A concise route for the synthesis of the novel compound class tetrazole-diketo-piperazine is described. The approach involves the use of two multicomponent reactions (MCRs), the Ugi tetrazole 4CR and the classical intramolecular Ugi 4CR. The tetrazole-diketo-piperazines comprise analogs of and are bioisosteric to the bioactive diketopiperazines, thus drawing attention for novel bioactive compound design. This scaffold is currently produced to fill the screening deck of the European lead factory.
2,5-Diketopiperazines (DKPs) are an interesting class of heterocycles, both naturally occurring and synthetic, demonstrating varied pharmacological activities.1 For example, compound 1 is a platelet-activating factor inhibitor,2 while conformationally restricted derivatives 2 are β-turn mimetics.3 The 2,5-diketopiperazine motif has also been found in structures of alkaloid natural products such as demethoxyfumitremorgin C 3.4 Another DKP Epelsiban 4 is developed for the treatment of premature ejaculation in men. It is an oral drug which acts as a selective, oxytocin receptor antagonist with >31000-fold selectivity over the related vasopressin receptors (Fig. 1).1b
Fig. 1.
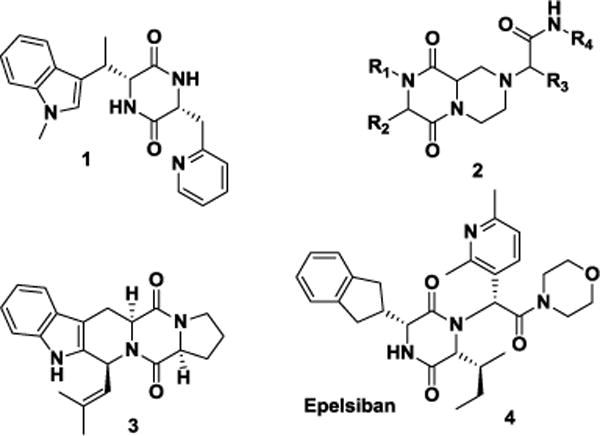
2,5-Diketopiperazines (DKPs)
The Ugi tetrazole reaction, originally reported in 1961, is one of the best and most general methods for the synthesis of highly substituted α-amino tetrazoles.5 It involves the Schiff base formation from the appropriately substituted aldehyde or ketone and primary amine, followed by its reaction with an isocyanide.6 The resulting intermediate nitrilium ion, then, reacts with hydrogen azide, affording highly substituted tetrazoles. In a recent review, more than 30 different scaffolds of differentially substituted piperazines, keto-piperazines and diketopiperazines, accessible by multicomponent reactions, have been enumerated.7 Several more recent syntheses are shown in Scheme 1. For example, Chauhan et al.8 reported on the synthesis of indole-fused diketopiperazines via a tandem Ugi-4CR and intramolecular cyclization to compounds 5. El Kaïm et al.9 synthesised polycyclic diketopiperazines 6 by an Ugi/Pictet-Spengler reaction from isocyanides and α-keto acids. Our laboratory is engaged in the further exploration of the bioisosteric nature of tetrazoles and carboxylic acids leading to new synthetic methods of tetrazole scaffolds.
Scheme 1.
