Abstract
Background
Bronchopleural fistulas (BPF) are conditions associated with prolonged hospital course, high morbidity, and possibly increased mortality. The presence of BPFs in critically ill patients may cause difficulty in ventilation and increased oxygen requirements. Intrabronchial valves (Spiration IBV) serve as a noninvasive therapeutic option for the closure of BPFs.
Methods
This report is a retrospective description of 3 patients transferred to our medical intensive care unit (ICU) with BPFs and persistent air leaks (PAL). One patient required high levels of oxygen supplementation through a nonrebreather face mask, whereas 2 required mechanical ventilation because of respiratory failure. IBVs were placed in each patient with the intention of closing their BPF and weaning them from respiratory support.
Results
The use of IBVs in ICU patients with BPFs and PALs resulted in 1 patient being weaned from the persistent need for a nonrebreather face mask to room air and also aided in the liberation from mechanical ventilation of 2 patients who had been failing spontaneous breathing trials.
Conclusions
The use of IBVs is safe and well tolerated in ICU patients with BPFs and PALs. The placement of IBVs results in significant clinical improvement, allowing for either weaning from high levels of oxygen support or liberation from mechanical ventilation.
Keywords: bronchoscopy, bronchopleural fistula, intrabronchial valve, persistent air leaks, respiratory failure
Bronchopleural fistulas (BPFs) are pathologic communications between the bronchial tree and the pleural space. These communications can result from pneumothoraces, pulmonary infections, trauma, tumors, and complications from thoracic surgery. BPFs are associated with high morbidity, increased mortality,1,2 and prolonged hospital course.3 This condition can be identified by direct bronchoscopic airway inspection by visualization of the communication or by the presence of a persistent air leak (PAL) characterized by bubbling in the chest tube drainage system water seal chamber. The flow of air through the communication into the pleural space delays healing of the fistulous site, inhibits lung expansion, and can hinder weaning from oxygen supplementation. Furthermore, when patients with BPFs are dependent on mechanical ventilation, positive pressure further delays fistula healing secondary to ease of flow through the low-resistance pathway into the pleural collecting system.
A myriad of algorithms exist for the treatment of PALs resulting from BPFs. Noninvasive therapeutic options include prolonged chest tube drainage with tailored ventilator strategies aimed at establishing acceptable ventilation and oxygenation while reducing fistula flow.4,5 When noninvasive approaches fail, clinicians can adopt more invasive strategies such as pleural abrasion, application of fibrin sealant,6 bronchial stump stapling, muscle flap construction,7 and, in extreme cases, surgical lobectomy.8
Critically ill patients suffering from BPFs and PALs often require prolonged intensive care stays to allow for fistula healing, with variable results.9 In addition, these patients may be considered high risk for surgical intervention such as lobectomy because of the high levels of oxygen support, mechanical ventilation, or other comorbidities. Intrabronchial valves (Spiration IBV) allow for a minimally invasive alternative to close BPFs. These 1-way valves are deployed in regional airways by a conventional flexible bronchoscope and result in the subsequent isolation of the associated lung segment. In our experience, the use of IBVs in critically ill patients requiring high levels of oxygen support or mechanical ventilation results in significant improvement or complete resolution of BPFs with reduction in respiratory support. This report demonstrates that the placement of IBVs in patients suffering from hypoxia or prolonged mechanical ventilation complicated by BPFs is a safe and effective therapy in intensive care unit (ICU) patients.
PATIENTS AND METHODS
This is a retrospective description of 3 patients transferred to our medical ICU between 2009 and 2010. All 3 patients presented initially from outside hospitals with respiratory distress. They were transferred to our institution because of the presence of BPFs and the inability to wean from either significant oxygen supplementation or mechanical ventilation.
IBVs are small umbrella-shaped valves that are placed by conventional flexible bronchoscopy. These valves are deployed into the airway and held in place by metal struts. After placement, air is unable to flow past the valve distally into the BPF, resulting in controlled collapse of the instrumented segment. Impaired flow through the BPF promotes healing of injured tissue and expedites resolution of the air leak. These valves are kept in place for at least 6 weeks and can be removed by bronchoscopy if desired.
