Abstract
Major depressive disorder (MDD) is a prevalent psychiatric condition in the child maltreatment population. However, not all children who have been maltreated will develop MDD or MDD symptoms, suggesting the presence of unique risk pathways that explain how certain children develop MDD symptoms when others do not. The current study tested several candidate risk pathways to MDD symptoms following child maltreatment: 1) neuroendocrine, 2) autonomic, 3) affective, and 4) emotion regulation. Female adolescents (N=110; Age range: 14–19) were recruited into a substantiated child maltreatment or comparison condition and completed a laboratory stressor, saliva samples, and measures of emotion regulation, negative affect, and MDD symptoms. MDD symptoms were reassessed eighteen months later. Mediational modeling revealed that emotion regulation was the only significant indirect effect of the relationship between child maltreatment and subsequent MDD symptoms, demonstrating that children exposed to maltreatment had greater difficulties managing affective states that in turn led to more severe MDD symptoms. These results highlight the importance of emotion dysregulation as a central risk pathway to MDD following child maltreatment. Areas of future research and implications for optimizing prevention and clinical intervention through the direct targeting of transdiagnostic risk pathways are discussed.
Keywords: child maltreatment, major depressive disorder, emotion dysregulation, transdiagnostic mechanisms
The life-long public health impact of child maltreatment in the United States is estimated at $124 billion (Fang, Brown, Florence, & Mercy, 2012) with psychiatric morbidity serving as a primary driver of healthcare utilization and costs in this population (Yanos, Czaja, & Widom, 2010). Major depressive disorder (MDD) is among the most commonly diagnosed psychiatric disorders in the child maltreatment population (Fergusson, Boden, & Horwood, 2008; MacMillan et al., 2001) with a lifetime prevalence rate of 25.0% (Widom, DuMont, & Czaja, 2007), considerably higher than the 16.6% and 11.7% rates found in the general adult (Kessler et al., 2005) and adolescent populations (Merikangas et al., 2010), respectively. There is broad support establishing child maltreatment as a risk factor for subsequent MDD (Gilbert et al., 2009). In fact, recent research suggests that the effect size magnitude of child maltreatment on subsequent MDD is larger than initially anticipated once contamination, or the presence of child maltreatment in a comparison condition, is controlled (Scott, Smith, & Ellis, 2010; Shenk, Noll, Peugh, Griffin, & Bensman, 2015). While the risk child maltreatment poses for MDD is clear, the etiological processes involved in the onset of this disorder remain largely unknown. For instance, not every child who is maltreated will develop MDD or MDD symptoms (Collishaw et al., 2007), suggesting the presence of unique risk pathways that lead to disorder onset for some and not others. Furthermore, MDD is highly comorbid with other psychiatric disorders in the child maltreatment population, namely posttraumatic stress disorder (PTSD) and substance dependence (Putnam, Harris, & Putnam, 2013; Widom et al., 2007), suggesting that child maltreatment affects transdiagnostic risk pathways that lead to taxonomically distinct yet functionally related psychiatric outcomes (Nolen-Hoeksema & Watkins, 2011). In addition to a greater understanding of the etiology of MDD in the child maltreatment population, efforts to identify risk pathways serve an important translational need where preventative and clinical interventions targeting identified pathways can be developed or modified in an effort to reduce the incidence of MDD and resulting healthcare costs.
A multiple-levels-of-analysis approach (Cicchetti & Blender, 2004; Cicchetti & Dawson, 2002), where researchers incorporate knowledge on candidate risk pathways across scientific disciplines, such as neurobiology and behavioral science, is a useful framework to advance a more complete perspective on the etiology of MDD in the child maltreatment population. In this vein, allostatic load (McEwen & Wingfield, 2003) has been advanced as a comprehensive framework for how chronic or severe stress, such as child maltreatment, alters the functioning of multiple interconnected, biological stress-mediating pathways that in turn lead to an increased risk for various psychiatric outcomes later in development (Juster, Bizik, et al., 2011). The neuroendocrine and autonomic systems are the primary biological systems responding to environmental challenge (Chrousos & Gold, 1992) and prior research has demonstrated that child maltreatment alters key markers of both neuroendocrine and autonomic activity (Gordis, Granger, Susman, & Trickett, 2008). Cortisol, a glucocorticoid biomarker of overall neuroendocrine functioning, has been examined extensively in the child maltreatment population with evidence suggesting that child maltreatment results in both hypersecretion and hyposecretion profiles during rest (Cicchetti & Rogosch, 2001; Trickett, Noll, Susman, Shenk, & Putnam, 2010) and laboratory challenge (Carpenter et al., 2007; Heim et al., 2000). Children who experience maltreatment and have greater depressive symptoms also exhibit changes in the diurnal profile of cortisol, yielding an attenuated or flattened profile that prolongs exposure to increased cortisol levels throughout the day (Cicchetti, Rogosch, Gunnar, & Toth, 2010). Although not always (MacMillan et al., 2009), various cortisol profiles, in particular the hypersecretion profile (Gillespie & Nemeroff, 2005), have been linked to an increased risk for MDD (Gotlib, Joormann, Minor, & Hallmayer, 2008; Miller, Chen, & Zhou, 2007), suggesting that dysregulation of the neuroendocrine system may be a risk pathway to MDD in the child maltreatment population.
Dysregulation of the sympathetic nervous system, a branch of the autonomic nervous system, may also serve as a candidate risk pathway to MDD in the child maltreatment population given its role in the short-term stimulation of physiological resources and organic activity following environmental challenge (Cacioppo, 1994). Salivary α-amylase is a non-invasive, surrogate biomarker of sympathetic activity (Ditzen, Ehlert, & Nater, 2014; Granger, Kivlighan, el-Sheikh, Gordis, & Stroud, 2007; Nater & Rohleder, 2009) indicative of adrenergic stimulation (Allwood, Handwerger, Kivlighan, Granger, & Stroud, 2011; van Stegeren, Rohleder, Everaerd, & Wolf, 2006). While research on a specific salivary α-amylase profile is still developing, existing studies have demonstrated that samples exposed to chronic or severe stress, including child maltreatment, exhibit dysregulation of salivary α-amylase performance (Gordis, Granger, Susman, & Trickett, 2006; Gordis et al., 2008; Rudolph, Troop-Gordon, & Granger, 2011) with dysregulated profiles, specifically the hypersecretion profile, occurring more often in those with MDD (Schumacher, Kirschbaum, Fydrich, & Strohle, 2013). Although not always (Juster, Sindi, et al., 2011), this research suggests that exposure to severe or chronic stress such as child maltreatment may lead to dysregulation of autonomic activity that in turn serves as a useful biomarker of MDD (Tanaka et al., 2012).
