Abstract
Background
Cardiovascular autonomic neuropathy (CAN) is recognized as a significant health risk, correlating with risk of heart disease, silent myocardial ischemia or sudden cardiac death. Beta-blockers are often prescribed to minimize risk.
Objectives
In this second of two articles, the effects on parasympathetic and sympathetic activity of the alpha/beta-adrenergic blocker, Carvedilol, are compared with those of the selective beta-adrenergic blocker, Metoprolol.
Methods
Retrospective, serial autonomic nervous system test data from 147 type 2 diabetes mellitus patients from eight ambulatory clinics were analyzed. Patients were grouped according to whether a beta-blocker was (1) introduced, (2) discontinued or (3) continued without adjustment. Group 3 served as the control.
Results
Introducing Carvedilol or Metoprolol decreased heart rate and blood pressure, and discontinuing them had the opposite effect. Parasympathetic activity increased with introducing Carvedilol. Sympathetic activity increased more after discontinuing Carvedilol, suggesting better sympathetic suppression. With ongoing treatment, resting parasympathetic activity decreased with Metoprolol but increased with Carvedilol.
Conclusion
Carvedilol has a more profound effect on sympathovagal balance than Metoprolol. While both suppress sympathetic activity, only Carvedilol increases parasympathetic activity. Increased parasympathetic activity may underlie the lower mortality risk with Carvedilol.
Keywords: Beta-blocker, Cardiovascular autonomic neuropathy, Heart rate variability, Patient outcomes, Respiratory analysis, Sympathovagal imbalance
Introduction
Autonomic neuropathy (AN) has long been recognized as a significant health risk and leads to reduced quality of life (QOL), increased mortality and morbidity and increased health care costs (1-2-3). Cardiovascular autonomic neuropathy (CAN) is an end-stage condition and is characterized by structural deficits due to loss of parasympathetic or sympathetic neurons that innervate the heart and blood vessels, resulting in abnormalities in heart rate (HR) control and vascular dynamics (3). CAN is associated with multiple cardiovascular disease states, including low ejection fraction (4, 5), atrial fibrillation (6), ventricular arrhythmias (7), cardiomyopathies (8, 9), symptomatic systolic heart failure (10) and myocardial ischemia (11), as well as many of the traditional and nontraditional risk factors for heart disease (12). In patients diagnosed with diabetes and coronary artery disease, CAN is strongly associated with silent ischemia (3). Serial AN testing of a large population (774) of patients with diabetes demonstrated that 26% progressively worsened over time, despite clinical surveillance, while only 3% improved (13). A meta-analysis of 15 studies of patients diagnosed with diabetes and CAN demonstrated a relative mortality risk of 3.65 over those without CAN (3).
The disease process of AN is poorly understood by many clinicians and the early stages of disease are often overlooked. The two branches of the autonomic nervous system (ANS), parasympathetic and sympathetic (P&S), act together to maintain sympathovagal balance (SB) (14). Establishing and maintaining appropriate SB slows the progression of AN, reducing morbidity and mortality and improving outcomes (3, 15, 16). Βeta1-adrenergic agonists (Beta-blockers) are known to reduce sympathetic activity (17) and thereby alter SB. They may also increase parasympathetic activity (15). Increased parasympathetic activity is known to be cardioprotective and is associated with greater longevity in geriatric and heart disease patients (16, 18). Propranolol and Metoprolol have been studied previously for their effect on diabetic autonomic nerve dysfunction, and these drugs affect only sympathetic inhibition (19, 20). The third-generation adrenergic blocker, Carvedilol, has both beta-blocker and alpha-blocker effects. The alpha-blocker acts as a vasodilator (21), and an antioxidant and endothelin biosynthesis suppressor (22). Thus, Carvedilol has a broader affect on the ANS, which may explain why it further reduces mortality (23).
Our hypotheses are that the beta-blocker, Metoprolol, would inhibit sympathetic tone less than the combination alpha- and beta-blocker, Carvedilol. Second, we hypothesize that Carvedilol will also result in an increase in parasympathetic activity.
Methods
This is a retrospective study. Patients who had undergone serial autonomic P&S testing (ANX-3.0; ANSAR Medical Technologies, Inc., Philadelphia, PA) based on clinical decisions were enrolled. The registry consists of 147 arrhythmia-free patients diagnosed with noninsulin-dependent (type 2) diabetes mellitus from eight ambulatory clinics located in New York, Pennsylvania, New Jersey, Maryland and Virginia. The patient selection method and demographics, and the three noninvasive autonomic measurement methods used for screening, are described in the companion article (24).
Three experimental groups (EG) were established. EG1, “(+)beta-blocker,” are those patients who were beta-blocker naïve at the beginning of the study and were started on beta-blocker during the study. EG2, “(–)beta-blocker,” consists of patients who started the study period on beta-blocker and had beta-blocker discontinued during the study. EG3, “(o)beta-blocker,” the control group, are patients who were on stable doses of beta-blockers that did not change throughout the study. EG3 patients were case-matched to the study population patients with respect to comorbid conditions such as hypertension (HTN) and cardiovascular disease (CVD), as well as age, gender, height and weight. Each experimental patient in the registry performed a baseline P&S study (Test n) the day of the prescribed change to their beta-blocker. To remain in the registry, there needed to be documentation of the patient’s compliance according to grouping, for at least 3 months, after which the patient performed a follow-up P&S study (Test n+1).