In October 2008, the “IBV Valve System” (Spiration Inc., Redmond, WA) was approved by the FDA under the Humanitarian Device Exemption for use in controlling certain post-surgical PALs. The Spiration valve is indicated in PALs diagnosed in surgical patients (post-segmentectomy, lobectomy, or post-lung volume reduction surgery) for an air leak present through postoperative day 7 unless present only during forced exhalation or cough. An air leak present on day 5 should be considered for treatment if it is continuous, present during the normal inhalation phase of inspiration, or present upon normal expiration and accompanied by subcutaneous emphysema or respiratory compromise. Our cases represent off-label uses of the devices, but we believed the mechanisms of BPF closure with IBV would be the same for these cases as seen with air leaks after surgery.
Two of our patients were intubated before transfer to our institution, and 1 patient, who was unable to be weaned from nonrebreather face mask, was electively intubated for the procedure. After a review of available computer tomography (CT) scans of the chest, the bronchoscope was advanced to the affected lung segment. A balloon was inflated to occlude each airway in the lung segment with observation of the chest tube water seal chamber to look for closure of the BPF represented by cessation of bubbling. Segmental airways were occluded for at least 20 seconds before assessing for cessation of bubbling. When the segment associated with the air leak was identified, the airway was sized using the Airway Sizing Kit. The valve deployment catheter was passed through the working channel of the bronchoscope into the airway with subsequent deployment of the valve. This method was repeated in multiple airways if necessary to ensure closure of the BPF. Postprocedure chest x-rays were obtained 1 hour after the procedure to ensure the absence of valve migration.
CASE REPORTS
Case 1
A 49-year-old man presented to the emergency room complaining of acute-onset shortness of breath. A chest x-ray revealed the absence of lung markings in the left thorax with mild shift of the trachea toward the right. Concern for a tension pneumothorax prompted the physicians to place a chest tube into the left pleural space. Shortly after placement, the patient began to desaturate, develop facial swelling, and chest wall crepitus. The patient’s oxygen saturation was 93% on a 100% nonrebreather mask. A subsequent CT of the chest showed the chest tube terminating in the left lung apex within a large apical bulla. The radiographic appearance in conjunction with significant bubbling in the chest tube water seal chamber was consistent with a BPF (Fig. 1). The patient continued to require significant oxygen support on a nonrebreather mask with failure to wean over the 1 week. He was transferred to our hospital for persistent hypoxia secondary to his BPF.
FIGURE 1.
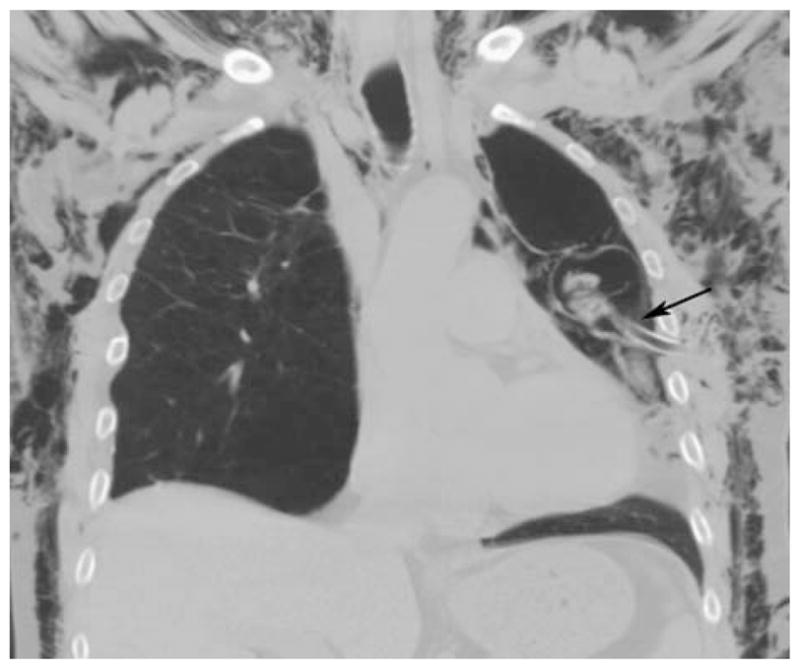
Bronchopleural fistula (arrow) before intrabronchial valve placement. Note: extensive subcutaneous emphysema.