One particular challenge in establishing neuroendocrine and autonomic pathways to MDD is the extent to which dysregulation in these systems functions uniquely and independently from alternative processes also affected by exposure to child maltreatment and related to MDD. For instance, child maltreatment increases the overall presence and intensity of negative affect (Bradley et al., 2011), subjective distress experienced across a variety of aversive states, such as anger, sadness, disgust, guilt, fear, and nervousness (Watson, Clark, & Tellegen, 1988), which are hallmark features of MDD (American Psychiatric Association, 2013). However, changes in negative affect during and after stress exposure often co-occur with changes in neuroendocrine and autonomic functioning (Waugh, Muhtadie, Thompson, Joormann, & Gotlib, 2012), making it difficult to know which pathway, biological or affective, is most influential in the onset of subsequent MDD. It is also unknown whether the presence or intensity of negative affect is a more potent risk pathway than one’s ability to manage or regulate such affect. Emotion dysregulation, or the difficulty in deploying behavioral strategies that effectively modulate the form, frequency or magnitude of an affective response (Gross, 1998), is also a candidate risk pathway given its role in influencing a variety of affective states and moods common to different forms of psychopathology (Kring, 2010; Southam-Gerow & Kendall, 2002). A substantial amount of research has established the temporal relations between child maltreatment and subsequent emotion dysregulation (Kim & Cicchetti, 2010; Shields & Cicchetti, 2001; Shipman, Zeman, Penza, & Champion, 2000) with emotion dysregulation playing a central role in the etiology and clinical presentation of MDD in children and adolescents (Aldao, Nolen-Hoeksema, & Schweizer, 2010; Kring & Werner, 2004; Rottenberg, Gross, & Gotlib, 2005; Silk, Steinberg, & Morris, 2003). Recent evidence indicates that emotion dysregulation mediates the relationship between psychological maltreatment in childhood and current MDD symptoms in young adult women (Coates & Messman-Moore, 2014). However, there is no indication whether emotion dysregulation continues to function as a mediator of MDD symptoms in the child maltreatment population when other co-occurring pathways, including biological and affective pathways, are included in the same mediational model.
The current study adopted a multiple-levels-of-analysis approach to test whether neuroendocrine, autonomic, affective, and emotion regulation pathways explain the relationship between child maltreatment and subsequent MDD symptoms. Female adolescents with a recently documented history of child maltreatment were recruited to participate in a prospective, longitudinal study examining the effects of child maltreatment on subsequent risk pathways and MDD symptoms. Child maltreatment occurring in adolescence was examined because it exerts strong effects on a variety of health outcomes in later adolescence (Flaherty et al., 2013; Thornberry, Ireland, & Smith, 2001). Female adolescents were selected as they are more likely to experience multiple forms of child maltreatment (U.S. Department of Health and Human Services, 2012), particularly sexual abuse (Sedlak et al., 2010), and are at a greater risk for MDD when compared to males (Merikangas et al., 2010). Testing several candidate pathways simultaneously has the advantage of assessing the cumulative effect of child maltreatment while minimizing the risk of attributing causal status to a single pathway when other relevant co-occurring pathways are omitted from the analysis. A simultaneous test of multiple pathways also allows each individual pathway to compete for variance in a specified outcome, leading to more rapid and effective identification of the putative risk pathways responsible for the development of MDD symptoms in the child maltreatment population. A final important advantage of this approach is that results have direct clinical utility for prevention and intervention programs. Identifying one or more risk pathways prioritizes clinical resources toward those pathways most influential in increasing the risk of subsequent MDD symptoms. This study tested whether: 1) the total indirect effect, or the sum of the specific indirect effects of each proposed pathway, mediated the relationship between child maltreatment and subsequent MDD symptoms, and 2) each proposed pathway constituted a specific indirect effect of the relationship between child maltreatment and MDD symptoms when simultaneously estimating the other risk pathways and after controlling relevant covariates.
Method
Sample
One hundred ten adolescent females between the ages of 14–19 years of age participated in this study. A child maltreatment condition (n = 51) was recruited from Child Protective Service (CPS) agencies investigating allegations of physical neglect or contact physical or sexual abuse. With the assistance of CPS caseworkers, a consecutive referral process identified families who had a child with a substantiated case of maltreatment occurring within the prior twelve months. A comparison condition where all members denied prior CPS involvement during an initial eligibility screen (n = 59) was recruited using posted flyers in a primary care outpatient medical clinic serving at-risk, adolescent females. The mean age of the total sample at study entry was 17.00 years (SD = 1.17), 58% of the adolescents were from single-caregiver homes, the median family income level was $20,000–$29,000, and the sample was 42% Caucasian, 51% African-American, 1% Hispanic and 6% Multi-racial. Demographic information is presented by condition membership in Table 1.
Table 1.
Demographic and Study Related Information at Study Entry
| Variable | Maltreated Condition | Comparison Condition | |
|---|---|---|---|
| M (SD) or n | M (SD) or n | ||
| Age | 16.78 (1.12) | 17.19 (1.20) | |
| Race | |||
| African-American | 24 | 32 | |
| Caucasian | 24 | 22 | |
| Hispanic | 0 | 1 | |
| Multi-racial | 3 | 3 | |
| Income | |||
| Under $10,000 | 14 | 14 | |
| $10,000 - $19,999 | 8 | 6 | |
| $20,000 - $29,999 | 9 | 9 | |
| $30,000 - $39,999 | 5 | 10 | |
| $40,000 - $49,999 | 4 | 6 | |
| $50,000 - $59,999 | 1 | 3 | |
| $60,000 - $69,999 | 3 | 3 | |
| $70,000 - $79,999 | 4 | 2 | |
| $80,000 - $89,999 | 2 | 2 | |
| $90,000 - $99,999 | 0 | 1 | |
| >$100,000 | 0 | 2 | |
| Family Environment | |||
| Single-caregiver home | 26 | 33 | |
| Dual-caregiver home | 20 | 23 | |
Procedure
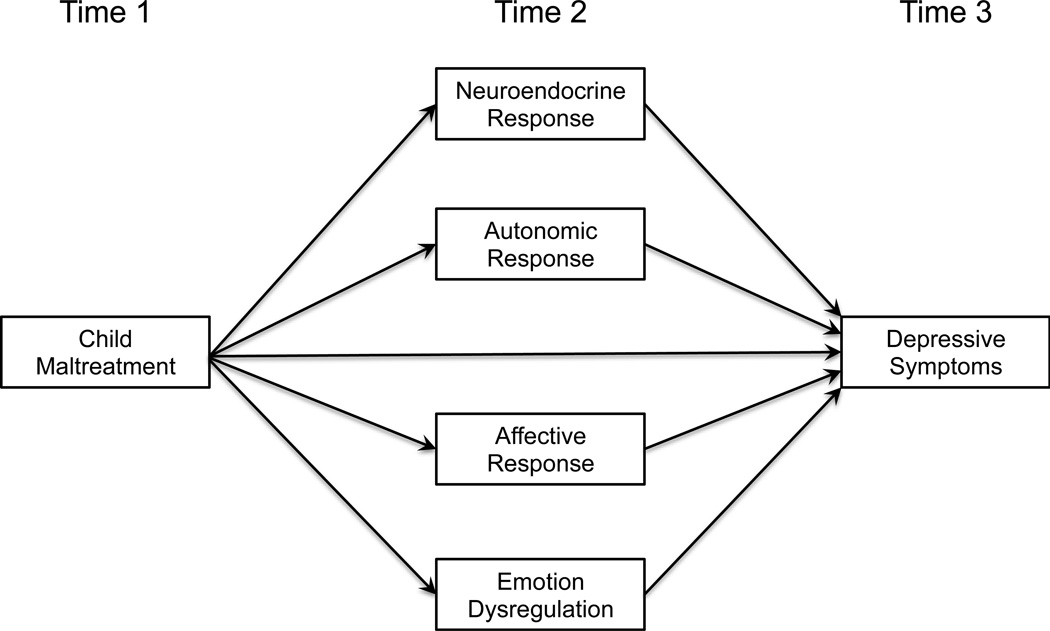
All study procedures were approved by the local Institutional Review Board prior to data collection. Causal inferences about risk pathways assessed in observational research are strengthened when there is proper temporal ordering of events that follow a logical or theoretical pattern (Maxwell & Cole, 2007; Preacher & Hayes, 2008). As such, adolescent females who experienced substantiated child maltreatment and a comparison condition were recruited to participate in an assessment of several candidate risk pathways of MDD symptoms. This same cohort was followed approximately eighteen months later to determine whether the set of risk pathways, as well as individual pathways, exerted significant indirect effects on subsequent MDD symptoms measured at the transition to adulthood (Mage = 18.66; SD = 0.66). To illustrate the actual temporal relationships among study-related variables, child maltreatment is presented as Time 1, the assessment of risk pathways as Time 2, and the subsequent assessment of MDD symptoms as Time 3 (see Figure 1).