Statistical analysis was performed with SPSS v14.0. All graphs consist of two curves: the broken lines represent the responses to Carvedilol, and the solid lines represent the responses to Metoprolol. In all figures, the responses are normalized to 1.0 in the baseline test to highlight the changes (Δ) in response to beta-blocker titration. The figure data present the average change (Δ) in a parameter from baseline to follow-up (Test n+1). Absolute values for the data in the figures are presented in the tables of the companion article (24).
Results
The average age, height and weight of the cohort are 61.8 ± 12 yrs, 166.7 ± 27 lb and 64.8 ± 6 in, respectively, and serial tests are separated by 4.1 (±1.2) months (24). Figure 1 displays the normalized, average hemodynamic responses from the two tests for each of the three subpopulations. Similarly, Figures 2 through 4 display the results from the three autonomic measurement methods: (1) P&S Method results, (2) spectral-domain HRV-alone results and (3) time-domain HRV-alone results, respectively.
Fig. 1 -.
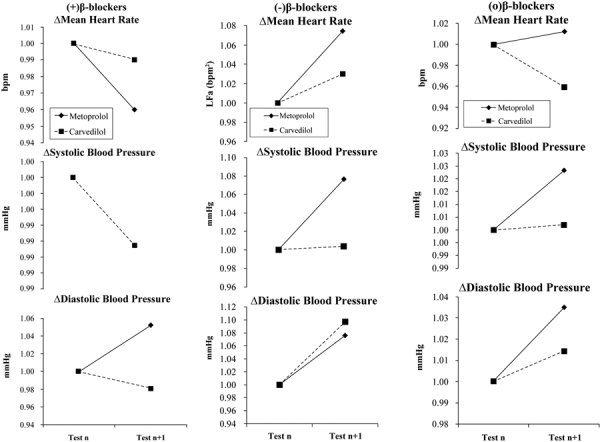
Hemodynamic responses to beta-blocker therapy at baseline and at follow-up. Therapy was changed as indicated after baseline testing. a) The naïve patient responses to the introduction of beta-blocker. b) Patients on stable beta-blocker therapy, which is then discontinued. c) Patients on stable beta-blocker therapy, which is then continued. (a) and (b) represent the experimental groups (1 and 2, respectively) and (c) represents the control group. (Please see text for details.)
Fig. 2 -.
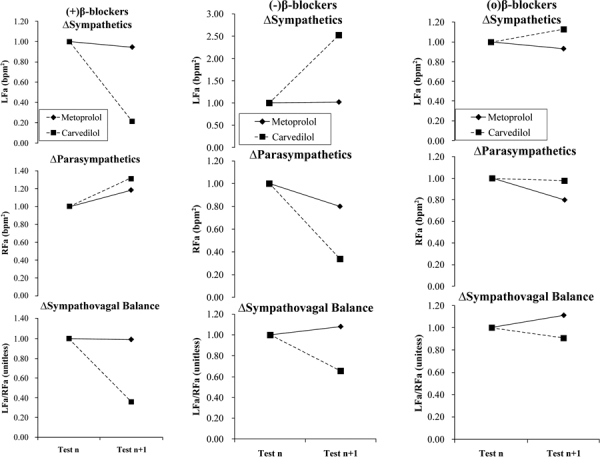
P&S responses to beta-blocker therapy at baseline and at follow-up. Therapy was changed as indicated after baseline testing. a) The naïve patient responses to the introduction of beta-blocker. b) Patients on stable beta-blocker therapy, which is then discontinued. c) Patients on stable beta-blocker therapy, which is then continued. (a) and (b) represent the experimental groups (1 and 2, respectively) and (c) represents the control group. (Please see text for details.)
In response to increasing either agent, the mean HR (mHR) and mean systolic BP (sBP) decreased. In response to increasing Metoprolol, the mHR decreases from 78 to 72 bpm and the average mHR response to increasing Carvedilol decreases from 76 to 70 bpm (both statistically significant, p<0.001). The average changes in sBP were not significant in response to increasing either agent, staying at 125 mmHg for Metoprolol and changing from 122 to 121 mmHg for Carvedilol. Only the mHR decreases are statistically significant (p<0.001). The diastolic BP (dBP) responses to either agent were not consistent. The Metoprolol-naïve subpopulation demonstrates an average increase in dBP from 73 to 74 mmHg, and the Carvedilol-naïve subpopulation demonstrates no change.
