Abstract
Background
Coronary heart disease (CHD) is a major health concern, affecting nearly half the middle-age population and responsible for nearly one-third of all deaths. Clinicians have responsibilities beyond diagnosing CHD, including risk stratification of patients for major adverse cardiac events (MACE), modifying the risks and treating the patient. In this first of a two-part review, identifying risk factors is reviewed, including more potential benefit from autonomic testing.
Methods
Traditional and non-traditional, and modifiable and non-modifiable risk factors for MACE where compared, including newer risk factors, such as inflammation, carotid intimal thickening, ankle-brachial index, CT calcium scoring, and autonomic function testing, specifically independent measurement of parasympathetic and sympathetic (P&S) activity.
Results
The Framingham Heart Study, and others, have identified traditional risk factors for the development of CHD. These factors effectively target high-risk patients, but a large number of individuals who will develop CHD and MACE are not identified. Many patients with CHD who appear to be well-managed by traditional therapies still experience MACE. In order to identify these patients, other possible risk factors have been explored. Advanced autonomic dysfunction, and its more severe form, cardiac autonomic neuropathy, have been strongly associated with an elevated risk of cardiac mortality and are diagnosable through P&S testing.
Conclusions
Independent measures of P&S activity, provides additional information and has the potential to incrementally add to risk assessment. This additional information enables physicians to (1) specifically target more high-risk patients and (2) titrate therapies, with autonomic testing guidance, in order to minimize risk of cardiac mortality and morbidity.
Keywords: Cardiac autonomic neuropathy, Cardiovascular risk factors, Heart disease, Mortality
Introduction
Heart disease has been the leading cause of mortality in the United States, with one of the highest hospitalization rates, imposing a tremendous financial burden on our health care system (1-2-3-4-5). Scientists have aggressively sought effective means of assessing and treating patients’ risk. Traditional and nontraditional risk factors have been identified. However, many are lacking in standardized guidelines, even though there are noninvasive tests developed to assess these risk factors. Even when there is a standard, such as beta-blocker use after a myocardial infarction (MI) (6), the efficacy is unclear.
This first of a two-part review series briefly discusses the traditional risk factors and then risk indications from quantified independent and simultaneous measures of parasympathetic and sympathetic (P&S) responses to disease and therapy. P&S monitoring has the potential to serve as a barometer, providing important additional information to identify higher-risk patients and guide more specific therapy to treat cardiac disease, such as documenting the individual patient’s response to beta-blockers or other autonomically active therapies. The second part discusses treating risk factors, including autonomic dysfunction, and expected outcomes.
Risk factors in heart disease
Risk factors in heart disease are based on the potential for developing atherosclerosis causing atherothrombosis (7). Epidemiological studies (8-9-10-11-12-13-14-15-16) confirm traditional risk factors for the development of atherosclerotic heart disease. They demonstrate that atherosclerosis often leads to coronary heart disease (CHD), cerebral vascular disease (including stroke and transient ischemic attack); peripheral artery disease (including intermittent claudication and ischemia to the lower extremities) and atherosclerosis of the aorta, which may lead to aneurysm formation. Risk factors in heart disease are categorized as follows:
-
➢
Traditional Risk Factors: 1) age (≥55 years for postmenopausal women and ≥45 years for men); 2) diabetes mellitus; 3) smoking; 4) high blood pressure (BP) or hypertension (BP >140/90 mmHg or history of antihypertensives); 5) dyslipidemia (high low-density lipoprotein, LDL, cholesterol >99 mg/dL), low high-density lipoprotein, HDL, cholesterol (<40 mg/dL), or hypertriglyceridemia (>150 mg/dL) and 6) family history of premature coronary artery disease (CAD, <65 years in females and <55 years in males).
-
➢
Nontraditional Risk Factors: 1) abnormal ankle–brachial index (ABI); 2) chronic inflammation as indicated by abnormal levels of C-reactive protein (CRP; CRP is an acute phase protein that is produced by the liver under the influence of cytokines, such as interleukin-6 and tumor necrosis factor-alpha), fibrinogen, lipoprotein (a), brain natriuretic peptide, or human immunodeficiency virus; 3) homocysteine elevation; 4) microproteinuria (urinary protein excretion between 80 and 300 mg/24 h, including albumin to creatinine ratio >30 mg/mmol or albumin concentration >200 mg/L); 5) microalbuminaria (albumin to creatinine ratio >2.5 mg/mmol in men or >3.5 mg/mmol in women, or albumin concentration >20 mg/L); 6) metabolic syndrome; 7) elevated serum insulin levels; 8) renal disease; 9) abnormal calcium score; 10) carotid intima–media thickness; 11) left ventricular (LV) hypertrophy; 12) psychosocial stresses; 13) alcohol; 14) abnormal diet; 15) clinical depression; 16) obesity (particularly of the abdominal male type); 17) sedentary lifestyle; 18) various types of infections and 19) collagen vascular diseases.
-
➢
Modifiable Risk Factors: (those that may be treated and negated, reversed or diminished): smoking, dyslipidemia, hypertension, sedentary lifestyle, diet, obesity, type 2 diabetes mellitus or impaired glucose tolerance and CRP.
-
➢
Nonmodifiable Risk Factors: age, gender, genetic abnormalities and family history of premature atherosclerosis.