Figure 1.
Conceptual Model for Multiple Levels of Analysis Approach
Time 1: Determination of Child Maltreatment
Child maltreatment was determined by a CPS investigation that resulted in a primary substantiated/indicated designation of child maltreatment. All substantiated/indicated designations of child maltreatment were made prior to the onset of this study. Of the 51 participants in the child maltreatment condition, 49% experienced sexual abuse, 45% experienced physical abuse, and 16% experienced physical neglect with 10% experiencing more than one type of maltreatment. Comparison females were screened for a history of child maltreatment by searching substantiated/indicated designations of child maltreatment in CPS records as well as through self-report of child maltreatment at each study visit. Cases of child maltreatment in the comparison condition (n = 29) were identified and controlled in statistical analyses by creating a dummy-coded variable that indicated the presence of contamination (Yes=1; No=0) in the comparison condition.
Time 2: Assessment of Risk Pathways
All appointments were scheduled between 11 a.m. and 5 p.m. Participants completed a general interview about current health habits, self-report measures assessing emotion dysregulation and MDD symptoms, and a laboratory stressor measuring reactivity in neuroendocrine, autonomic, and affective systems. The laboratory stressor involved participants first completing a five-minute resting condition where each participant sat comfortably in a chair while listening to soft music and watching slow-moving images on a computer screen. Participants then completed a combined stressor task to elicit reactivity across different physiological and affective systems. A combined performance and interpersonal stressor was chosen given varying neuroendocrine and autonomic responses to different stressor types (Stroud et al., 2009). The performance aspect of the stressor paradigm involved each participant completing a series of affect recognition tasks (Porges, Cohn, Bal, & Lamb, 2007). Participant responses were timed and each participant was asked to identify the expressed emotion as quickly as they could while not making any mistakes before the time elapsed. The average length of time to complete the affect recognition tasks was 7.45 minutes (SD = 1.19). The interpersonal stressor involved participants viewing a series of video-clips of parent-adolescent conflict. The time required to view all videos was 8 minutes.
Time 3: Assessment of Subsequent MDD Symptoms
Approximately eighteen months following Time 2, study participants were re-contacted to complete the same measure of MDD symptoms at the transition to young adulthood. Five percent (n = 6) of the original sample was unable to complete the subsequent MDD symptoms assessment, indicating a 95% retention rate throughout the course of the study.
Measures
General demographics and health habits form
Demographic information was assessed via self- and caregiver-report and included age, race, family income and family constellation (single-caregiver vs. dual-caregiver homes). Health habits were measured at Time 2 to control their potential influence on neuroendocrine and autonomic activity. Specific health habits assessed were: pregnancy status, use of steroids (topical, oral and inhaled), cigarettes, over-the counter (aspirin, ibuprofen) and prescription drugs (psychotropic, seasonal allergies, oral contraceptive), whether participants ate anything one hour prior to their appointment, and whether participants exercised or drank caffeine on the day of their appointment.
Cortisol and Salivary α-Amylase
Cortisol and salivary α-amylase reactivity were assessed across five, whole saliva samples to obtain resting and stress response estimates for each biomarker. Each participant was instructed not to eat or drink anything one hour prior to participation. Upon arrival, each participant was asked to rinse their mouth with water prior to beginning their appointment. The first sample was collected approximately 25 minutes (M = 25.30, SD = 0.24) after participants began their study appointment to give them time to acclimate to the research environment and procedures. Samples two through five were collected 5-, 10-, 20- and 30-minutes post stressor to detect response to the combined stressor task. Unstimulated saliva was obtained in 20 mL polypropylene vials and stored at −80°C until assayed. Cortisol was assayed in duplicate using a commercially available, high-sensitivity enzyme immunoassay (Salimetrics®). The test has a lower limit sensitivity of <.003 µg/dl and average intra-and inter-assay coefficients of variation 3.35% - 3.65% and 3.75% - 6.41%, respectively. Saliva samples were also assayed for salivary α-amylase using a commercially available kinetic reaction assay (Salimetrics®) with intra- and inter-assay coefficients of variation ranging from 2.5% - 7.2% U/ml and 3.6% - 5.8% U/ml, respectively.
Positive and Negative Affect Schedule (PANAS; Watson et al., 1988)
The PANAS is a twenty-item self-report questionnaire assessing the subjective intensity of current positive and negative affective states. Reliability of the PANAS indicates the measure has strong internal consistency in both the positive (α = .89) and negative affect scales (α = .85) with good concurrent validity with measures of depressive symptoms (Crawford & Henry, 2004). Intensity ratings for the negative affect scale of the PANAS were collected in the current study to assess changes in current affect across four different measurements at Time 2: 1) immediately before the resting condition, 2) immediately after the combined stressor task, 3) thirty minutes post-stressor, and 4) forty minutes post-stressor. Total scores obtained from the negative affect scale of the PANAS were examined in statistical analyses given their relevance to affective reactions to stress and depressive symptoms. Internal consistency of the PANAS negative affect scale in the current sample ranged from α = .71–.84.
Difficulties in Emotion Regulation Scale (DERS; Gratz & Roemer, 2004)
The DERS is a 36-item, self-report questionnaire assessing several aspects of emotion dysregulation: non-acceptance of emotions, difficulties engaging in goal-oriented behavior, difficulties with impulse control, lack of emotional awareness, limited access to emotion regulation strategies, and lack of emotional clarity. The DERS has excellent internal consistency (α = .93) and strong construct validity with other measures of emotion regulation in young adult (Gratz & Roemer, 2004) and adolescent samples (Weinberg & Klonsky, 2009). Internal consistency for the DERS in the current sample is α = .93. Total scores obtained from the DERS were used in statistical analyses and represent an individual’s overall difficulty in regulating emotions with higher scores reflecting greater emotion dysregulation.
The Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996)
The BDI-II is a 21-item, well-established measure of depressive symptoms in adolescence and adulthood. The BDI-II was administered at Time 2 and Time 3 with α’s ranging from .90–.96. Total BDI-II scores at Time 3 were used as the primary outcome in statistical analyses and indicate overall MDD symptom severity subsequent to child maltreatment.
Data Analytic Strategy
A multiple mediator model (Preacher & Hayes, 2008) was employed to identify the total and specific indirect effects of neuroendocrine, autonomic, affective, and emotion dysregulation pathways when explaining the relationship between child maltreatment and subsequent MDD symptoms. To do so, area under the curve with respect to ground (AUCg; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) was calculated for cortisol (CortAUCg), salivary α-amylase (α-amylaseAUCg), and negative affect (PANASAUCg) to obtain a single estimate that reflects the total output of each pathway across all repeated measurements, including resting and stress response. DERS total scores were estimated simultaneously with all other pathways. The multiple mediator model was performed using PROCESS v2.13 within SPSS v22. PROCESS is a regression-based, path analytic approach to testing the indirect effects of several proposed mediators simultaneously (Hayes, 2013). This method of testing mediation is particularly useful in the current study because it tests the indirect effects of each proposed mediator simultaneously through the use of bootstrapping. Bootstrapping is a non-parametric, re-sampling procedure for estimating indirect effects and their corresponding confidence intervals with optimal accuracy (MacKinnon, Lockwood, & Williams, 2004). By randomly sampling from n observations and estimating indirect effects k times with replacement of observations, estimates of the total and specific indirect effects, and their corresponding confidence intervals, can be obtained. There is also the opportunity to enter variables into the multiple mediator model as covariates, allowing for statistical control of variables affecting both mediators and outcomes. Results of the current multiple mediator model are based on k = 5000 bootstrap samples with bias-corrected, 95% confidence intervals (BC 95% CI) and address two important hypotheses: 1) whether the total indirect effect, or sum of indirect effects, mediates the relationship between child maltreatment and subsequent MDD symptoms, and 2) whether there are specific indirect effects that constitute individual mediation of subsequent MDD symptoms.
Results
Data Screening
Participants who reported being pregnant (n = 6) were excluded from statistical analyses. Chi-squared and serial analysis of variance (ANOVA) tests were used to evaluate differences between child maltreatment and comparison conditions on all demographic and health-related variables measured at Time 2 that might affect risk pathways, particularly cortisol and salivary α-amylase. Results indicated significant between-group differences on the use of prescribed steroid medication (Steroid use = 1; No steroid use = 0), χ2(1) = 5.27, p = .02, ϕ = 0.23, and cigarette use (Cigarette use = 1; No cigarette use = 0), χ2(1) = 7.81, p = .01, ϕ = 0.28, with the child maltreatment condition using steroid medication and cigarettes more often than the comparison condition. There were no significant differences between groups on race, income, family constellation, medication use (prescription, over-the-counter), the use of caffeine prior to the study, whether participants exercised on the day of their appointment, or ate in the hour prior to the study. The ANOVA for participant age indicated that the child maltreatment condition was marginally younger than the comparison condition at the Time 2 assessment, F(1, 102) = 3.37, p = .069, η2 = .03. There was no significant group difference in the time of day the first saliva sample was collected. Of note, there was a significant zero-order correlation between child maltreatment status and BDI-II scores obtained at the Time 3 assessment, r = 0.21, p = .037.
Cortisol concentrations continue to decline throughout the afternoon and into the evening (Kiess et al., 1995). As such, the relationship between the time the first saliva sample was collected and corresponding cortisol concentrations was examined. Although there was no significant difference between the child maltreatment and comparison conditions as to the time of day when the first saliva sample was obtained, the time of day when the first sample was collected was systematically related to the cortisol concentrations obtained in the first sample, r = −.22, p = .024. This result indicated that samples collected later in the day had cortisol estimates that were lower than samples collected earlier in the day. Because age, steroid use, and cigarette use were either marginally or significantly different between groups at Time 2, and because the time of day in which the first cortisol sample was collected was related to observed cortisol concentrations, each of these variables were used as covariates in subsequent statistical analysis. A multivariate analysis of covariance assessing group differences on each proposed mediator after controlling for each of the aforementioned covariates, the dummy-coded indicator variable controlling contamination, and baseline MDD symptoms, is presented in Table 2. A partial correlation matrix of child maltreatment, proposed mediators, and subsequent MDD symptoms after accounting for all identified covariates is presented in Table 3.
Table 2.
Multivariate Analysis of Covariance Testing for Group Differences on Mediational Pathways
| Variable | Child Maltreatment | Comparison | F | df | ηp2 |
|---|---|---|---|---|---|
| M (SD) | M (SD) | ||||
| CortAUCg | 6.52 (5.00) | 7.72 (5.19) | 3.15† | 1, 97 | .034 |
| α-amylaseAUCg | 4891.38 (3405.21) | 5562.31 (4634.77) | 1.01 | 1, 97 | .011 |
| PANASAUCg | 649.77 (215.26) | 575.50 (124.04) | 0.01 | 1, 97 | .000 |
| DERS | 91.44 (23.49) | 77.61 (22.09) | 5.00* | 1, 97 | .053 |
Note. AUCg = Area under the curve with respect to ground; PANAS = Positive and Negative Affect Schedule; DERS = Difficulties in Emotion Regulation Scale. Covariates in model are: age, steroid use, cigarette use, time of day first saliva sample was collected, contamination, and baseline depressive symptoms. All variables in model were assessed at Time 2.
= p < .05;
= p < .08.
Table 3.
Partial Correlation Matrix for Variables Used in Multiple Mediator Analysis
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Maltreatment | - | |||||
| 2. CortisolAUCg | −0.19† | - | ||||
| 3. α-amylaseAUCg | 0.09 | 0.01 | - | |||
| 4. PANASAUCg | −0.02 | 0.13 | 0.17 | - | ||
| 5. DERS | 0.24* | 0.13 | 0.09 | 0.32** | - | |
| 6. BDI-II | 0.16 | 0.08 | −0.06 | 0.15 | 0.29** | - |
Note. Age, steroid use, cigarette use, time of day first saliva sample was collected, contamination, and baseline depressive symptoms measured at Time 2 were controlled in matrix. Maltreatment coded as 0 = Comparison, 1 = Maltreatment. AUCg = Area under the curve with respect to ground; PANAS = Positive and Negative Affect Schedule; DERS = Difficulties in Emotion Regulation Scale; BDI-II = Beck Depression Inventory-II measured at Time 3.
p < .01,
p < .05,
p < .08.