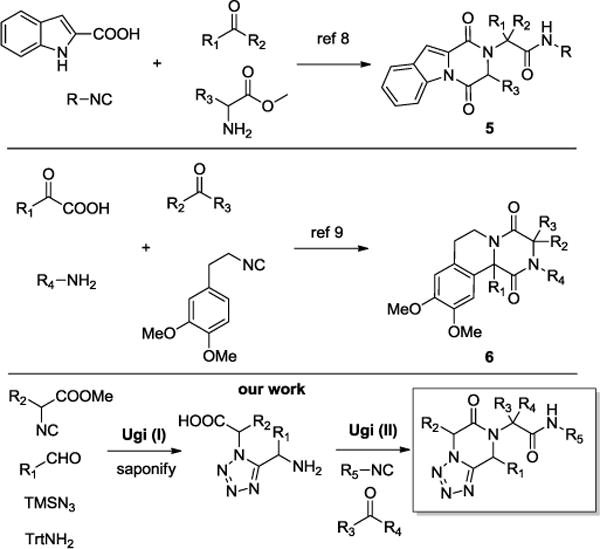
Reported Synthesis of Fused Diketopiperazines and Our Work
Here, we report on the synthesis of tetrazole-fused keto-piperazines which are potentially bioisosteres to the Epelsiban 4 scaffold. Surprisingly, a related scaffold has been described in literature only once before.10 Based on our recently published approach to amino tetrazoles, the desired tetrazole-fused keto-piperazines were synthesized in two steps.11 Firstly, we synthesized the N-unsubstituted α-aminotetrazoles using an Ugi tetrazole reaction and then employed in a second intramolecular Ugi 4CR reaction affording the desired products in moderate to good yields.12 The Ugi tetrazole synthesis was initially performed under Ugi azide conditions with tritylamine as the amine component, various aldehydes and isocyanides derived from α-aminoacids and azidotrimethylsilane, producing the desired tetrazoles 7, Scheme 2.
Scheme 2.
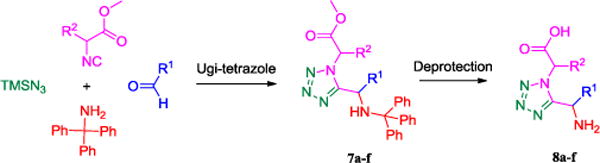
Two step synthesis of N-unsubstituted ω-carboxyl α-aminotetrazoles
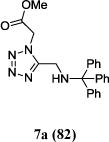
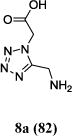
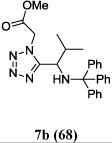
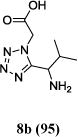
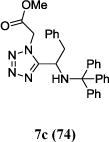
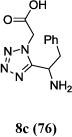
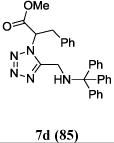
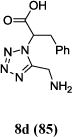
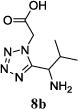
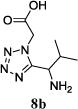
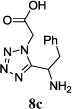
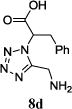
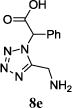
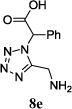
We were able to crystalize one of the tetrazoles and obtain its crystal structure 7e (Fig. 3). The N-unsubstituted ω-carboxyl α-aminotetrazoles 8 were accessed after treating the tetrazoles 7 with aqueous HCl such cleaved both the trityl group and the methyl ester derived from the isocyanide component in one step Table 1.
Fig 3.
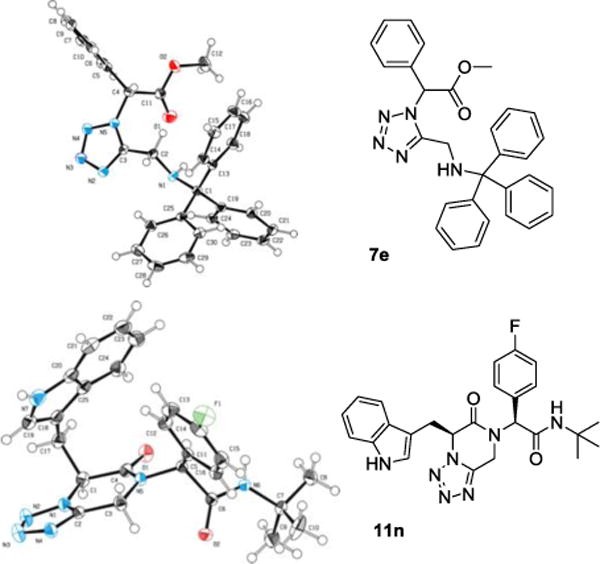
X-ray ORTEP plots of compounds 7e and 11n
Table 1.