Risk scores
For many decades, physicians and epidemiologists have attempted to develop equations, scoring systems and algorithms to risk-stratify and predict which individual patients are at risk for cardiac events. The first landmark system was derived from prospective follow-up of approximately 20 years of a cohort of individuals that resided in Framingham, MA. The Framingham Risk Score projects future risk of cardiovascular disease (CVD) for up to 10 years (13). This risk score system incorporates several risk factors which are commonly seen in a large cohort of individuals (traditional risk factors). These risk factors include diabetes, hypertension, lipid elevations, cigarette smoking and age. While the Framingham Risk Score was an excellent beginning and is still widely used in clinical medicine today, it has a number of shortcomings. Current longer life expectancies need a prediction model that extends beyond 10 years. Furthermore, large subpopulations develop complications from heart disease and are not identified by these scoring systems (16-17-18). Family history of premature CAD, a risk factor not incorporated into the Framingham Risk Score, is an addition important factor in risk stratification for cardiac events.
The family history of premature CAD with inflammation (e.g., as measured by CRP) added to the Framingham Score (the Reynolds risk scoring system), improves upon the Framingham system. The Reynolds risk scoring system is based on age, BP, cigarette smoking, CRP and family history of cardiac events prior to 60 years. The improvement in this scoring system is based on the fact that the Reynolds scoring system reclassifies almost half of the intermediate-risk women into high- and low-risk groups (16). Here again, however, the Reynolds risk scoring system only predicts out to 10 years.
Recently, research into risk scoring has focused on nontraditional risk factors which have been shown to improve scoring. In addition to CRP, ultrasound, Doppler and other imaging-derived measurements, such as carotid intimal thickness, ABI and cardiac CT scan calcium scores, have also yielded addition information in risk stratification (16-17-18-19-20-21-22). Another risk factor which predisposes patients to adverse cardiac events is autonomic neuropathy, specifically cardiovascular autonomic neuropathy (CAN) (23-24-25-26-27-28-29-30-31-32-33-34-35-36-37-38). CAN is associated with other risk factors (39), including 1) low ejection fraction (25, 26); 2) poor cardiac output (40); 3) arrhythmias (27, 28); 4) cardiomyopathies (29, 30), including chronic heart failure (31); 5) poor circulation (32), including poor cardiac circulation (angina or CAD) (33); 6) greater mortality (24) and 7) greater morbidity (41), including silent MI and early cardiac death (24, 34). Often, very low parasympathetic activity leads to the need for cardiac intervention or an implanted cardiac device. With supplemental information from parasympathetic and sympathetic monitoring, which identifies CAN, appropriate treatment modalities, including pharmacological and cardiac device therapy, may reduce adverse cardiac outcomes. By restoring proper P&S balance (42), morbidity and mortality may be reduced (23, 24, 43).
Parasympathetic and sympathetic function assessment
Heart rate (HR) alone does not provide a reliable diagnostic criterion of CAN (44-45-46). Historically, autonomic monitoring (including for CAN) has only measured general autonomic function from analyses of just the heart beat interval (HBI, including HR variability (HRV) alone and beat-to-beat BP) (47, 48). These measures force assumption or approximation to differentiate parasympathetic from sympathetic activity. “Functional imbalances between the sympathetic and parasympathetic nervous systems are discerned with respiratory modulation.” (24) This observation is supported by a large body of literature (e.g., (35-36-37-38, 44-45-46)). Newer technology is available to specify P&S activity without assumption or approximation. It is based on HRV coupled with analysis of concurrent respiratory activity (49-50-51-52-53-54). Respiratory activity (e.g., from impedance plethysmography) helps to identify the cardiovagal response which is respiratory sinus arrhythmia (RSA). Conceptually, this technique separates RSA from the other HR changes that are observed in the cardiogram. This technique is sensitive enough to identify RSA even in sick patients when it is not visible to the human in the cardiogram, irrespective of patient history, state or activity. Specific P&S function testing has the ability to provide the clinician with supplemental information to document and differentiate which agents or therapeutic modalities are needed. For example, more is not always better, such as intensive glucose control for diabetic patients (55-56-57).
Risks associated with autonomic neuropathy
Autonomic neuropathy is associated with cardiac mortality risk
Decreased HRV, specifically very low resting parasympathetic activity, defines CAN (24, 50, 58, 59). Meta-analyses strengthen the association of CAN with cardiac mortality (24, 50, 58). When more measures defining CAN are fulfilled, the mortality rate is higher (24, 50, 58, 74). Curtis and O’Keefe (23) show that associations of CAN with high mortality rates are consistent across study groups, patient cohorts, testing modalities, autonomic dysfunction and disease definitions. Subsequent studies demonstrate the association with multivariant analyses (58, 60, 61). Some of these researchers find that CAN is treatable with more information from P&S monitoring (39, 42).
Epidemiological studies strengthen the association between CAN and mortality risk (62-63-64, 66). After assessing for age, gender, cigarette smoking, diabetes and other relevant risk factors, autonomic measurements offer significant prognostic information beyond that provided by evaluation of traditional cardiovascular risk factors. Tsuji and coworkers studied all-cause mortality in elderly participants, and subsequently addressed the general population (59). A predicted risk increase for sudden cardiac event was found in 2,501 men and women who were without clinically apparent heart disease and with reduced autonomic activity. A biologically feasible mechanism for this is based on the fact that patients who have heart disease with increased sympathetic activity, or decreased parasympathetic activity, are predisposed to ventricular fibrillation.
The first prospective study to identify an association between reduced autonomic function and heart disease risk in a community-based population (65-66-67) demonstrated the independent value of HR turbulence (a type of HRV analysis). As a measure of autonomic function, HR turbulence predicts fatal and nonfatal cardiac arrest in a low-risk, post-acute MI population. While it is unclear from their study which patients might benefit from more advanced therapy, including defibrillators (due to very low resting parasympathetic activity), it is well known that post-MI patients diagnosed with diabetes have higher mortality rates than nondiabetic post-MI patients. Twelve studies of diabetic patients, with and without CAN, show that CAN diabetics are 280% more likely to suffer silent MI than non-CAN diabetics (Fig. 1) (24).