Manipulation Check
Paired sample t-tests were performed to examine whether significant change occurred between resting estimates of cortisol, salivary α-amylase, and negative affect and the maximum subsequent estimate obtained following the stressor task. Results indicated that the t-test for cortisol did not achieve statistical significance, t(101) = 0.69, p = 0.495, suggesting that the stressor did not activate the neuroendocrine system. However, previous research indicates that children who have been maltreated can display a hypocortisol profile in response to challenge and in contrast to controls (e.g., Hart, Gunnar, & Cicchetti, 1995), thereby potentially obscuring main effect changes in response to a stressor task. The relationship between child maltreatment status and resting and maximum cortisol estimates was therefore examined to determine whether this phenomenon occurred in the present study. Correlations between child maltreatment status and cortisol estimates obtained during rest, r = −0.19, p = .062, and the maximum subsequent estimate obtained in response to the stressor, r = −0.23, p = .024, revealed that children who were maltreated did in fact have a response to the stressor task but in a manner consistent with the hypocortisol response observed in prior research. The paired sample t-tests for salivary α-amylase, t(102) = 6.90, p < .001, and PANAS, t(103) = 2.70, p = 0.008, did achieve statistical significance, demonstrating that the combined stressor task elicited changes in the expected directions for these candidate risk pathways.
Multiple Mediator Model of Subsequent Depressive Symptoms
Covariates in the multiple mediator model were age, steroid use, cigarette use, the time of day the first saliva sample was collected, contamination, and Time 2 BDI-II scores. The first hypothesis tested whether the set of potential mediators mediated the relationship between child maltreatment and subsequent MDD symptoms. As shown in Table 4, the estimate of the total indirect effect was not significantly different from zero, Point Estimate = 0.78, BC 95% CI = −1.59 - 3.72, indicating that the set of proposed variables did not mediate the relationship between child maltreatment and subsequent MDD symptoms. However, the significance of the total indirect effect can be influenced by variation in the directionality of each specific indirect effect, whereas non-significant total indirect effects are more likely to occur when specific indirect effects confer risk for subsequent MDD symptoms in different ways (Preacher & Hayes, 2008). Thus, inspection of the significance of each specific indirect effect is needed to determine whether an individual mediator accounts for the relationship between child maltreatment and MDD symptoms.
Table 4.
Results of Multiple Mediator Analysis
| Point Estimate |
BC 95% CI |
|||
|---|---|---|---|---|
| Lower | Upper | |||
| Indirect Effects | ||||
| Total | 0.78 | −1.59 | 3.72 | |
| CortisolAUCg | −0.28 | −2.10 | 0.73 | |
| α-amylaseAUCg | −0.23 | −2.09 | 0.29 | |
| PANASAUCg | −0.05 | −1.16 | 0.40 | |
| DERS | 1.35* | 0.09 | 4.70 | |
| Pairwise Contrasts | ||||
| CortisolAUCg vs. α-amylaseAUCg | −0.05 | −1.73 | 1.73 | |
| CortisolAUCg vs. PANASAUCg | −0.23 | −2.10 | 1.00 | |
| CortisolAUCg vs. DERS | −1.63 | −4.64 | 0.16 | |
| α-amylaseAUCg vs. PANASAUCg | −0.18 | −2.00 | 0.76 | |
| α-amylaseAUCg vs. DERS | −1.58 | −5.26 | 0.01 | |
| PANASAUCg vs. DERS | −1.40* | −4.54 | −0.05 | |
Note.
p < .05.
BC = Bias-corrected; AUCg = Area under the curve with respect to ground; PANAS = Positive and Negative Affect Schedule; DERS = Difficulties in Emotion Regulation Scale.
As such, the multiple mediator model also tested whether CortAUCg, α-amylaseAUCg, PANASAUCg, and DERS scores each constituted a specific indirect effect of the relationship between child maltreatment and subsequent MDD symptoms. Results demonstrated that the confidence interval around the point estimate for the specific indirect effect of the DERS did not include zero, Point Estimate = 1.35, BC 95% CI = 0.09 - 4.70, indicating that the DERS was a significant indirect effect of the relationship between child maltreatment and subsequent MDD symptoms when simultaneously estimating the contributions of CortAUCg, α-amylaseAUCg, and PANASAUCg. Inspection of the unstandardized beta coefficients indicates that child maltreatment significantly predicted higher DERS scores at the Time 2 assessment, b = 13.32, p = .029, with higher DERS scores significantly predicting increased BDI-II scores at the Time 3 assessment, b = .10, p = .046. The specific indirect effects for CortAUCg, α-amylaseAUCg, and PANASAUCg were not significantly different from zero.
Pairwise contrasts comparing the strength of each specific indirect effect relative to all other indirect effects in the model were estimated for each proposed mediator. Results demonstrated that the BC 95% CI around the contrast comparing the indirect effects for the DERS and PANASAUCg was statistically significant, Point Estimate = 1.40, BC 95% CI = 0.05 - 4.54, indicating that the indirect effect for the DERS was significant stronger when compared to the indirect effect of PANASAUCg. There were no other pairwise contrasts that achieved statistical significance.
Clinical Levels of Major Depressive Disorder Symptoms
Average BDI-II scores measured at Time 3 were 11.84 (SD = 10.85) for the child maltreatment condition and 7.77 (SD = 8.12) for the comparison condition. A score of 21 or higher on the BDI-II was used identify clinical levels of MDD symptoms at Time 3, consistent with prior research demonstrating that a BDI-II total score of 21 or higher provided maximal clinical efficiency in distinguishing adolescents with and without MDD (Kumar, Steer, Teitelman, & Villacis, 2002). Using this cut-off, 20.0% of those in the child maltreatment condition experienced clinical levels of MDD symptoms at the final study assessment compared to 7.6% of those in the comparison condition. These rates are comparable to previous studies examining MDD in the child maltreatment population that range from 14.2%-15.1% for past year prevalence of MDD in a maltreated group and 7.8%-10.6% in a comparison condition (Scott et al., 2010; Widom et al., 2007), especially when considering the higher rates of sexual and physical abuse in the current study’s child maltreatment condition. However, its important to note that the 7.6% rate of clinical levels of MDD symptoms in the comparison condition reduces to 0.0% when contamination is controlled by removing cases of child maltreatment from the comparison condition.
Discussion
The need for multiple-levels-of-analysis research on the etiology of psychiatric disorders in child maltreatment population coincides with recent changes at the National Institute of Mental Health (NIMH) where funding priorities are being given to basic science research on genomics, neuroscience, pathophysiology, and behavioral processes in an effort to identify the putative mechanisms and pathways involved in the onset of psychiatric outcomes (Cuthbert & Insel, 2010; Insel et al., 2010). This effort takes an agnostic, bottom-up approach to developing a framework of psychiatric disorders that is focused on the identification of transdiagnostic risk pathways that lead to multiple psychiatric conditions, unlike existing taxonomies, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), that take a top-down approach by first arbitrarily defining individual disorders based on symptom presentation and then attempting to identify etiological pathways unique to each disorder. A particular advantage of any bottom-up approach is that the identification of risk pathways affecting one or more psychiatric outcomes serves an important translational need. Specifically, risk pathways affecting the onset of one or more psychiatric disorders then become the focus of interventions aimed at preventing or treating those outcomes in the most effective and efficient manner possible. Not only does this approach connect basic science research to clinical intervention but also avoids the inherent paradox in existing taxonomies of needing to develop a unique intervention for each distinct psychiatric disorder when in practice one intervention can be used to treat multiple disorders, such as exposure and response prevention (Foa & Rothbaum, 1998; Franklin & Foa, 2002). Thus, focusing on identifying the common, transdiagnostic risk pathways of psychiatric outcomes in basic research with the child maltreatment population holds considerable promise in more effectively and efficiently optimizing interventions through the direct targeting of etiological pathways.
