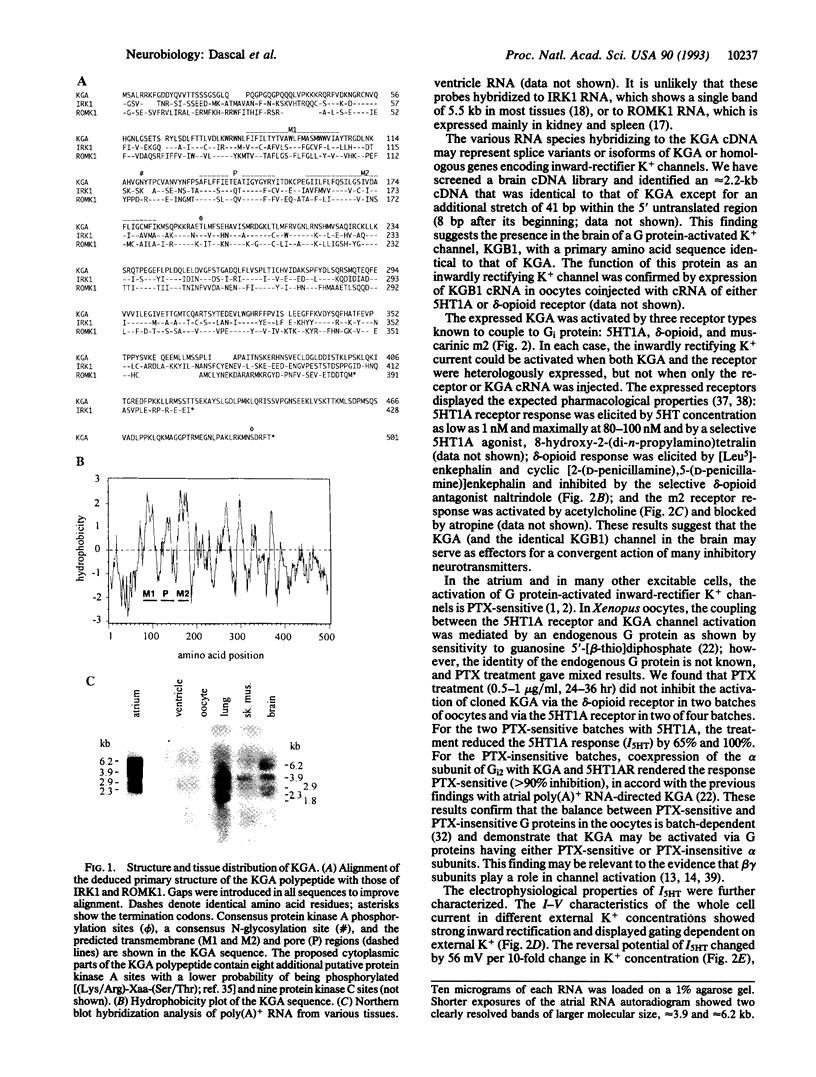
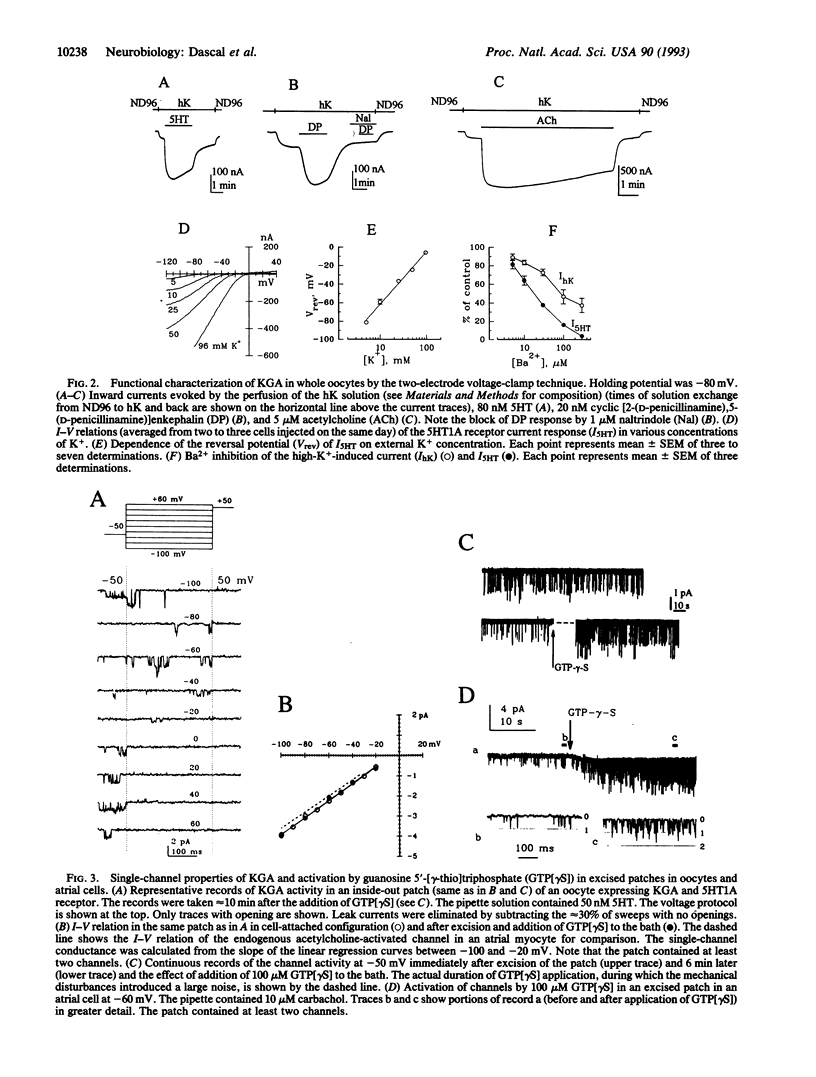
Abstract
Activity of several ion channels is controlled by heterotrimeric GTP-binding proteins (G proteins) via a membrane-delimited pathway that does not involve cytoplasmic intermediates. The best studied example is the K+ channel activated by muscarinic agonists in the atrium, which plays a crucial role in regulating the heartbeat. To enable studies of the molecular mechanisms of activation, this channel, denoted KGA, was cloned from a rat atrium cDNA library by functional coupling to coexpressed serotonin type 1A receptors in Xenopus oocytes. KGA displays regions of sequence homology to other inwardly rectifying channels as well as unique regions that may govern G-protein interaction. The expressed KGA channel is activated by serotonin 1A, muscarinic m2, and delta-opioid receptors via G proteins. KGA is activated by guanosine 5'-[gamma-thio]triphosphate in excised patches, confirming activation by a membrane-delimited pathway, and displays a conductance equal to that of the endogenous channel in atrial cells. The hypothesis that similar channels play a role in neuronal inhibition is supported by the cloning of a nearly identical channel (KGB1) from a rat brain cDNA library.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechem M., Pott L. Removal of Ca current inactivation in dialysed guinea-pig atrial cardioballs by Ca chelators. Pflugers Arch. 1985 May;404(1):10–20. doi: 10.1007/BF00581485. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Ionic channels and their regulation by G protein subunits. Annu Rev Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Dascal N., Ifune C., Hopkins R., Snutch T. P., Lübbert H., Davidson N., Simon M. I., Lester H. A. Involvement of a GTP-binding protein in mediation of serotonin and acetylcholine responses in Xenopus oocytes injected with rat brain messenger RNA. Brain Res. 1986 Dec;387(3):201–209. doi: 10.1016/0169-328x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Dascal N., Lim N. F., Schreibmayer W., Wang W., Davidson N., Lester H. A. Expression of an atrial G-protein-activated potassium channel in Xenopus oocytes. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6596–6600. doi: 10.1073/pnas.90.14.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., KUFFLER S. W., TRAUTWEIN W. Changes in membrane characteristics of heart muscle during inhibition. J Gen Physiol. 1956 Sep 20;40(1):135–145. doi: 10.1085/jgp.40.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy H. R., Conti F. Pursuing the structure and function of voltage-gated channels. Trends Neurosci. 1990 Jun;13(6):201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H210–H214. doi: 10.1152/ajpheart.1987.253.1.H210. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Schoeffter P. 5-HT receptors: subtypes and second messengers. J Recept Res. 1991;11(1-4):197–214. doi: 10.3109/10799899109066399. [DOI] [PubMed] [Google Scholar]