Fig. 1 -.
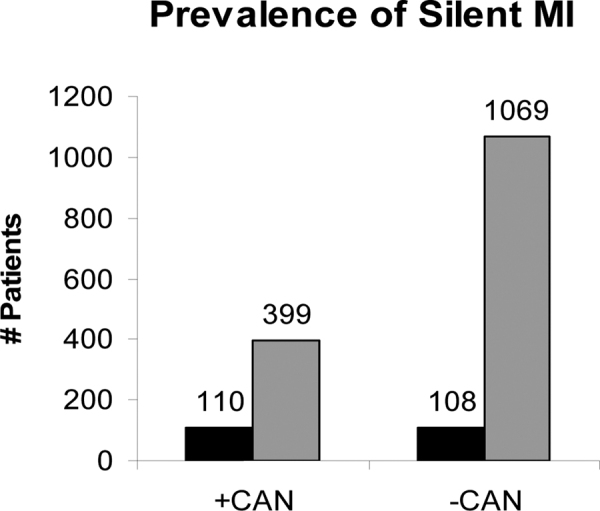
Prevalence rate ratios and 95% confidence intervals for association between CAN and silent myocardial ischemia in 12 studies. Adapted with permission from (24). See text for details.
Using a definition of severe autonomic failure that includes abnormalities of autonomic reflex function, Barthel and coworkers (68) identify at-risk patients and demonstrate very poor prognoses. In their risk model, autonomic dysfunction predicts history of previous MI, arrhythmia on Holter monitoring, poor glucose control and LV ejection fraction less than 30%. This highlights the importance, even in the low-risk patients, of performing P&S testing to risk-stratify for major adverse cardiac events (MACE), including cardiac death. In general, abnormal cardiac autonomic activity as assessed by autonomic monitoring is associated with a post-MI mortality, sudden death and all-cause mortality.
In a population-based prospective study, Liao and coworkers (69) demonstrate that autonomic dysfunction, especially lower parasympathetic activity, is associated with the risk of developing CHD. This expands the application of monitoring autonomic dysfunction to a much larger patient base and the general population. Liao et al find that autonomic dysfunction may be a predictor of subsequent development of CAD. This is an extremely important finding, highlighting autonomic dysfunction as a potentially important risk factor for newly developing CAD. Therefore, not only is identifying abnormal autonomic function and CAN important for secondary prevention, it is also important for primary prevention. Furthermore, autonomic dysfunction is correlated with progression of CAD (70), and with silent ischemia, which leads to sudden unexpected cardiac death and unexpected MI. Wackers and coworkers (71) find that myocardial ischemia is associated with abnormal Valsalva response with a risk ratio of 5.6. Males demonstrate a risk ratio of 2.5, and patients diagnosed with diabetes demonstrated a risk ratio of 5.2. All other traditional cardiac risk factors, including inflammatory and prothrombotic markers, are not predictive. The emerging cardiac risk factors in this thorough study are not associated with abnormal stress tests or computed tomography imaging. By contrast, CAD is a strong predictor of ischemia.
CAN is associated with a denervated heart, leaving patients unaware of cardiac events. This demonstrates a compelling need to assess P&S function in asymptomatic patients, especially given silent ischemia or sudden cardiac death (SCD). Without P&S monitoring, critical information concerning the asymptomatic patient’s risk of silent ischemia will be lacking, including clinical trending information to document patients’ responses to therapy. The fact that coronary atherosclerosis may progress with CAN (71), and that silent ischemia may occur with a higher incidence with CAN (23, 43, 70-71-72), suggests that CAN is either a risk factor or an etiological factor for these subclinical events. Asymptomatic patients, despite having other traditional risk factors, should have autonomic function assessed.
Stratifying autonomic neuropathy risk
CAN indicates an autonomic condition in which a sympathetically mediated ventricular tachyrhythm may not be sufficiently slowed by parasympathetic activity to prevent ventricular fibrillation or worse. CAN may be normal for geriatric and long-standing chronic disease patients (43). For example, based on Framingham risk factors, an 85-year-old has a greater mortality risk than a 45-year-old. More, but not excessive, parasympathetic activity relative to sympathetic activity is known to be cardioprotective and reduce mortality risk (43). Chronic sympathetic activation is known to increase cardiovascular risk (23). Depression is known to elevate mortality risk in heart disease (72), and depression is associated with abnormally high levels of parasympathetic activity relative to sympathetic activity.
The relationship between P&S activity at rest is known as sympathovagal balance (SB) (73). CAN risk (the risk associated with very low parasympathetic activity with respect to sympathetic activity) may be stratified based on SB. High SB indicates relative resting sympathetic excess. CAN with high SB is considered high risk (25, 28, 29). Low SB indicates a relative resting parasympathetic excess. Very low SB (<0.4) is associated with (subclinical) depression and elevates CAN risk (72). Normal SB, indicating a balanced ANS, is associated with much lower CAN risk (23). Low-normal SB, indicating more parasympathetic activity, is associated with minimal CAN risk (43).
Diabetes risk and autonomic neuropathy
It is well established that diabetes mellitus is a major risk factor for heart disease. Diabetic autonomic neuropathy (DAN) is a very serious and common complication in diabetes (24). Symptoms of DAN include 1) resting tachycardia, 2) exercise intolerance, 3) orthostatic hypotension and 4) also a glycemic autonomic failure (74). DAN is often misperceived as asymptomatic and the symptoms considered in isolation. DAN imposes a burden on an individual whose cardiac reserve may be compromised by underlying atherosclerosis or LV abnormalities. The most studied and clinically important advanced form of DAN is CAN (24). CAN may be present at diagnosis of diabetes (one in three), and prevalence increases with age, duration of diabetes and poor glycemic control. CAN encompasses damage to the autonomic nerve fibers that innervate the heart and blood vessels, resulting in abnormalities in heart control and vascular dynamics. Autonomic neuropathy is not restricted to diabetics. Advanced autonomic dysfunction (i.e., a form of DAN in nondiabetics) may occur in those without diabetes, with similar burdens, including CAN. A symptom of CAN is an increased threshold to chest pain during MI (silent MI), which can lead to SCD.