The current study adopted a multiple-levels-of-analysis approach across neuroendocrine, autonomic, affective, and emotion regulation pathways to identify transdiagnostic mechanisms leading to MDD symptoms in the child maltreatment population. Selecting several candidate pathways provides a strong theoretical and empirical test that increases the likelihood of identifying one or more pathways after accounting for the indirect effects of other, related pathways. Results of this study add to a robust literature on the role emotion dysregulation plays in the onset of MDD (Aldao et al., 2010; Silk et al., 2003) with particular relevance to the child maltreatment population (Coates & Messman-Moore, 2014). After accounting for the indirect effects of neuroendocrine, autonomic, and affective pathways, child maltreatment predicted higher subsequent rates of emotion dysregulation. Examination of emotion dysregulation scores across the child maltreatment and comparison conditions in this study indicated that the comparison condition had scores that were comparable to those obtained in normative, female adolescent samples (Weinberg & Klonsky, 2009), whereas the child maltreatment condition had significantly higher emotion dysregulation scores. These higher scores of emotion dysregulation predicted a greater severity of MDD symptoms approximately eighteen months later or over two years after the child maltreatment allegation was substantiated, on average. Thus, emotion dysregulation appears to play a critical role in the onset of MDD symptoms for adolescent females who experienced child maltreatment and are entering young adulthood. It is important to note that this indirect effect for emotion dysregulation was detected after adjusting for relevant covariates, MDD symptom severity at study entry and while accounting for each of the other indirect effects included in the model. This suggests that the key risk pathway to MDD for maltreated females lies in their ability to engage in behaviors that modulate, change, or accept aversive affective experiences, and not in the neuroendocrine, autonomic, or affective changes following an environmental challenge. In other words, changes in stress physiology or even increases in negative affect per se play a less critical role in the expression of subsequent MDD symptoms in the child maltreatment population than behavioral pathways responsible for the overall management of emotional experience. This has important implications for bottom-up approaches of classifying psychiatric disorders, where including and estimating key behavioral processes in etiological models are essential for identifying putative risk pathways.
Contrary to expectations, neuroendocrine, autonomic, and affective pathways did not exert significant indirect effects in this study. One explanation for this may come from longitudinal research showing that women who were sexually abused have higher resting cortisol concentrations during childhood, comparable estimates to non-sexually abused peers during adolescence, and significantly lower concentrations in adulthood (Trickett et al., 2010). Thus, an adjustment of neuroendocrine functioning across development may be occurring in samples experiencing child maltreatment with profiles assessed in younger and older developmental stages tied more strongly to MDD symptoms. Like cortisol, there is variation in findings reported across studies examining the relationship between salivary α-amylase and MDD using child and adolescent samples experiencing early life trauma, including child maltreatment (Gordis et al., 2008; Rudolph et al., 2011). Thus, there may be a similar adjustment in autonomic processes during adolescence that may make detection of a relationship between child maltreatment and MDD symptoms difficult. It is also possible that salivary α-amylase is not a useful biomarker for distinguishing chronic stress groups or increasing one’s risk for MDD symptoms in the child maltreatment population (Juster, Sindi, et al., 2011). Another explanation may be that the stressor used in this study had mixed effects in achieving levels of reactivity, particularly in activating the neuroendocrine response. Main effect tests indicated that salivary α-amylase and negative affect did change significantly from resting to stressor conditions while cortisol did not. However, closer inspection revealed that the maltreated group displayed a hypocortisol profile during the stressor condition when compared to the control group, consistent with previous research (Hart et al., 1995) and therefore providing an adequate test of the neuroendocrine pathway to subsequent MDD symptoms.
Several limitations must also be considered when interpreting the results of this study. First, mediational modeling was used to examine the contributions of several variables in a single statistical model to identify risk pathways to MDD symptoms following child maltreatment. While these variables have both theoretical and empirical relevance for child maltreatment and MDD symptom development, additional pathways likely exist yet were not tested in this study. Alternative models testing emotion dysregulation with other potential mediators, such as quality of parenting (Collishaw et al., 2007), hold considerable promise in future research. Second, the effects of child maltreatment in this sample are limited to those with a substantiated case of maltreatment. Thus, results cannot necessarily generalize to other methods of assessing child maltreatment. Third, the sample for this study consists entirely of females with the maltreated group experiencing higher rates of sexual abuse than the general maltreatment population. This study targeted females specifically to identify pathways of MDD symptom development in the subpopulation of child maltreatment at greatest risk for MDD. It is important to emphasize that the findings of this study therefore only generalize to the adolescent female subpopulation and the extent to which the current results generalize to males who have been maltreated can only be determined by future research. Finally, time of waking and timing within the menstrual cycle was not assessed yet can influence hormone concentrations, particularly cortisol.
Future Directions
Results from this study contribute to the growing effort to identify the relevant etiological pathways for those at greatest risk of psychiatric disorders in the child maltreatment population. In addition to emotion dysregulation, there are likely other person-specific factors that moderate or mediate the effects of child maltreatment on the risk for MDD and related disorders. For example, recent findings from the Psychiatric Genetics Consortium appear to support a bottom-up approach to the classification of psychiatric disorders and describe shared genetic etiology across a variety of psychiatric disorders (Cross-disorder Group of the Psychiatric Genomics Consortium, 2013). Prior research has shown specific genetic polymorphisms moderate the effects of child maltreatment on later mental health (Kim-Cohen et al., 2006). MDD may also be a complex trait where specific polymorphisms across multiple genes, namely 5-HTTLPR, a serotonin transporter, and CRHR1, a corticotropin releasing hormone gene, determine rates of internalizing symptomatology subsequent to child maltreatment (Cicchetti, Rogosch, & Oshri, 2011). The molecular basis of such gene by environment effects has been well characterized for the glucocorticoid co-chaperone FKBP5 (Klengel et al., 2013). A history of child maltreatment predicts FKBP5 DNA methylation in an allele-specific manner, which in turn predicts symptoms of glucocorticoid insensitivity and a range of clinical phenotypes, suggesting that epigenetic processes may serve as candidate risk pathways in future mediational analyses. Likewise, marked differences in genome-wide DNA methylation and gene expression have been described between diagnostically similar groups of PTSD patients with and without a history of child maltreatment (Mehta et al., 2013). Such results suggest that existing diagnostic classifications alone may not be informative for understanding pertinent biological processes of relevance for psychopathology. The challenge then becomes to determine how other sources of data, both molecular and behavioral, can be integrated into prediction models to identify those at greatest risk and in need of direct clinical intervention.