The patient had a bronchoscopy performed for closure of his BPF. The patient was electively intubated for the procedure. After airway sizing, 3 intrabronchial valves (Spiration IBV valves) were deployed in the segmental airways of the left upper lobe (Figs. 2, 3). There was an immediate cessation of bubbling in the water seal chamber of the chest tube collecting system after placement of the valves. The patient was successfully extubated without complication. After the procedure, the patient was transitioned to nasal cannula and was subsequently stable on room air later that day. After 3 days, there were no signs of an air leak, and the chest tube was removed (Fig. 4). He was transferred out of the medical ICU and discharged home.
FIGURE 2.
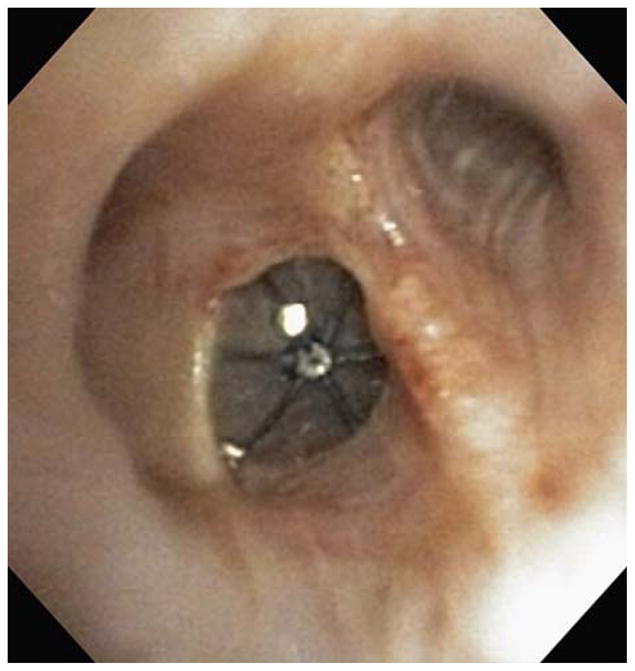
Bronchoscopic view of intrabronchial valves in the left upper lobe.
FIGURE 3.
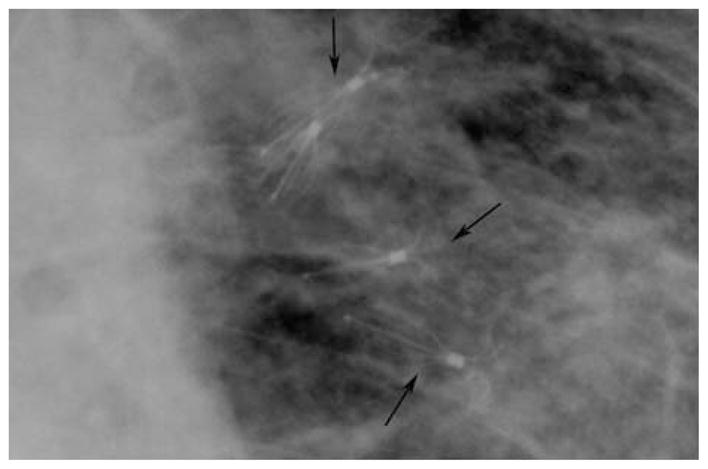
Cone down view of intrabronchial valves on chest x-ray. The multiple intrabronchial valves can be viewed on standard chest x-ray.
FIGURE 4.