Various tests of autonomic function have been used to define CAN and have been studied by numerous investigators who compared mortality risk among diabetic patients with and without CAN (75). Tests may include the provocative Ewing challenges (76): changes in posture, Valsalva maneuvers and paced breathing. These autonomic challenges have been shown to stimulate one or the other or both branches of the autonomic nervous system through changes in HBI and respiratory activity. The Ewing challenges have become the standard for clinical autonomic testing [low, 1997]. Fifteen studies of 2,900 patients with and without CAN showed a 230% higher risk of mortality for the CAN diabetics (Fig. 2) (24). These data are supported by Ewing’s findings. He demonstrated a 53% mortality risk after 5 years in patients with CAN (76). He also compared the mortality rate of abnormal autonomic function tests to a mortality rate of only 15% over a 5-year period among diabetic patients with normal autonomic function tests. Half of the deaths of individuals that have abnormal autonomic function were from renal failure and 29% from SCD. CAN increases morbidity and mortality in diabetes and may have greater predictive power than traditional risk factors for cardiovascular events. Significant morbidity and mortality is attributed to dysregulation of cardiovascular function from P&S imbalance. Consider frequent screening for and treating P&S imbalance (dysfunction) (77, 78).
Fig. 2 -.
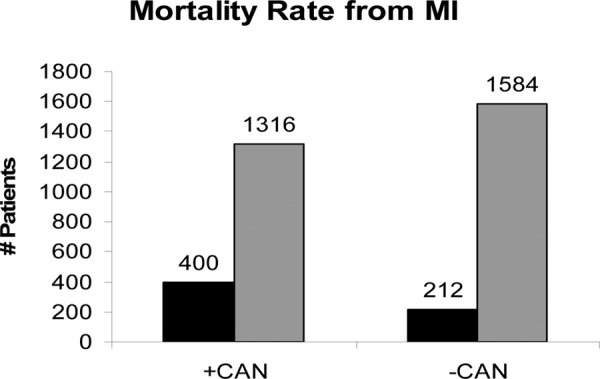
Mortality rate of patients with and without CAN. Relative risks and 95% confidence intervals for association between cardiovascular autonomic neuropathy and mortality in 15 studies. Adapted with permission from (24).
Nontraditional risk factors and autonomic neuropathy
CRP is a useful marker of increased long-term risk of SCD. After 17 years of follow-up study, including homocysteine and lipid values, CRP was the only significant biomarker that had predictive potential with SCD (79). CRP is associated with decreased autonomic function, even after controlling for traditional risk factors that decrease CAD. Autonomic dysregulation may represent one pathway leading to CAD, even with treatment of risk factors to prevent the development of CAD (80). Dyslipidemia (a traditional risk factor) significantly contributes to atherosclerosis in some cases. Inflammation is also a significant contributor toward atherosclerosis and is a nontraditional risk factor with incremental value (80). The association of diminished autonomic function with elevated CRP levels is potentially significant. In multivariant analysis, autonomic variables remain independently associated with CRP while norepinephrine concentrations did not (80). In a recent work by Vinik, inflammatory markers were correlated with diminished HRV measures and independent measures of low P&S activity with high SB (Fig. 3) (24).
Fig. 3 -.
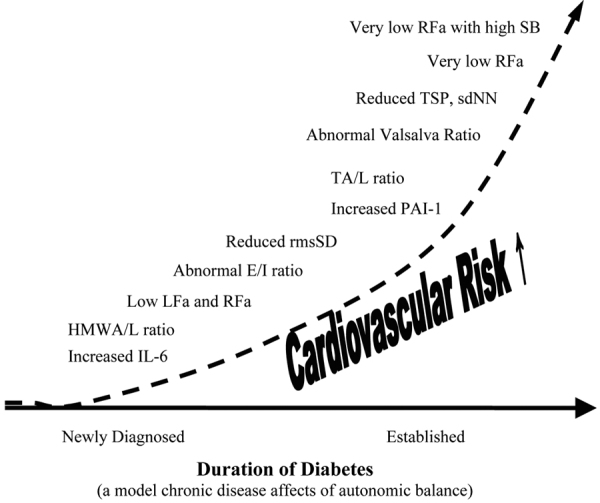
The natural history of autonomic balance, based on diabetes as a model of the affect of chronic disease on the autonomic nervous system. IL-6 = Interleukin-6, an inflammatory marker; HMWA/L = high-molecular weight adiponectin-to-leptin ratio, an inflammatory marker; LFa = low frequency area, a pure measure of sympathetic activity (based on concurrent spectral analyses of continuous measures of both respiratory activity and HRV); RFa = respiratory frequency area, a pure measure of parasympathetic activity (based on concurrent spectral analyses of continuous measures of both respiratory activity and HRV); E/I ratio = the ratio of the peak exhalation R-R interval to the peak inhalation R-R interval (R-R interval is the interval between two consecutive heart beats, and is a qualitative measure of more or less parasympathetic activity; rmsSD = root mean square of standard deviation, a statistical measure of heart rate variability (HRV), and is a qualitative measure of more or less parasympathetic activity; PAI-1 = plasminogen activator Inhibitor 1, an inflammatory marker; TA/L ratio = total adiponectin/leptin ratio, an inflammatory marker; Valsalva ratio = the ratio of the longest to shortest R-R interval during a 15 second Valsalva maneuver, a qualitative measure of more or less parasympathetic activity; TSP = total spectral power, a measure of gross autonomic activity (parasympathetic plus sympathetic activity); sdNN = standard deviation of the beat-to-beat (R-R) intervals, a measure of gross autonomic activity (parasympathetic plus sympathetic activity); RFa = respiratory frequency area, a pure measure of parasympathetic activity (based on concurrent spectral analyses of continuous measures of both respiratory activity and HRV); SB = Sympathovagal Balance = ratio of resting sympathetic activity to resting parasympathetic activity. Very low RFa is a definition of Cardiovascular Autonomic Neuropathy (CAN), increased indicating mortality risk. CAN with high SB is associated with high mortality risk (see text) (24).