Clinical and Translational Implications
There are clear implications for the development and implementation of evidence-based behavioral interventions for maltreated females resulting from this study. Universal prevention of child maltreatment remains a topic public health priority (World Health Organization, 2014). However, child maltreatment continues to affect 1.2 million children each year in the United States alone (Sedlak et al., 2010). Thus, selective prevention programs (Institute of Medicine, 1994), especially those aiming to reduce the incidence of psychiatric disorders, represent the earliest form of intervention available for this population. This study indicates that such selective prevention programs are likely to have optimal therapeutic effect when a specific emphasis is made to improve and utilize emotion regulation abilities following the investigation and substantiation of child maltreatment. Commonly used treatment components, such as psychoeducation and relaxation, are likely to provide a benefit for the acute physiological reactions following such a trauma. However, prevention of MDD and related psychiatric disorders will occur most effectively by targeting the emotion dysregulation pathway explicitly, either through individual skill building or by including caregivers or both. Evidence-based, selective prevention programs for children exposed to trauma exist (Berkowitz, Stover, & Marans, 2011), however the mechanisms of action for these programs, and whether they target emotion dysregulation in particular, remain unknown. Research supporting the modification of existing or the development of new selective prevention programs with a specific focus on emotion dysregulation is likely to provide optimal benefit to children experiencing maltreatment through the targeting of this risk pathway; a mission consistent with treatment optimization priorities at NIMH (National Advisory Mental Health Council's Workgroup, 2010). Similarly, treating MDD in the child maltreatment population is likely to have the greatest effect when emotion dysregulation is minimized though improved changes in regulatory functioning that can be achieved and maintained in an interpersonal or familial context. There are several well-established, transdiagnostic behavioral interventions that target emotion dysregulation in adult populations (Barlow, Allen, & Choate, 2004; Linehan, 1993) and adaptations of these interventions for those with a trauma or child maltreatment history (Bohus et al., 2013; Geddes, Dziurawiec, & Lee, 2013), and in significantly reducing depressive symptoms among adolescents (Bilek & Ehrenreich-May, 2012; Mehlum et al., 2014), have a growing empirical base. Thus, continued adaptation of these evidence-based approaches focusing on emotion dysregulation as a core, transdiagnostic pathway to MDD and comorbid conditions has the potential to optimize the efficacy of behavioral interventions for females experiencing child maltreatment.
Acknowledgments
This manuscript was supported by a grant awarded to Dr. Shenk (KL2TR000078-05) and a fellowship awarded to Ms. Griffin (T32DA017629). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Allwood MA, Handwerger K, Kivlighan KT, Granger DA, Stroud LR. Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biological Psychology. 2011;88:57–64. doi: 10.1016/j.biopsycho.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statisical Manual of Mental Disorders. 5th. Washington, DC: American Psychiatric Press; 2013. [Google Scholar]
- Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behavior Therapy. 2004;35:205–230. doi: 10.1016/j.beth.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Berkowitz SJ, Stover CS, Marans SR. The Child and Family Traumatic Stress Intervention: Secondary prevention for youth at risk of developing PTSD. Journal of Child Psychology & Psychiatry. 2011;52:676–685. doi: 10.1111/j.1469-7610.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilek EL, Ehrenreich-May J. An open trial investigation of a transdiagnostic group treatment for children with anxiety and depressive symptoms. Behavior Therapy. 2012;43:887–897. doi: 10.1016/j.beth.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Bohus M, Dyer AS, Priebe K, Kruger A, Kleindienst N, Schmahl C, Niedtfeld I, Steil R. Dialectical Behaviour Therapy for post-traumatic stress disorder after childhood sexual abuse in patients with and without borderline personality disorder: A randomised controlled trial. Psychotherapy and Psychosomatics. 2013;82:221–233. doi: 10.1159/000348451. [DOI] [PubMed] [Google Scholar]
- Bradley B, DeFife JA, Guarnaccia C, Phifer J, Fani N, Ressler KJ, Westen D. Emotion dysregulation and negative affect: Association with psychiatric symptoms. Journal of Clinical Psychiatry. 2011;72:685–691. doi: 10.4088/JCP.10m06409blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT. Social neuroscience: Autonomic, neuroendocrine, and immune responses to stress. Psychophysiology. 1994;31:113–128. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis approach to the study of developmental processes in maltreated children. Proceedings of the National Academy of Sciences (USA) 2004;101:17325–17326. doi: 10.1073/pnas.0408033101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Dawson G. Multiple levels of analysis. Development and Psychopathology. 2002;14:417–420. doi: 10.1017/s0954579402003012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010;81:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Oshri A. Interactive effects of corticotropin releasing hormone receptor 1, serotonin transporter linked polymorphic region, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Developmenent and Psychopathology. 2011;23:1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates AA, Messman-Moore TL. A structural model of mechanisms predicting depressive symptoms in women following childhood psychological maltreatment. Child Abuse & Neglect. 2014;38:103–113. doi: 10.1016/j.chiabu.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B. Resilience to adult psychopathology following childhood maltreatment: Evidence from a community sample. Child Abuse & Neglect. 2007;31:211–229. doi: 10.1016/j.chiabu.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Cross-disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B, Insel T. Classification issues in women's mental health: Clinical utility and etiological mechanisms. Archives of Womens Mental Health. 2010;13:57–59. doi: 10.1007/s00737-009-0132-z. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Ehlert U, Nater UM. Associations between salivary alpha-amylase and catecholamines--a multilevel modeling approach. Biological Psychology. 2014;103:15–18. doi: 10.1016/j.biopsycho.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Fang X, Brown DS, Florence CS, Mercy JA. The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse & Neglect. 2012 doi: 10.1016/j.chiabu.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Exposure to childhood sexual and physical abuse and adjustment in early adulthood. Child Abuse & Neglect. 2008;32:607–619. doi: 10.1016/j.chiabu.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Flaherty EG, Thompson R, Dubowitz H, Harvey EM, English DJ, Proctor LJ, Runyan DK. Adverse childhood experiences and child health in early adolescence. JAMA Pediatrics. 2013;167:622–629. doi: 10.1001/jamapediatrics.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E, Rothbaum B. Treating the trauma of rape: Cognitive-behavioral therapy for PTSD. New York, NY: Guilford Press; 1998. [Google Scholar]
- Franklin ME, Foa E. Cognitive behavioral treatments for obsessive complusive disorder. In: Nathan PE, Gorman JM, editors. A Guide to Treatments that Work. New York: Oxford University Press; 2002. pp. 367–386. [Google Scholar]
- Geddes K, Dziurawiec S, Lee CW. Dialectical Behaviour Therapy for the treatment of emotion dysregulation and trauma symptoms in self-injurious and suicidal adolescent females: A pilot programme within a community-based child and adolescent mental health service. Psychiatry Journal. 2013;2013:145219. doi: 10.1155/2013/145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosomatic Medicine. 2005;67(Suppl 1):S26-288. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones and Behavior. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, el-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: Recent developments and applications. Annals of the New York Academy of Sciences. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology & Behavioral Assessment. 2004;26:41–54. [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2:271–299. [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Development and Psychopathology. 1995;7:11–26. [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press; 2013. [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Mrazek PJ, Haggerty RJ, editors. Institute of Medicine. Reducing Risks for Mental Disorders: Frontiers for Preventative Intervention Research. Washington, DC: National Academy Press; 1994. [PubMed] [Google Scholar]