Yields of Ugi Tetrazoles (7) and deprotected Tetrazoles (8)
| No. | Ugi-tetrazole 7 (%) | Deprotected tetrazole 8* (%) |
|---|---|---|
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
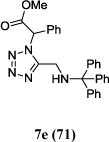
|
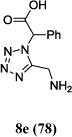
|
| 6 |
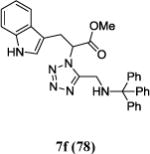
|
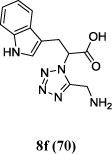
|
Isolated yield at neutral pH of amino acid.
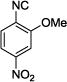
The synthesis of the desired tetrazole-fused keto-piperazines 11, (Fig. 2) proved to be difficult in the beginning, since classic room temperature conditions did not favour the Ugi 4CR reaction even after prolonged time (3 days). The reaction was also performed under reflux conditions, using MeOH as solvent, in a way similar to the first attempt. However, the results were still not satisfactory, and thus microwave irradiation was employed giving very good results, in short time, with moderate to good yields. In order to further optimize the reaction conditions, different solvents were screened; such as EtOH, toluene, MeOH/H2O and finally trifluoroethanol (TFE). The last one proved to be the optimum solvent for the reaction. As a final step of the optimization process, a Lewis acid (ZnCl2) was tested as an additive in the reaction, in an effort to improve the yield of the reaction, without, however, any significant increase in yield. No substantial byproducts were detected, but only unreacted starting material was recovered. Optimized conditions for the synthesis of tetrazole-fused keto-piperazines 11 were 1 eq of each of the reagents, TFE as the solvent and microwave irradiation for 30 min at 120°C. The diversity of the reaction can be seen in Table 2.
Fig. 2.
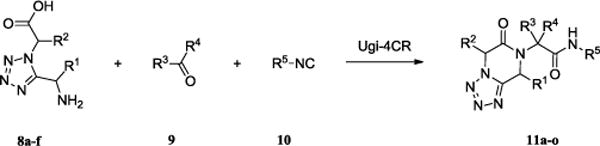
Synthesis of tetrazole-fused keto-piperazines (5)
Table 2.
Yields and d.r. ratios of tetrazole-fused keto-piperazines (11)
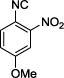
| No. | Amino Acid (8) | R3R4C=O (9) | R5-NC (10) | Product (Yield %)* | d.r. Ratio |
|---|---|---|---|---|---|
| 1 |
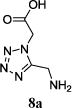
|
|
|
11a (45) | – |
| 2 |
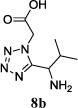
|
|
|
11b (53) | 9:1 |
| 3 |
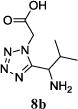
|
|
|
11c (33) | 8:1 |
| 4 |
|
|
|
11d (58) | 8:1 |
| 5 |
|
|
|
11e (32) | – |
| 6 |
|
|
|
11f (72) | 2:1 |
| 7 |
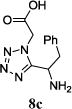
|
|
|
11g (36) | 3:1 |
| 8 |
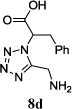
|
|
|
11h (37) | 4:1 |
| 9 |
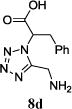
|
|
|
11i (33) | 1:1 |
| 10 |
|
|
|
11j (40) | 5:3 |
| 11 |
|
|
|
11k (44) | 3:2 |
| 12 |
|
|
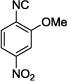
|
11l (71) | – |
| 13 |
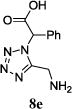
|
|
|
11m (42) | 1:1 |
| 14 |
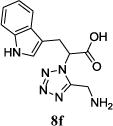
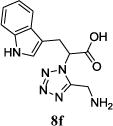
|
|
|
11n (20) | 9:1 |
| 15 |
|
|
|
11o (49) | – |
Yields reported for mixture of diastereomers
The reaction works with aromatic and aliphatic aldehydes along with ketones such as cyclohexanone. Many different isocyanides, both aromatic and aliphatic, were used in the reaction. The reaction proceeded with discriminating diastereo-selectivity since most of the products exhibited a major preference for only one diastereoisomer. An example of this preference is 11n whose crystal structure is shown in (Fig. 3). Both the indole moiety deriving from the α-amino acid-derived isocyanide component of the Ugi 4CR and the 4-F-benzaldehyde prefer to be on the same side of the plane that is formed by the main body of the tetrazole-fused keto-piperazine.