Microalbuminuria has been associated with an increased risk of cardiovascular mortality independently of other known coronary artery risk factors (81). Endothelial function and low-grade inflammation have been proposed to explain the increased risk of cardiovascular mortality in individuals with microalbuminuria (82). The Hoorn study (83) (498 individuals, ages 50 to 75 years, followed for a median period of 13.6 years) demonstrated that with an albumin to creatinine ratio greater than 20 mg/mmol, patients’ CAN was independently associated with cardiovascular mortality. Their conclusions suggest that microalbuminuria and CAD are associated with cardiovascular mortality in an elderly Caucasian population of individuals with normal glucose tolerance.
Sudden cardiac death
Lastly, one cannot discuss diagnosis and treatment of CVDs without addressing SCD (84). Approximately 67% of symptoms of SCD are related to CHD (85-86-87), affecting 450,000 individuals per year in the United States (88), and this is probably an underestimate. The risk is three times greater in men than in women (89). Important risk factors for SCD are underlying CAD, heart failure, LV dysfunction and prior MI. The risk factors for CAD are the same as those for SCD. Heart failure is also a significant risk factor for SCD. Significant genetic factors for SCD (90) showed that parental SCD is an independent risk factor for SCD in middle-aged men. Familial SCD risk factors help explain high-risk subjects and enable prevention early on. A study of twins (91) showed a greater risk in younger than in older patients. Diabetes and glucose levels also influence the risk of SCD. Diabetes is a strong risk factor for SCD and the importance of glucose level at every stage of diabetes severity should be examined (92). The Framingham Study (93) established CHD factors reflecting ischemic myocardial damage and cardiac failure as the chief predictors of SCD. Despite a national decline in the overall component of heart disease mortality rates, the proportion of CHD deaths presenting as SCD has not declined.
Patients with LV dysfunction are at high risk for SCD. This risk is used as an index for aggressive treatment for devices such as defibrillators. However, a community-wide study (94) shows that only one third of the evaluated SCD patients having severe LV dysfunction meet the criteria for prophylactic cardioverter defibrillator implantations. A greater number of patients with SCD have normal LV function, and present with several distinguishable clinical features: 1) they are younger in age, 2) a higher proportion are female, 3) there is a higher prevalence of seizure disorders and 4) there is a lower prevalence of established CAD. Prophylactically implanted cardiac device trials represent a minority of SCD population (95). Therefore, screening patients for SCD based on LV dysfunction is not a very sensitive technique and will miss approximately two thirds of SCD patients.
A study of 5,713 asymptomatic men concludes that HR profile during exercise and recovery is a predictor of SCD (96). Subjects demonstrating an increase in HR during exercise of less than 89 bpm have a relative risk of 6.18. Subjects that failed to decrease HR by 25 beats in the first minute after exercise have a relative risk of 2.2. The risk from SCD is also increased in patients with a resting HR of more than 75 bpm (relative risk is 3.92) (77, 96). The recovery of HR immediately after exercise is a parasympathetic function. Poor HR recovery is associated with insufficient parasympathetic activity. Parasympathetic insufficiency is associated with increased mortality risk (43, 97). Again, sufficiently sensitive testing for risk factors and specific predictors of SCD is lacking. However, it may be useful, when treating patients with normal LV systolic function, to risk-stratify. Abnormal physiological HR responses, with P&S dysfunction, translate into a significant prognostic risk factor, which results in further follow-up, especially in individuals with normal LV systolic function.
In a review article (88), Myerburg states that SCD is an unresolved problem despite more insight into the mechanisms and therapeutic advances. Prediction and prevention of SCD should not be restricted to assessing an individual for the presence of CAD, coronary ischemia, LV dysfunction or heart failure. This is a much more complicated issue underlying various diseases and risk factors. It is apparent that independent, simultaneous P&S testing for CAD provides additional information to understand these issues, to guide therapy and treatment and enable improved outcomes. P&S testing allows for the risk assessment of patients for MACE, even when they are asymptomatic and have no clinical CAD. Subclinical CAN is associated with CAD. Testing for CAN and SB may be extremely productive in identifying and treating high-risk patients for cardiac events (42, 77, 78).
Conclusion
Clinical studies, epidemiological data and biologically feasible mechanisms support the need to test for CAN, not only in diabetics, but also in the general population as they age and their risk of incidence of heart disease increases. It is unequivocally established that CAN is associated with increased cardiac morbidity and mortality. Identifying and addressing CAN early, especially in a subclinical cardiac patient, will further differentiate which asymptomatic patients require more aggressive therapy. The results from P&S testing documenting CAN may be used as a baseline. While further studies are indicated, the clinical and epidemiological data are too compelling not to test for, diagnose and aggressively treat CAN with abnormal SB to guard the patient’s well-being, not only in diabetics (77, 78), but in all patients with risk factors for heart disease.
