [DOI] [PubMed] [Google Scholar]
- 26.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor sans xenobiotics: endogenous function in genetic model systems. Mol Pharmacol. 2007;72:487–98. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- 27.Reiss R, Mackay N, Habig C, Griffin J. An ecological risk assessment for triclosan in lotic systems following discharge from wastewater treatment plants in the United States. Environ Toxicol Chem. 2002;21:2483–92. [PubMed] [Google Scholar]
- 28.Halden RU, Paull DH. Co-occurrence of triclocarban and triclosan in U.S. water resources. Environ Sci Technol. 2005;39:1420–26. doi: 10.1021/es049071e. [DOI] [PubMed] [Google Scholar]
- 29.Anger CT, Sueper C, Blumentritt DJ, McNeill K, Engstrom DR, Arnold WA. Quantification of triclosan, chlorinated triclosan derivatives, and their dioxin photoproducts in lacustrine sediment cores. Environ Sci Technol. 2013;47:1833–43. doi: 10.1021/es3045289. [DOI] [PubMed] [Google Scholar]
- 30.Heidler J, Halden RU. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere. 2007;66:362–69. doi: 10.1016/j.chemosphere.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 31.Chalew TE, Halden RU. Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J Am Water Works Assoc. 2009;45:4–13. doi: 10.1111/j.1752-1688.2008.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coogan MA, La Point TW. Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a North Texas, USA, stream affected by wastewater treatment plant runoff. Environ Toxicol Chem. 2008;27:1788–93. doi: 10.1897/07-374.1. [DOI] [PubMed] [Google Scholar]
- 33.Houtman CJ, Van Oostveen AM, Brouwer A, Lamoree MH, Legler J. Identification of estrogenic compounds in fish bile using bioassay-directed fractionation. Environ Sci Technol. 2004;38:6415–23. doi: 10.1021/es049750p. [DOI] [PubMed] [Google Scholar]
- 34.Fair PA, Lee HB, Adams J, Darling C, Pacepavicius G, et al. Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environ Pollut. 2009;157:2248–54. doi: 10.1016/j.envpol.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Wilson BA, Smith VH, deNoyelles F, Jr, Larive CK. Effects of three pharmaceutical and personal care products on natural freshwater algal assemblages. Environ Sci Technol. 2003;37:1713–19. doi: 10.1021/es0259741. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira R, Domingues I, Koppe GC, Soares AM. Effects of triclosan on zebrafish early-life stages and adults. Environ Sci Pollut Res Int. 2009;16:679–88. doi: 10.1007/s11356-009-0119-3. [DOI] [PubMed] [Google Scholar]
- 37.Yang LH, Ying GG, Su HC, Stauber JL, Adams MS, Binet MT. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ Toxicol Chem. 2008;27:1201–8. doi: 10.1897/07-471.1. [DOI] [PubMed] [Google Scholar]
- 38.Bester K. Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Arch Environ Contam Toxicol. 2005;49:9–17. doi: 10.1007/s00244-004-0155-4. [DOI] [PubMed] [Google Scholar]
- 39.Ying GG, Yu XY, Kookana RS. Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling. Environ Pollut. 2007;150:300–5. doi: 10.1016/j.envpol.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Browne MA, Niven SJ, Galloway TS, Rowland SJ, Thompson RC. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr Biol. 2013;23:2388–92. doi: 10.1016/j.cub.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116:303–7. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Queckenberg C, Meins J, Wachall B, Doroshyenko O, Tomalik-Scharte D, et al. Absorption, pharmacokinetics, and safety of triclosan after dermal administration. Antimicrob Agents Chemother. 2010;54:570–72. doi: 10.1128/AAC.00615-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin YJ. Buccal absorption of triclosan following topical mouthrinse application. Am J Dent. 2000;13:215–17. [PubMed] [Google Scholar]
- 44.Black JG, Howes D, Rutherford T. Percutaneous absorption and metabolism of Irgasan DP300. Toxicology. 1975;3:33–47. doi: 10.1016/0300-483x(75)90006-2. [DOI] [PubMed] [Google Scholar]