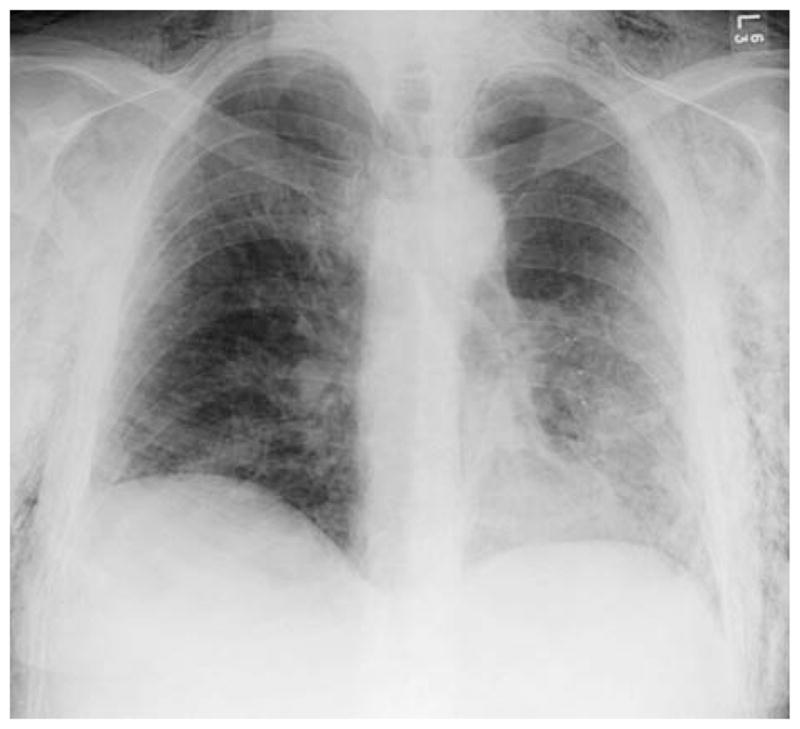
Chest x-ray after intrabronchial valve placement.
Case 2
A 73-year-old woman presented to the emergency room with shortness of breath. She had an oxygen saturation of 50% and an elevated PaCO2. The patient was emergently intubated for hypoxic and ventilatory failure. She had a medical history of lung cancer status post left pneumonectomy 10 years earlier. A BPF complicated the pneumonectomy, with failed attempts at closure with bronchoscopic glue application 7 years earlier. A chest radiograph showed pronounced volume loss in the left chest with a mediastinal shift and hyperexpansion of the right lung. A fluid collection was noted to be tracking into the apex of the left pleural space. Cultures from a bronchoalveolar lavage grew methicillin-resistant Staphylococcus aureus and Stenotrophomonas species. In addition, cultures from the fluid in the left pleural space grew 2 strains of multidrug-resistant Pseudomonas aeruginosa species. The patient had been on appropriate antibiotics for a prolonged course with a need to close her BPF and drain the pleural space. She continued to fail spontaneous breathing trials because of the inability to produce adequate tidal volumes.
Bronchoscopy was performed for placement of IBVs. The BPF was visualized at the left mainstem bronchus stump at the site of the previous pneumonectomy. One 5-mm IBV was placed into the fistula, resulting in complete closure of the BPF. The following day, the patient was able to generate appropriate tidal volumes on her spontaneous breathing trial without signs of fatigue. She was successfully extubated with further improvement in her ventilation and oxygenation.
Case 3
A 67-year-old man presented to the emergency room with shortness of breath, fever, and a productive cough. A CT scan at the time of admission showed extensive subcutaneous air through the chest and the abdomen, with evidence of a ruptured pneumatocele in the left lung. The patient had a history of non–small-cell lung cancer in the upper lobe of the left lung and had undergone resection of the tumor 6 months earlier with positive margins. The patient underwent transthoracic radiofrequency ablation for the remaining tumor in his lingula 1 week before presentation. A chest tube was placed into the left pleural space with significant bubbling in the water seal chamber indicating an air leak. Over the following days, the patient’s condition declined, resulting in respiratory failure and intubation. A chest radiograph showed extensive subcutaneous emphysema affecting the entire thorax. Spontaneous breathing trials continued to fail because of an inability to produce adequate tidal volumes.