Disclosures
Financial support: No grants or funding have been received for this study.
Conflict of interest: Dr DePace, Ms. Mears and Mr. Yayac have no conflicts of interest. Dr. Colombo is medical director, executive vice president, board member and part owner of ANSAR Medical Technologies, Inc., Philadelphia, PA, USA, a researcher, developer, manufacturer and distributor of autonomic function testing technology.
References
- 1.Rosamond W, Flegal K, Furie K et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke Statistics—2008 update. Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM et al.; WRITING GROUP MEMBERS; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services (HHS). Prevention Makes Common “Cents.”. U.S. Department of Health and Human Services, 2003 Sep::35 p. [Google Scholar]
- 5.Ausubel JH, Meyer PS, Wernick IK. Death and the human environment: the United States in the 20th century. Technol Soc. 2001;23(2):131–146. [Google Scholar]
- 6.Bangalore S, Steg G, Deedwania P et al.; REACH Registry Investigators. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308(13):1340–1349. doi: 10.1001/jama.2012.12559. [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Abbas AK, Fausto N, Mitchell RS eds. 8th ed. Philadelphia, PA: Saunders Elsevier; Robbins basic pathology. 2007:345. [Google Scholar]
- 8.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 11.Meigs JB, Larson MG, D’Agostino RB et al. Coronary artery calcification in type 2 diabetes and insulin resistance: the Framingham offspring study. Diabetes Care. 2002;25(8):1313–1319. doi: 10.2337/diacare.25.8.1313. [DOI] [PubMed] [Google Scholar]
- 12.Karim R, Hodis HN, Detrano R, Liu CR, Liu CH, Mack WJ. Relation of Framingham risk score to subclinical atherosclerosis evaluated across three arterial sites. Am J Cardiol. 2008;102(7):825–830. doi: 10.1016/j.amjcard.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannel WB, Evans JC, Piper S, Murabito JM. Angina pectoris is a stronger indicator of diffuse vascular atherosclerosis than intermittent claudication: Framingham study. J Clin Epidemiol. 2008;61(9):951–957. doi: 10.1016/j.jclinepi.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyama N, Gona P, Salton CJ et al. Differential impact of age, sex, and hypertension on aortic atherosclerosis: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008;28(1):155–159. doi: 10.1161/ATVBAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- 15.Junyent M, Zambón D, Gilabert R, Núñez I, Cofán M, Ros E. Carotid atherosclerosis and vascular age in the assessment of coronary heart disease risk beyond the Framingham Risk Score. Atherosclerosis. 2008;196(2):803–809. doi: 10.1016/j.atherosclerosis.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118(22):2243–2251,. doi: 10.1161/CIRCULATIONAHA.108.814251. 4p, 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359(18):1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 18.Albert MA, Glynn RJ, Ridker PM. Plasma concentration of C-reactive protein and the calculated Framingham coronary heart disease risk score. Circulation. 2003;108(2):161–165. doi: 10.1161/01.CIR.0000080289.72166.CF. [DOI] [PubMed] [Google Scholar]
- 19.Roberts WL, Moulton L, Law TC et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47(3):418–425. [PubMed] [Google Scholar]
- 20.Visonà A, Pesavento R, Lusiani L et al. Intimal medial thickening of common carotid artery as indicator of coronary artery disease. Angiology. 1996;47(1):61–66. doi: 10.1177/000331979604700109. [DOI] [PubMed] [Google Scholar]
- 21.Murphy TP, Dhangana R, Pencina MJ, D’Agostino RB., Sr Ankle–brachial index and cardiovascular risk prediction: an analysis of 11,594 individuals with 10-year follow-up. Atherosclerosis. 2012;220(1):160–167. doi: 10.1016/j.atherosclerosis.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 22.Grayburn PA. Interpreting the coronary-artery calcium score. N Engl J Med. 2012;366(4):294–296. doi: 10.1056/NEJMp1110647. [DOI] [PubMed] [Google Scholar]
- 23.Curtis BM, O’Keefe JH., Jr Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc. 2002;77(1):45–54. doi: 10.4065/77.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 25.Bullinga JR, Alharethi R, Schram MS, Bristow MR, Gilbert EM. Changes in heart rate variability are correlated to hemodynamic improvement with chronic CARVEDILOL therapy in heart failure. J Card Fail. 2005;11(9):693–699. doi: 10.1016/j.cardfail.2005.06.435. [DOI] [PubMed] [Google Scholar]
- 26.Fantoni C, Raffa S, Regoli F et al. Cardiac resynchronization therapy improves heart rate profile and heart rate variability of patients with moderate to severe heart failure. J Am Coll Cardiol. 2005;46(10):1875–1882. doi: 10.1016/j.jacc.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 27.Chen PS, Chou CC, Tan AY et al. The mechanisms of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(s3)(Suppl 3):S2–S7. doi: 10.1111/j.1540-8167.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 28.Copie X, Lamaison D, Salvador M et al.; VALID Investigators. Heart rate variability before ventricular arrhythmias in patients with coronary artery disease and an implantable cardioverter defibrillator. Ann Noninvasive Electrocardiol. 2003;8(3):179–184. doi: 10.1046/j.1542-474X.2003.08302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alter P, Grimm W, Vollrath A, Czerny F, Maisch B. Heart rate variability in patients with cardiac hypertrophy—relation to left ventricular mass and etiology. Am Heart J. 2006;151(4):829–836. doi: 10.1016/j.ahj.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Debono M, Cachia E. The impact of Cardiovascular Autonomic Neuropathy in diabetes: is it associated with left ventricular dysfunction? Auton Neurosci. 2007;132(1-2):1–7. doi: 10.1016/j.autneu.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Just H. Peripheral adaptations in congestive heart failure: a review. Am J Med. 1991;90(5)(5B):23S–26S. doi: 10.1016/0002-9343(91)90269-4. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Matsumura K, Kobayashi S, Kaneko T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci Res. 2005;51(1):1–8. doi: 10.1016/j.neures.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Manfrini O, Morgagni G, Pizzi C, Fontana F, Bugiardini R. Changes in autonomic nervous system activity: spontaneous versus balloon-induced myocardial ischaemia. Eur Heart J. 2004;25(17):1502–1508. doi: 10.1016/j.ehj.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Clarke BF, Ewing DJ, Campbell IW. Diabetic autonomic neuropathy. Diabetologia. 1979;17(4):195–212. doi: 10.1007/BF01235856. [DOI] [PubMed] [Google Scholar]