With this general synthesis of tetrazole-fused keto-piperazines in hand we created a virtual library of 1000 randomly generated tetrazole-fuzed keto-piperazines and analyzed some general features against their related diketopiperazine (DKP) counterparts, also synthesized via a variation of the Ugi reaction.13 Looking first at just the backbone of each scaffold, (Fig. 4A), we notice that they have many similarities, from a medicinal chemistry point of view. Looking at the pharmacophore points we see a hydrogen bond donor, two hydrogen bond acceptors and a hydrophobic area in common between the two scaffolds. Their major difference stems from the piperazine ring where the DKP scaffold has one hydrogen bond donor and acceptor while our tetrazole-fused keto-piperazine contains 3 hydrogen bond acceptors.
Fig 4.
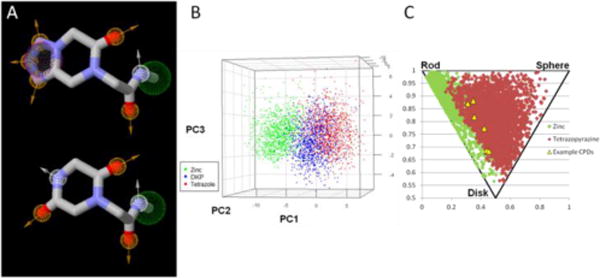
(a) Pharmacophore point comparison of our tetrazole-fuzed keto-piperazines (top) and their diketopiperazine counterparts, synthesized via a variation of the Ugi reaction (green ball = hydrophobic, orange ball = hydrogen bond acceptor, white ball = hydrogen bond donor) ; (b) 3D PCA of 1000 randomly selected tetrazoles (red), DKPs (blue), and Zinc compounds (green) (c) PMI plot depicting 1000 randomly selected tetrazole compounds (red) overlapped with the PMI plot of 1000 randomly selected compounds from the ZINC database (green). Six of the compounds from the paper have also been plotted (yellow triangles).
To visualize the distribution in a 3D chemical space, unbiased molecular descriptors were analyzed by principal component analysis (PCA), (Fig. 4B). Our tetrazole-fused compounds (red) were compared with a virtually generated diketopiperazine (blue) library and 1000 randomly selected compounds from the ZINC14 database (green). As can be seen they are widely distributed in a 3D chemical space compared to the ZINC database, but retain some similarities to the DKP scaffold. The molecular diversity of the different libraries was differentiated mainly by van der Waals (VDW) volume, logP, and heteroaromatic ring count. We performed the principal moment of inertia (PMI)15 analysis to compare the shape distribution of our virtual library of small molecules to that of 1000 randomly selected compounds from the ZINC library (Fig. 4C). As can be seen our tetrazole compounds (red) are much more 3D-like in nature compared to compounds from the ZINC library. Six of the compounds from this paper (11 a,c,g,h,l,n) are also plotted in yellow triangles for reference.
Conclusions
In conclusion, we have developed an effective procedure for the novel syntheses of highly substituted tetrazole-fused keto-piperazines via Ugi-tetrazole/deprotection and Ugi-4CR. We will report, soon, on the biological properties of this interesting new scaffold.
Supplementary Material
Acknowledgments
Funding is provided for AD by the National Institute of Health (1R01GM097082-01), European Lead Factory (IMI under grant agreement no 115489) and the Qatar National Research Foundation (NPRP6-065-3-012).