The patient was transferred to our ICU for closure of his BPF with IBVs. Two IBVs were deployed in the airways leading to the superior and the inferior segments of the lingual. The placement of valves resulted in immediate resolution of bubbling in the water seal chamber. The patient passed his spontaneous breathing trial and was extubated the next morning. Unfortunately, the patient required reintubation the following day for unclear reasons but was successfully extubated again 5 days later. A repeat CT scan of the chest showed complete resolution of the patient’s intrapleural air. He was transferred to an acute rehabilitation facility after 3 more days in the hospital.
DISCUSSION
Our study represents one of the few reports documenting the use of IBVs in ICU patients requiring high levels of oxygen support or mechanical ventilation. Traditional approaches toward the treatment of BPFs range from watchful waiting with prolonged chest tube placement to more invasive measures such as lobectomy. In the setting of critical illness, extended ICU stays with prolonged chest tube placement and major surgical interventions are often high risk. Prolonged mechanical ventilation increases the risk for infection, volume overload, tracheal bleeding, ileus, renal failure, pneumothorax, laryngeal edema, and seizures.10–13 Our study demonstrates that in patients with BPFs requiring high levels of oxygen support or even mechanical ventilation, IBVs serve as an alternative to invasive surgical interventions. In our experience, these valves can be placed safely with significant improvement or complete resolution of BPFs in an ICU setting.
To our knowledge, the few studies describing the use of airway valves for the correction of PALs involve hemodynamically stable patients without critical care needs.14–17 El-Sameed and colleagues have recently described the use of IBVs in patients suffering from prolonged air leaks stemming from the loss of structural lung integrity due to bronchiectasis or cavitary lung disorders. The mean time of chest tube removal after the placement of IBVs for PALs in this case series of 4 patients was 8 days.18 Our case series is unique by describing the treatment of PALs contributing to the need for significant oxygen support or mechanical ventilation in ICU patients. In the past 10 years, the use of endobronchial Emphasys valves (Emphasys Medical Inc., Redwood City, CA) to correct PALs from BPFs have been documented.14–17 Most recently, Travaline et al19 showed in a multicenter experience that the Zephyr valve is an effective, nonsurgical, minimally invasive device for treating PALs. In addition, Abu-Hijleh and Blundin20 were able to describe the emergency use of endobronchial 1-way valves in the management of BPFs and alveolar-pleural fistulas. Their report described the use of 1-way endobronchial valves in the management of severe air leaks with rapid progressive massive subcutaneous emphysema and respiratory failure, likely secondary to radiofrequency ablation for the management of lung cancer. These studies, along with our case series, provide intensive care clinicians with a minimally invasive alternative to surgery for the treatment of PALs. Because the placement of IBVs requires expertise and specialized training, clinicians should refer patients needing IBVs to a tertiary care institution with interventional pulmonologists comfortable in performing the procedure.
In conclusion, our case series describes the successful use of Spiration IBVs in the treatment of BPFs in ICU patients requiring high levels of oxygen support or mechanical ventilation. Previous studies have chronicled the use of airway valves for the treatment of PALs in non-ICU patients. Considering the many complications that can develop with prolonged ICU stays, our study demonstrates that IBVs can be placed in the ICU setting to provide an expeditious, safe, and minimally invasive therapeutic option for critically ill patients with BPFs.
Footnotes
Disclosure: There is no conflict of interest or other disclosures.