- Juster RP, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trepanier L, Marin MF, Wan N, Sekerovic Z, Lord C, Fiocco AJ, Plusquellec P, McEwen BS, Lupien SJ. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Development and Psychopathology. 2011;23:725–776. doi: 10.1017/S0954579411000289. [DOI] [PubMed] [Google Scholar]
- Juster RP, Sindi S, Marin MF, Perna A, Hashemi A, Pruessner JC, Lupien SJ. A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology. 2011;36:797–805. doi: 10.1016/j.psyneuen.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, Schwarz HP, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: Relation with age, pubertal stage, and weight. Pediatric Research. 1995;37:502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Kim J, Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology and Psychiatry. 2010;51:706–716. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: New evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM. The future of emotion research in the study of psychopathology. Emotion Review. 2010;2:225–228. [Google Scholar]
- Kring AM, Werner KH, editors. Emotion Regulation and Psychopathology. Mahwah, NJ,US: Lawrence Erlbaum Associates Publishers; 2004. [Google Scholar]
- Kumar G, Steer RA, Teitelman KB, Villacis L. Effectiveness of Beck Depression Inventory-II subscales in screening for major depressive disorders in adolescent psychiatric inpatients. Assessment. 2002;9:164–170. doi: 10.1177/10791102009002007. [DOI] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press; 1993. [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, Duku EK, Walsh CA, Wong MY, Beardslee WR. Childhood abuse and lifetime psychopathology in a community sample. American Journal of Psychiatry. 2001;158:1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Meulen J, Boyle MH, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the youth mood project. Biological Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Mehlum L, Tormoen AJ, Ramberg M, Haga E, Diep LM, Laberg S, Larsson BS, Stanley BH, Miller AL, Sund AM, Groholt B. Dialectical behavior therapy for adolescents with repeated suicidal and self-harming behavior: A randomized trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:1082–1091. doi: 10.1016/j.jaac.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, Rex-Haffner M, Loeschner A, Gonik M, Mercer KB, Bradley B, Muller-Myhsok B, Ressler KJ, Binder EB. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proceedings of the National Academy of Sciences USA. 2013;110:8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- National Advisory Mental Health Council's Workgroup. From Discovery to Cure: Accelerating the Development of New and Personalized Interventions for Mental Illnesses. Washington, DC: Department of Health & Human Services; 2010. [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspectives on Psychological Science. 2011;6:589–609. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- Porges SW, Cohn JF, Bal E, Lamb D. The Dynamic Affect Recognition Evaluation (DARE) software. Chicago, IL: University of Illinois-Chicago; 2007. [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Putnam KT, Harris WW, Putnam FW. Synergistic childhood adversities and complex adult psychopathology. Journal of Traumatic Stress. 2013;26:435–442. doi: 10.1002/jts.21833. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Individual differences in biological stress responses moderate the contribution of early peer victimization to subsequent depressive symptoms. Psychopharmacology. 2011;214:209–219. doi: 10.1007/s00213-010-1879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher S, Kirschbaum C, Fydrich T, Strohle A. Is salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders?--A review of preliminary findings and the interactions with cortisol. Psychoneuroendocrinology. 2013;38:729–743. doi: 10.1016/j.psyneuen.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Scott KM, Smith DR, Ellis PM. Prospectively ascertained child maltreatment and its association with DSM-IV mental disorders in young adults. Archives of General Psychiatry. 2010;67:712–719. doi: 10.1001/archgenpsychiatry.2010.71. [DOI] [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A. Fourth National Incidence Study of Child Abuse and Neglect (NIS-4): Report to Congress. Washington, DC: US Dept. of Health and Human Services, Administration for Children and Families, Administration on Children, Youth and Families, National Center on Child Abuse and Neglect; 2010. [Google Scholar]
- Shenk CE, Noll JG, Peugh JL, Griffin AG, Bensman HE. Contamination in the prospective study of child maltreatment and female adolescent health. Journal of Pediatric Psychology. 2015 doi: 10.1093/jpepsy/jsv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Parental maltreatment and emotion dysregulation as risk factors for bullying and victimization in middle childhood. Journal of Clinical Child Psychology. 2001;30:349–363. doi: 10.1207/S15374424JCCP3003_7. [DOI] [PubMed] [Google Scholar]
- Shipman K, Zeman J, Penza S, Champion K. Emotion management skills in sexually maltreated and nonmaltreated girls: A developmental psychopathology perspective. Development & Psychopathology. 2000;12:47–62. doi: 10.1017/s0954579400001036. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents' emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Southam-Gerow MA, Kendall PC. Emotion regulation and understanding: Implications for child psychopathology and therapy. Clinical Psychology Review. 2002;22:189–222. doi: 10.1016/s0272-7358(01)00087-3. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Ishitobi Y, Maruyama Y, Kawano A, Ando T, Okamoto S, Kanehisa M, Higuma H, Ninomiya T, Tsuru J, Hanada H, Kodama K, Isogawa K, Akiyoshi J. Salivary alpha-amylase and cortisol responsiveness following electrical stimulation stress in major depressive disorder patients. Progress in Neuropsychopharmacology & Biological Psychiatry. 2012;36:220–224. doi: 10.1016/j.pnpbp.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Thornberry TP, Ireland TO, Smith CA. The importance of timing: The varying impact of childhood and adolescent maltreatment on multiple problem outcomes. Development and Psychopathology. 2001;13:957–979. [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.SDepartment of Health and Human Services, Administration for Children and Families, Administration on Children, Youth, and Families, Children's Bureau. Child Maltreatment, 2011. 2012 Retrieved from http://www.acf.hhs.gov/programs/cb/research-data-technology/statistics-research/child-maltreatment.
- van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of beta blockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality & Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Muhtadie L, Thompson RJ, Joormann J, Gotlib IH. Affective and physiological responses to stress in girls at elevated risk for depression. Development and Psychopathology. 2012;24:661–675. doi: 10.1017/S0954579412000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Klonsky ED. Measurement of emotion dysregulation in adolescents. Psychological Assessment. 2009;21:616–621. doi: 10.1037/a0016669. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Archives of General Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Investing in Children: The European Child and Adolescent Health Strategy 2015–2020 and the European Child Maltreatment Prevntion Action Plan 2015–2020; Paper presented at the Regional Committee for Europe, 64th Session; Copenhagen, Denmark. 2014. Sep 15–18, 2014. [Google Scholar]
- Yanos PT, Czaja SJ, Widom CS. A prospective examination of service use by abused and neglected children followed up into adulthood. Psychiatric Services. 2010;61:796–802. doi: 10.1176/ps.2010.61.8.796. [DOI] [PubMed] [Google Scholar]