- 45.Moss T, Howes D, Williams FM. Percutaneous penetration and dermal metabolism of triclosan (2,4,4′-trichloro-2′-hydroxydiphenyl ether) Food Chem Toxicol. 2000;38:361–70. doi: 10.1016/s0278-6915(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 46.Fang JL, Vanlandingham M, da Costa GG, Beland FA. Absorption and metabolism of triclosan after application to the skin of B6C3F1 mice. Environ Toxicol. 2014 doi: 10.1002/tox.22074. [DOI] [PubMed] [Google Scholar]
- 47.Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 2006;69:1861–73. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- 48.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 49.Long J, Zhang S, Fang X, Luo Y, Liu J. Association of neonatal hyperbilirubinemia with uridine diphosphate-glucuronosyltransferase 1A1 gene polymorphisms: meta-analysis. Pediatr Int. 2011;53:530–40. doi: 10.1111/j.1442-200X.2011.03337.x. [DOI] [PubMed] [Google Scholar]
- 50.Nagar S, Remmel RP. Uridine diphosphoglucuronosyltransferase pharmacogenetics and cancer. Oncogene. 2006;25:1659–72. doi: 10.1038/sj.onc.1209375. [DOI] [PubMed] [Google Scholar]
- 51.Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, et al. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Sonoda J, Pei L, Evans RM. Nuclear receptors: decoding metabolic disease. FEBS Lett. 2008;582:2–9. doi: 10.1016/j.febslet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson EF, Hsu MH, Savas U, Griffin KJ. Regulation of P450 4A expression by peroxisome proliferator activated receptors. Toxicology. 2002;181–82:203–6. doi: 10.1016/s0300-483x(02)00282-2. [DOI] [PubMed] [Google Scholar]
- 54.Jinno H, Hanioka N, Onodera S, Nishimura T, Ando M. Irgasan® DP 300 (5-chloro-2-(2,4-dichlorophenoxy)-phenol) induces cytochrome P450s and inhibits haem biosynthesis in rat hepatocytes cultured on Matrigel. Xenobiotica. 1997;27:681–92. doi: 10.1080/004982597240271. [DOI] [PubMed] [Google Scholar]
- 55.Paul KB, Thompson JT, Simmons SO, Vanden Heuvel JP, Crofton KM. Evidence for triclosan-induced activation of human and rodent xenobiotic nuclear receptors. Toxicol In Vitro. 2013;27:2049–60. doi: 10.1016/j.tiv.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Paul KB, Hedge JM, DeVito MJ, Crofton KM. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in young Long-Evans rats. Toxicol Sci. 2010;113:367–79. doi: 10.1093/toxsci/kfp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pollock T, Tang B, deCatanzaro D. Triclosan exacerbates the presence of 14C-bisphenol A in tissues of female and male mice. Toxicol Appl Pharmacol. 2014;278:116–23. doi: 10.1016/j.taap.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 58.Hovander L, Malmberg T, Athanasiadou M, Athanassiadis I, Rahm S, et al. Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma. Arch Environ Contam Toxicol. 2002;42:105–17. doi: 10.1007/s002440010298. [DOI] [PubMed] [Google Scholar]
- 59.Dayan AD. Risk assessment of triclosan [Irgasan®] in human breast milk. Food Chem Toxicol. 2007;45:125–29. doi: 10.1016/j.fct.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 60.Arbuckle TE, Marro L, Davis K, Fisher M, Ayotte P, et al. Exposure to free and conjugated forms of bisphenol A and triclosan among pregnant women in the MIREC cohort. Environ Health Perspect. 2014;123:277–84. doi: 10.1289/ehp.1408187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geens T, Neels H, Covaci A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere. 2012;87:796–802. doi: 10.1016/j.chemosphere.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115:116–21. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ. 2006;372:87–93. doi: 10.1016/j.scitotenv.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G. Human exposure to triclosan via toothpaste does not change CYP3A4 activity or plasma concentrations of thyroid hormones. Basic Clin Pharmacol Toxicol. 2009;105:339–44. doi: 10.1111/j.1742-7843.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 65.Philippat C, Botton J, Calafat AM, Ye X, Charles MA, Slama R. Prenatal exposure to phenols and growth in boys. Epidemiology. 2014;25:625–35. doi: 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rotroff DM, Wetmore BA, Dix DJ, Ferguson SS, Clewell HJ, et al. Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening. Toxicol Sci. 2010;117:348–58. doi: 10.1093/toxsci/kfq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aylward LL, Hays SM. Consideration of dosimetry in evaluation of ToxCast data. J Appl Toxicol. 2011;31:741–51. doi: 10.1002/jat.1626. [DOI] [PubMed] [Google Scholar]