References
- 1.Williams N, Lewis C. Bronchopleural fistula: a review of 86 cases. Br J Surg. 1976;63:520–522. doi: 10.1002/bjs.1800630706. [DOI] [PubMed] [Google Scholar]
- 2.Malave G, Foster E, Wilson J, et al. Bron-chopleural fistula—present-day study of an old problem: a review of 52 cases. Ann Thorac Surg. 1971;11:1–10. doi: 10.1016/s0003-4975(10)65404-5. [DOI] [PubMed] [Google Scholar]
- 3.Chee C, Abisheganaden J, Yeo J, et al. Persistent air-leak in spontaneous pneuomothrax—clinical course and outcome. Respir Med. 1998;92:757–761. doi: 10.1016/s0954-6111(98)90008-7. [DOI] [PubMed] [Google Scholar]
- 4.Kempainen R, Pierson D. Persistent air leaks in patients receiving mechanical ventilation. Semin Respir Crit Care Med. 2001;22:675–684. doi: 10.1055/s-2001-18804. [DOI] [PubMed] [Google Scholar]
- 5.Tobin M, editor. Principle and Practice of Mechanical Ventilation. 2. New York: McGraw-Hill; 2006. [Google Scholar]
- 6.Onotera R, Unruh H. Closure of a post-pneumonectomy bronchopleural fistula with fibrin sealant (Tisseel) Thorax. 1988;43:1015–1016. doi: 10.1136/thx.43.12.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abolhoda A, Bui T, Milliken J, et al. Pedicled latissimus dorsi muscle flap. Tex Heart Inst J. 2009;36:298–302. [PMC free article] [PubMed] [Google Scholar]
- 8.Puskas J, Mathisen D, Grillo H, et al. Treatment strategies for bronchopleural fistulas. J Thorac Cardiovasc Surg. 1995;109:989–995. doi: 10.1016/S0022-5223(95)70325-X. [DOI] [PubMed] [Google Scholar]
- 9.Puckas JD, Mathisen DJ, Grillo HC, et al. Treatment strategies for bronchopleural fistula. J Thorac Cardiovasc Surg. 1995;109:989–996. doi: 10.1016/S0022-5223(95)70325-X. [DOI] [PubMed] [Google Scholar]
- 10.Chatila W, Criner G. Complications of long-term mechanical ventilation. Respir Care Clin N Am. 2002;8:631–647. doi: 10.1016/s1078-5337(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 11.Kalb T, Lorin S. Infections in the chronically critically ill: unique risk profile in a newly defined population. Crit Care Clin. 2002;18:529–552. doi: 10.1016/s0749-0704(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 12.Scheinhorn D, Hassenpflug M, Votto J. Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007;131:85–93. doi: 10.1378/chest.06-1081. [DOI] [PubMed] [Google Scholar]
- 13.Chung Y, Chao T, Chiu C, et al. The cuff-leak test is a simple tool to verify severe laryngeal edema in patients undergoing long-term mechanical ventilation. Crit Care Med. 2006;34:409–414. doi: 10.1097/01.ccm.0000198105.65413.85. [DOI] [PubMed] [Google Scholar]
- 14.Kristopher MM, Boley TM, Hazelrigg SR. Endobronchial valves for treatment of bronchopleural fistula. Ann Thorac Surg. 2006;81:1129–1131. doi: 10.1016/j.athoracsur.2005.02.074. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson J, Sprenger K, Van Natta T. Closure of a bronchopleural fistula using bronchoscopic placement of an endobronchial valve designed for the treatment of emphysema. Chest. 2006;129:479–481. doi: 10.1378/chest.129.2.479. [DOI] [PubMed] [Google Scholar]
- 16.Anile M, Ventura F, De Giacomo T, et al. Treatment of persistent air leakage with endobronchial one-way valves. J Thorac Cardiovasc Surg. 2006;132:711–712. doi: 10.1016/j.jtcvs.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Feller-Kopman D, Bechara R, Garland R, et al. Use of a removable endobronchial valve for the treatment of bronchopleural fistula. Chest. 2006;130:273–275. doi: 10.1378/chest.130.1.273. [DOI] [PubMed] [Google Scholar]
- 18.El-Sameed Y, Waness A, Al Shamsi I, et al. Endobronchial valves in the management of broncho-pleural and alveolo-pleural fistulae. Lung. 2011;184 doi: 10.1007/s00408-011-9369-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Travaline J, McKenna R, Jr, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest. 2009;136:355–360. doi: 10.1378/chest.08-2389. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Hijleh M, Blundin M. Emergency use of an endobronchial one-way valve in the management of severe air leak and massive subcutaneous emphysema. Lung. 2010;188:253–257. doi: 10.1007/s00408-009-9204-0. [DOI] [PubMed] [Google Scholar]