- 35.Eckberg DL. Physiological basis for human autonomic rhythms. Ann Med. 2000;32(5):341–349. doi: 10.3109/07853890008995937. [DOI] [PubMed] [Google Scholar]
- 36.Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol (1985) 1993;75(5):2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- 37.Novak V, Novak P, de Champlain J, Le Blanc AR, Martin R, Nadeau R. Influence of respiration on heart rate and blood pressure fluctuations. J Appl Physiol (1985) 1993;74(2):617–626. doi: 10.1152/jappl.1993.74.2.617. [DOI] [PubMed] [Google Scholar]
- 38.Parati G, Rizzoni D. Assessing the prognostic relevance of blood pressure variability: discrepant information from different indices. J Hypertens. 2005;23(3):483–486. doi: 10.1097/01.hjh.0000160200.51158.9a. [DOI] [PubMed] [Google Scholar]
- 39.DePace NL, Mears JP, Yayac M, Colombo J. Cardiac autonomic testing and treating heart disease. “A clinical perspective.” Heart Int. 2014 doi: 10.5301/heartint.5000216. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fathizadeh P, Shoemaker WC, Wo CC, Colombo J. Autonomic activity in trauma patients based on variability of heart rate and respiratory rate. Crit Care Med. 2004;32(6):1300–1305. doi: 10.1097/01.ccm.0000127776.78490.e4. [DOI] [PubMed] [Google Scholar]
- 41.Vinik AI, Maser RE, Nakave AA. Diabetic cardiovascular autonomic nerve dysfunction. US Endocrine Disease. 2007 Dec::2–9. [Google Scholar]
- 42.Vinik AI, Murray GL. Autonomic neuropathy is treatable. US Endocrinol. 2008;2:82–84. [Google Scholar]
- 43.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31(3):593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 44.Cammann H, Michel J. How to avoid misinterpretation of heart rate variability power spectra? Comput Methods Programs Biomed. 2002;68(1):15–23. doi: 10.1016/s0169-2607(01)00154-7. [DOI] [PubMed] [Google Scholar]
- 45.Badra LJ, Cooke WH, Hoag JB et al. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280(6):H2674–H2688. doi: 10.1152/ajpheart.2001.280.6.H2674. [DOI] [PubMed] [Google Scholar]
- 46.Hayano J, Mukai S, Sakakibara M, Okada A, Takata K, Fujinami T. Effects of respiratory interval on vagal modulation of heart rate. Am J Physiol. 1994;267(1 Pt 2):H33–H40. doi: 10.1152/ajpheart.1994.267.1.H33. [DOI] [PubMed] [Google Scholar]
- 47.Malik M; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 48.Malik M, Bigger JT, Camm AJ et al.; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 49.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 50.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249(4 Pt 2):H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 51.Akselrod S, Eliash S, Oz O, Cohen S. Hemodynamic regulation in SHR: investigation by spectral analysis. Am J Physiol. 1987;253(1 Pt 2):H176–H183. doi: 10.1152/ajpheart.1987.253.1.H176. [DOI] [PubMed] [Google Scholar]
- 52.Akselrod S. Spectral analysis of fluctuations in cardiovascular parameters: a quantitative tool for the investigation of autonomic control. Trends Pharmacol Sci. 1988;9(1):6–9. doi: 10.1016/0165-6147(88)90230-1. [DOI] [PubMed] [Google Scholar]
- 53.Aysin B, Aysin E. Effect of respiration in heart rate variability (HRV) analysis. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1776–1779. doi: 10.1109/IEMBS.2006.260773. [DOI] [PubMed] [Google Scholar]
- 54.Aysin B, Aysin E, Colombo J. IEEE Engineering in Medicine and Biology Conference. Lyons, France: Comparison of HRV analysis methods during orthostatic challenge: HRV with respiration or without? 2007. [DOI] [PubMed] [Google Scholar]
- 55.Gerstein HC, Miller ME, Genuth S et al.; ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pop-Busui R, Evans GW, Gerstein HC et al.; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(7):1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calles-Escandón J, Lovato LC, Simons-Morton DG et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(4):721–727. doi: 10.2337/dc09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26(6):1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 59.Tsuji H, Venditti FJ, Jr,, Manders ES et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 60.Barakat HA, Mooney N, O’Brien K et al. Coronary heart disease risk factors in morbidly obese women with normal glucose tolerance. Diabetes Care. 1993;16(1):144–149. doi: 10.2337/diacare.16.1.144. [DOI] [PubMed] [Google Scholar]
- 61.Ziegler D, Zentai CP, Perz S et al.; KORA Study Group. Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care. 2008;31(3):556–561. doi: 10.2337/dc07-1615. [DOI] [PubMed] [Google Scholar]
- 62.Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29(2):334–339. doi: 10.2337/diacare.29.02.06.dc05-1242. [DOI] [PubMed] [Google Scholar]
- 63.Istenes I, Keresztes K, Hermányi Z et al. Relationship between autonomic neuropathy and hypertension—are we underestimating the problem? Diabet Med. 2008;25(7):863–866. doi: 10.1111/j.1464-5491.2008.02458.x. [DOI] [PubMed] [Google Scholar]