References
- 1.(a) Borthwick AD. Chem Rev. 2012;112:3641. doi: 10.1021/cr200398y. [DOI] [PubMed] [Google Scholar]; (b) Borthwick AD, et al. J Med Chem. 2012;55:783. doi: 10.1021/jm201287w. [DOI] [PubMed] [Google Scholar]; (c) Zabrocki J, David Smith G, Dunbar JB, Iijima H, Marshall GR. J Am Chem Soc. 1988;110:5875. [Google Scholar]
- 2.Shimazaki N, Hemmi K, Nakaguti O, Miyazaki Y, Hashimoto M. EP 243,122. European Patent Application. 1987 Oct 28;:53.
- 3.Golebiowski A, Klopfenstein SR, Chen JJ, Shao X. Tetrahedron Lett. 2000;41:4841. [Google Scholar]
- 4.Wang H, Ganesan A. Org Lett. 1999;1:1647. [Google Scholar]
- 5.(a) Ugi I, Steinbrückner C. Chem Ber. 1961;94:734. [Google Scholar]; (b) Ugi I. Angew Chem Int Ed Engl. 1962;1:8. [Google Scholar]; (c) Ugi I, Meyr R, Fetzer U, Steinbrückner C. Angew Chem. 1959;71:386. [Google Scholar]
- 6.a) Dömling A, Beck B, Fuchs T, Yazbak A. J Comb Chem. 2006;8:872. doi: 10.1021/cc060068w. [DOI] [PubMed] [Google Scholar]; b) Gulevich AV, Zhdanko AG, Orru RVA, Nenajdenko VG. Chem Rev. 2010;110:5235. doi: 10.1021/cr900411f. [DOI] [PubMed] [Google Scholar]
- 7.Dömling A, Huang Y. Synthesis. 2010;17:2859. [Google Scholar]
- 8.Chauhan PMS, Pandey S, Khan S, Singh A, Gauniyal HM, Kumar B. J Org Chem. 2012;77:10211. doi: 10.1021/jo3018704. [DOI] [PubMed] [Google Scholar]
- 9.El Kaïm L, Gageat M, Gaultier L, Grimud L. Synlett. 2007:500. [Google Scholar]
- 10.Nixey T, Kelly M, Hulme C. Tet Let. 2000;41:8729. [Google Scholar]
- 11.Zhao T, Boltjes A, Herdtweck E, Dömling A. Org Lett. 2013;15:639. doi: 10.1021/ol303348m. [DOI] [PubMed] [Google Scholar]
- 12.(a) Dömling A, Ugi I. Angew Chem Int Ed. 1993;32:563. [Google Scholar]; (b) Elders N, van der Born D, Hendrickx LJD, Timmer BJJ, Krause A, Janssen E, de Kanter FJJ, Ruijter E, Orru RVA. Angew Chem Int Ed. 2009;48:5856. doi: 10.1002/anie.200902683. [DOI] [PubMed] [Google Scholar]
- 13.(a) Szardenings A, Burkoth TS, Lu HH, Tien DW, Campbell DA. Tetrahedron. 1997;53:6573. [Google Scholar]; (b) Szardings A, et al. J Med Chem. 1999;42:1348. [Google Scholar]; (c) Cho S, Keum G, Kang SB, Han SY, Kim Y. Molec Div. 2003;6:283. doi: 10.1023/b:modi.0000006812.16141.b5. [DOI] [PubMed] [Google Scholar]
- 14.(a) Irwin JJ, Shoichet BK. J Chem Inf Model. 2005;45:177. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Irwin JJ, Sterling T, Mysinger MM, Bolstad ES, Coleman RG. J Chem Inf Model. 2012;52:1757. doi: 10.1021/ci3001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer WH, Schwarz MK. J Chem Inf Comp Sci. 2003;43:987. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