- Ito H., Tung R. T., Sugimoto T., Kobayashi I., Takahashi K., Katada T., Ui M., Kurachi Y. On the mechanism of G protein beta gamma subunit activation of the muscarinic K+ channel in guinea pig atrial cell membrane. Comparison with the ATP-sensitive K+ channel. J Gen Physiol. 1992 Jun;99(6):961–983. doi: 10.1085/jgp.99.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibara M., Nakajima T., Irisawa H., Giles W. Regulation of spontaneous opening of muscarinic K+ channels in rabbit atrium. J Physiol. 1991 Feb;433:589–613. doi: 10.1113/jphysiol.1991.sp018445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin A., Ho B. Y., Labarca C., Elroy-Stein O., Moss B., Davidson N., Lester H. A. Heterologously expressed serotonin 1A receptors couple to muscarinic K+ channels in heart. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5694–5698. doi: 10.1073/pnas.88.13.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kieffer B. L., Befort K., Gaveriaux-Ruff C., Hirth C. G. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. Modulation of acetylcholine-activated K+ channel function in rat atrial cells by phosphorylation. J Physiol. 1991 Jun;437:133–155. doi: 10.1113/jphysiol.1991.sp018588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Yatani A., Codina J., Birnbaumer L., Brown A. M. Alpha-subunit of Gk activates atrial K+ channels of chick, rat, and guinea pig. Am J Physiol. 1988 Jun;254(6 Pt 2):H1200–H1205. doi: 10.1152/ajpheart.1988.254.6.H1200. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Frielle T., Collins S., Yang-Feng T., Kobilka T. S., Francke U., Lefkowitz R. J., Caron M. G. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature. 1987 Sep 3;329(6134):75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T. Positive cooperativity in activation of the cardiac muscarinic K+ channel by intracellular GTP. Pflugers Arch. 1990 Apr;416(1-2):216–218. doi: 10.1007/BF00370247. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Tung R. T., Ito H., Nakajima T. G protein activation of cardiac muscarinic K+ channels. Prog Neurobiol. 1992 Sep;39(3):229–246. doi: 10.1016/0301-0082(92)90017-9. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Mager S., Naeve J., Quick M., Labarca C., Davidson N., Lester H. A. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993 Feb;10(2):177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- North R. A. Twelfth Gaddum memorial lecture. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989 Sep;98(1):13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K., Yatani A., Brown A. M. The nature and origin of spontaneous noise in G protein-gated ion channels. J Gen Physiol. 1991 Jun;97(6):1279–1293. doi: 10.1085/jgp.97.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S., Sultana M., Takemori A. E. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988 Jan 27;146(1):185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Yatani A., Mattera R., Codina J., Graf R., Okabe K., Padrell E., Iyengar R., Brown A. M., Birnbaumer L. The G protein-gated atrial K+ channel is stimulated by three distinct Gi alpha-subunits. Nature. 1988 Dec 15;336(6200):680–682. doi: 10.1038/336680a0. [DOI] [PubMed] [Google Scholar]