- 64.Astrup AS, Nielsen FS, Rossing P et al. Predictors of mortality in patients with type 2 diabetes with or without diabetic nephropathy: a follow-up study. J Hypertens. 2007;25(12):2479–2485. doi: 10.1097/HJH.0b013e3282f06428. [DOI] [PubMed] [Google Scholar]
- 65.Bilchick KC, Fetics B, Djoukeng R et al. Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs’ Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure). Am J Cardiol. 2002;90(1):24–28. doi: 10.1016/s0002-9149(02)02380-9. [DOI] [PubMed] [Google Scholar]
- 66.Batchvarov V, Hnatkova K, Ghuran A, Poloniecki J, Camm AJ, Malik M. Ventricular gradient as a risk factor in survivors of acute myocardial infarction. Pacing Clin Electrophysiol. 2003;26(1 Pt 2):373–376. doi: 10.1046/j.1460-9592.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 67.Ghuran A, Reid F, La Rovere MT et al.; ATRAMI Investigators. Heart rate turbulence-based predictors of fatal and nonfatal cardiac arrest (The Autonomic Tone and Reflexes After Myocardial Infarction substudy). Am J Cardiol. 2002;89(2):184–190. doi: 10.1016/s0002-9149(01)02198-1. [DOI] [PubMed] [Google Scholar]
- 68.Barthel P, Bauer A, Müller A et al. Reflex and tonic autonomic markers for risk stratification in patients with type 2 diabetes surviving acute myocardial infarction. Diabetes Care. 2011;34(8):1833–1837. doi: 10.2337/dc11-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao D, Cai J, Rosamond WD et al. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145(8):696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 70.Huikuri HV, Jokinen V, Syvänne M et al. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19(8):1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 71.Wackers FJT, Young LH, Inzucchi SE et al.; Detection of Ischemia in Asymptomatic Diabetics Investigators. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27(8):1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 72.Lichtman JH, Bigger JT, Jr,, Blumenthal JA et al.; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 73.Low P ed. 2nd ed. Philadelphia, PA: Lippincott-Raven Publishers; Clinical autonomic disorders: evaluation and management. 1997. [Google Scholar]
- 74.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 75.Malik M ed. Clinical guide to cardiac autonomic tests. Kluwer Academic Publishers, Dordrecht, Netherlands; 1998. [Google Scholar]
- 76.Ewing DJ, Campbell IW, Clarke BF. Assessment of cardiovascular effects in diabetic autonomic neuropathy and prognostic implications. Ann Intern Med. 1980;92(2 Pt 2):308–311. doi: 10.7326/0003-4819-92-2-308. [DOI] [PubMed] [Google Scholar]
- 77.Vinik AI, Maser RE, Ziegler D. Neuropathy: the crystal ball for cardiovascular disease? Diabetes Care. 2010;33(7):1688–1690. doi: 10.2337/dc10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabet Med. 2011;28(6):643–651. doi: 10.1111/j.1464-5491.2010.03184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105(22):2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 80.Su S, Lampert R, Zhao J et al. Pleiotropy of C-reactive protein gene polymorphisms with C-reactive protein levels and heart rate variability in healthy male twins. Am J Cardiol. 2009;104(12):1748–1754. doi: 10.1016/j.amjcard.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerstein HC, Mann JFE, Yi Q et al.; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 82.Hermans MMH, Henry R, Dekker JM et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol. 2007;18(6):1942–1952. doi: 10.1681/ASN.2006111217. [DOI] [PubMed] [Google Scholar]
- 83.Hermans MMH, Henry RMA, Dekker JM, Nijpels G, Heine RJ, Stehouwer CDA. Albuminuria, but not estimated glomerular filtration rate, is associated with maladaptive arterial remodeling: the Hoorn Study. J Hypertens. 2008;26(4):791–797. doi: 10.1097/HJH.0b013e3282f50066. [DOI] [PubMed] [Google Scholar]
- 84.Fauci AS, Braunwald E, Kasper DL et al. 17th ed. New York, NY:: McGraw-Hill Professional; Harrison’s principles of internal medicine. 2008. [Google Scholar]
- 85.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 86.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97(21):2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention (CDC). Decline in deaths from heart disease and stroke—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48(30):649–656. [PubMed] [Google Scholar]
- 88.Myerburg RJ. Scientific gaps in the prediction and prevention of sudden cardiac death. J Cardiovasc Electrophysiol. 2002;13(7):709–723. doi: 10.1046/j.1540-8167.2002.00709.x. [DOI] [PubMed] [Google Scholar]
- 89.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110(5):522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 90.Jouven X, Desnos M, Guerot C, Ducimetière P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99(15):1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 91.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330(15):1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 92.Jouven X, Lemaître RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26(20):2142–2147. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 93.Kannel WB, Cupples LA, D’Agostino RB. Sudden death risk in overt coronary heart disease: the Framingham Study. Am Heart J. 1987;113(3):799–804. doi: 10.1016/0002-8703(87)90722-8. [DOI] [PubMed] [Google Scholar]
- 94.Stecker EC, Vickers C, Waltz J et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 95.Buxton AE, Ellison KE, Kirk MM et al. Primary prevention of sudden cardiac death: trials in patients with coronary artery disease. J Interv Card Electrophysiol. 2003;9(2):203–206. doi: 10.1023/a:1026236524273. [DOI] [PubMed] [Google Scholar]
- 96.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 97.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]