- 68.DeSalva SJ, Kong BM, Lin YJ. Triclosan: a safety profile. Am J Dent. 1989;2:185–96. (Spec. No.) [PubMed] [Google Scholar]
- 69.Ciniglia C, Cascone C, Giudice RL, Pinto G, Pollio A. Application of methods for assessing the geno- and cytotoxicity of triclosan to C. ehrenbergii. J Hazard Mater. 2005;122:227–32. doi: 10.1016/j.jhazmat.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Binelli A, Cogni D, Parolini M, Riva C, Provini A. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of triclosan in zebra mussel hemocytes. Aquat Toxicol. 2009;91:238–44. doi: 10.1016/j.aquatox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–41. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Latch DE, Packer JL, Stender BL, VanOverbeke J, Arnold WA, McNeill K. Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products. Environ Toxicol Chem. 2005;24:517–25. doi: 10.1897/04-243r.1. [DOI] [PubMed] [Google Scholar]
- 73.Jaiswal AK. Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radic Biol Med. 2000;29:254–62. doi: 10.1016/s0891-5849(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 74.Ma H, Zheng L, Li Y, Pan S, Hu J, et al. Triclosan reduces the levels of global DNA methylation in HepG2 cells. Chemosphere. 2013;90:1023–29. doi: 10.1016/j.chemosphere.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 75.Gou N, Yuan S, Lan J, Gao C, Alshawabkeh AN, Gu AZ. A quantitative toxicogenomics assay reveals the evolution and nature of toxicity during the transformation of environmental pollutants. Environ Sci Technol. 2014;48:8855–63. doi: 10.1021/es501222t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin D, Zhou Q, Xie X, Liu Y. Potential biochemical and genetic toxicity of triclosan as an emerging pollutant on earthworms (Eisenia fetida) Chemosphere. 2010;81:1328–33. doi: 10.1016/j.chemosphere.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 77.Wang X, Liu Z, Wang W, Yan Z, Zhang C, et al. Assessment of toxic effects of triclosan on the terrestrial snail (Achatina fulica) Chemosphere. 2014;108:225–30. doi: 10.1016/j.chemosphere.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 78.Lin D, Xie X, Zhou Q, Liu Y. Biochemical and genotoxic effect of triclosan on earthworms (Eisenia fetida) using contact and soil tests. Environ Toxicol. 2012;27:385–92. doi: 10.1002/tox.20651. [DOI] [PubMed] [Google Scholar]
- 79.Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human diseases. Biochim Biophys Acta. 2007;1775:138–62. doi: 10.1016/j.bbcan.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Winitthana T, Lawanprasert S, Chanvorachote P. Triclosan potentiates epithelial-to-mesenchymal transition in anoikis-resistant human lung cancer cells. PLOS ONE. 2014;9:e110851. doi: 10.1371/journal.pone.0110851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee HR, Hwang KA, Nam KH, Kim HC, Choi KC. Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem Res Toxicol. 2014;27:834–42. doi: 10.1021/tx5000156. [DOI] [PubMed] [Google Scholar]
- 82.Kim JY, Yi BR, Go RE, Hwang KA, Nam KH, Choi KC. Methoxychlor and triclosan stimulates ovarian cancer growth by regulating cell cycle- and apoptosis-related genes via an estrogen receptor-dependent pathway. Environ Toxicol Pharmacol. 2014;37:1264–74. doi: 10.1016/j.etap.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 83.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ Health Perspect. 2011;119:390–96. doi: 10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tamura I, Kanbara Y, Saito M, Horimoto K, Satoh M, et al. Triclosan, an antibacterial agent, increases intracellular Zn2+ concentration in rat thymocytes: its relation to oxidative stress. Chemosphere. 2012;86:70–75. doi: 10.1016/j.chemosphere.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 86.Udoji F, Martin T, Etherton R, Whalen MM. Immunosuppressive effects of triclosan, nonylphenol, and DDT on human natural killer cells in vitro. J Immunotoxicol. 2010;7:205–12. doi: 10.3109/15476911003667470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Foran CM, Bennett ER, Benson WH. Developmental evaluation of a potential non-steroidal estrogen: triclosan. Mar Environ Res. 2000;50:153–56. doi: 10.1016/s0141-1136(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 88.Kumar V, Chakraborty A, Kural MR, Roy P. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod Toxicol. 2009;27:177–85. doi: 10.1016/j.reprotox.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 89.Gee RH, Charles A, Taylor N, Darbre PD. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol. 2008;28:78–91. doi: 10.1002/jat.1316. [DOI] [PubMed] [Google Scholar]
- 90.Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect. 2008;116:1203–10. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Honkisz E, Zieba-Przybylska D, Wojtowicz AK. The effect of triclosan on hormone secretion and viability of human choriocarcinoma JEG-3 cells. Reprod Toxicol. 2012;34:385–92. doi: 10.1016/j.reprotox.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 92.Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, et al. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol. 2004;67:167–79. doi: 10.1016/j.aquatox.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Stoker TE, Gibson EK, Zorrilla LM. Triclosan exposure modulates estrogen-dependent responses in the female Wistar rat. Toxicol Sci. 2010;117:45–53. doi: 10.1093/toxsci/kfq180. [DOI] [PubMed] [Google Scholar]
- 94.Jung EM, An BS, Choi KC, Jeung EB. Potential estrogenic activity of triclosan in the uterus of immature rats and rat pituitary GH3 cells. Toxicol Lett. 2012;208:142–48. doi: 10.1016/j.toxlet.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 95.James MO, Li W, Summerlot DP, Rowland-Faux L, Wood CE. Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ Int. 2010;36:942–49. doi: 10.1016/j.envint.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, et al. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol. 2006;80:217–27. doi: 10.1016/j.aquatox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 97.Helbing CC, van Aggelen G, Veldhoen N. Triclosan affects thyroid hormone–dependent metamorphosis in anurans. Toxicol Sci. 2011;119:417–18. doi: 10.1093/toxsci/kfq343. [DOI] [PubMed] [Google Scholar]
- 98.Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, et al. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 2009;107:56–64. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]
- 99.Rodriguez PE, Sanchez MS. Maternal exposure to triclosan impairs thyroid homeostasis and female pubertal development in Wistar rat offspring. J Toxicol Environ Health A. 2010;73:1678–88. doi: 10.1080/15287394.2010.516241. [DOI] [PubMed] [Google Scholar]
- 100.Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, et al. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology. 2012;300:31–45. doi: 10.1016/j.tox.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Axelstad M, Boberg J, Vinggaard AM, Christiansen S, Hass U. Triclosan exposure reduces thyroxine levels in pregnant and lactating rat dams and in directly exposed offspring. Food Chem Toxicol. 2013;59:534–40. doi: 10.1016/j.fct.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 102.Qatanani M, Zhang J, Moore DD. Role of the constitutive androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology. 2005;146:995–1002. doi: 10.1210/en.2004-1350. [DOI] [PubMed] [Google Scholar]
- 103.Cullinan MP, Palmer JE, Carle AD, West MJ, Seymour GJ. Long term use of triclosan toothpaste and thyroid function. Sci Total Environ. 2012;416:75–79. doi: 10.1016/j.scitotenv.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 104.Cookson BD, Farrelly H, Stapleton P, Garvey RP, Price MR. Transferable resistance to triclosan in MRSA. Lancet. 1991;337:1548–49. doi: 10.1016/0140-6736(91)93242-2. [DOI] [PubMed] [Google Scholar]
- 105.Yazdankhah SP, Scheie AA, Høiby EA, Lunestad BT, Heir E, et al. Triclosan and antimicrobial resistance in bacteria: an overview. Microb Drug Resist. 2006;12:83–90. doi: 10.1089/mdr.2006.12.83. [DOI] [PubMed] [Google Scholar]
- 106.Beier RC, Duke SE, Ziprin RL, Harvey RB, Hume ME, et al. Antibiotic and disinfectant susceptibility profiles of vancomycin-resistant Enterococcus faecium (VRE) isolated from community wastewater in Texas. Bull Environ Contam Toxicol. 2008;80:188–94. doi: 10.1007/s00128-007-9342-0. [DOI] [PubMed] [Google Scholar]
- 107.Seaman PF, Ochs D, Day MJ. Small-colony variants: a novel mechanism for triclosan resistance in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2007;59:43–50. doi: 10.1093/jac/dkl450. [DOI] [PubMed] [Google Scholar]
- 108.Bayston R, Ashraf W, Smith T. Triclosan resistance in methicillin-resistant Staphylococcus aureus expressed as small colony variants: a novel mode of evasion of susceptibility to antiseptics. J Antimicrob Chemother. 2007;59:848–53. doi: 10.1093/jac/dkm031. [DOI] [PubMed] [Google Scholar]
- 109.Birošova L, Mikulášová M. Development of triclosan and antibiotic resistance in Salmonella enterica serovar Typhimurium. J Med Microbiol. 2009;58:436–41. doi: 10.1099/jmm.0.003657-0. [DOI] [PubMed] [Google Scholar]
- 110.Aiello AE, Marshall B, Levy SB, Della-Latta P, Larson E. Relationship between triclosan and susceptibilities of bacteria isolated from hands in the community. Antimicrob Agents Chemother. 2004;48:2973–79. doi: 10.1128/AAC.48.8.2973-2979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cole EC, Addison RM, Rubino JR, Leese KE, Dulaney PD, et al. Investigation of antibiotic and antibacterial agent cross-resistance in target bacteria from homes of antibacterial product users and nonusers. J Appl Microbiol. 2003;95:664–76. doi: 10.1046/j.1365-2672.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 112.Braoudaki M, Hilton AC. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J Clin Microbiol. 2004;42:73–78. doi: 10.1128/JCM.42.1.73-78.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brenwald NP, Fraise AP. Triclosan resistance in methicillin-resistant Staphylococcus aureus (MRSA) J Hosp Infect. 2003;55:141–44. doi: 10.1016/s0195-6701(03)00222-6. [DOI] [PubMed] [Google Scholar]
- 114.Drury B, Scott J, Rosi-Marshall EJ, Kelly JJ. Triclosan exposure increases triclosan resistance and influences taxonomic composition of benthic bacterial communities. Environ Sci Technol. 2013;47:8923–30. doi: 10.1021/es401919k. [DOI] [PubMed] [Google Scholar]
- 115.Cherednichenko G, Zhang R, Bannister RA, Timofeyev V, Li N, et al. Triclosan impairs excitation-contraction coupling and Ca2+ dynamics in striated muscle. PNAS. 2012;109:14158–63. doi: 10.1073/pnas.1211314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schultz MM, Bartell SE, Schoenfuss HL. Effects of triclosan and triclocarban, two ubiquitous environmental contaminants, on anatomy, physiology, and behavior of the fathead minnow (Pimephales promelas) Arch Environ Contam Toxicol. 2012;63:114–24. doi: 10.1007/s00244-011-9748-x. [DOI] [PubMed] [Google Scholar]
- 117.Pugliese G, Favero MS. EPA charges illegal claim for antibacterial-impregnated consumer products. Infect Control Hosp Epidemiol. 1998;19:140. doi: 10.1017/s0195941700000382. [DOI] [PubMed] [Google Scholar]
- 118.Kriebel D, Tickner J, Epstein P, Lemons J, Levins R, et al. The precautionary principle in environmental science. Environ Health Perspect. 2001;109:871–76. doi: 10.1289/ehp.01109871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yueh MF, Nguyen N, Famourzadeh M, Strassburg CP, Oda Y, et al. The contribution of UDP-glucuronosyltransferase 1A9 on CYP1A2-mediated genotoxicity by aromatic and heterocyclic amines. Carcinogenesis. 2001;22:943–50. doi: 10.1093/carcin/22.6.943. [DOI] [PubMed] [Google Scholar]
- 120.Raffensperger C, Tickner J, editors. Protecting Public Health and the Environment: Implementing the Precautionary Principle. Washington, DC: Island Press; 1999. [Google Scholar]