In response to discontinuing either agent, all hemodynamic measures increase. However, these increases are not clinically significant. There is a statistically significant increase in mHR in response to discontinuing Carvedilol (from 73 to 77 bpm) as compared with Metoprolol (68 to 69 bpm; p<0.001). The average sBP increase to discontinuing either agent (from 115 to 116 mmHg and 122 to 123 mmHg for Metoprolol and Carvedilol, respectively) is also statistically significant (p<0.001), but not clinically significant. The average dBP increases in response to discontinuing either agent are not significant (from 67 to 69 mmHg for Metoprolol and 69 to 70 mmHg for Carvedilol). In response to continuing either agent, none of the hemodynamic responses are significant. The normalized data (to highlight the respective changes in hemodynamic responses) are displayed in Figure 1.
From Figure 2, the results of the P&S Method show that when either beta-blocker is introduced (Fig. 2a) there is a decrease in sympathetic activity (see top graph). However, only the (greater) decrease for Carvedilol is significant (p<0.001). A significant increase in parasympathetic activity (Fig. 2a, middle) is measured (p<0.001) in response to either agent, with the average parasympathetic increase in response to Carvedilol greater than that for Metoprolol. A significant decrease in SB (Fig. 2a, bottom; p<0.010) is recorded in response to introducing either agent. The decrease in Metoprolol response is less than that for Carvedilol. For patients in whom a beta-blocker is discontinued (Fig. 2b), the opposite occurred. The average sympathetic response (Fig. 2b, top) to discontinuing either agent increases significantly (p<0.001 for Carvedilol response and p<0.010 for Metoprolol response), and the average parasympathetic responses decrease significantly (p<0.001; Fig. 2b, middle). For both P and S, the change in response to Carvedilol is greater than that for Metoprolol. The average SB responses are mixed and both are statistically significant (Fig. 2b, bottom; p<0.010). The average SB response to discontinuing Carvedilol increases, whereas the average SB response to discontinuing Metoprolol decreases. For those patients continuing beta-blocker therapy without any adjustment (Fig. 2c), there is very little change in P&S Method results: p = 0.747 for sympathetic activity, p = 0.301 for parasympathetic activity and p = 0.275 for SB, overall.
The results of the spectral domain HRV-alone analysis [low frequency (LF), high frequency [HF] and LF/HF ratio) are presented in Figure 3. The total spectral power (TSP) is presented in Figure 4. Normalized LF (LFnu) increases in response to discontinuing Carvedilol, and decreases in response to discontinuing Metoprolol (Fig. 3b, top). HF decreases significantly (Fig. 3b, middle; p<0.010) in response to Carvedilol, and increases in response to Metoprolol. LF/HF ratio decreases in response to Carvedilol, and increases in response to Metoprolol (Fig. 3b, bottom; p<0.010). TSP decreases in response to discontinuing either agent, with only the Metoprolol change being significant (Fig. 4b, top; p<0.010). These data show that average TSP changes to either agent are statistically significant (see Fig. 4, top row). In response to increasing beta-blocker, TSP increases in response to Carvedilol (p<0.001) and decreases in response to Metoprolol (p<0.010) (see Fig. 4a, top). While the absolute value of the change in TSP in response to Carvedilol is much greater than that for Metoprolol (240.9 vs −42.2 ms2, respectively), the relative changes with respect to baseline (Test n) are similar. For patients who continued beta-blocker without adjustment (Fig. 3c), Carvedilol further decreases the LF measure significantly (ΔLFnu = −0.34, p<0.010) and increases the HF measure significantly (ΔHFnu = +0.77, p<0.010), whereas only Metoprolol decreases the HF measure significantly (ΔHFnu = −0.08, p<0.010).
Fig. 3 -.
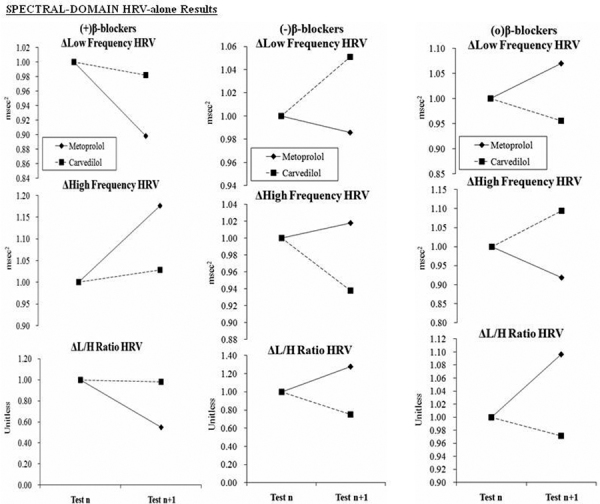
Spectral-domain HRV-alone responses to beta-blocker therapy at baseline and at follow-up. Therapy was changed as indicated after baseline testing. a) The naïve patient responses to the introduction of beta-blocker. b) Patients on stable beta-blocker therapy, which is then discontinued. c) Patients on stable beta-blocker therapy, which is then continued. (a) and (b) represent the experimental groups (1 and 2, respectively) and (c) represents the control group. (Please see text for details.)
Fig. 4 -.
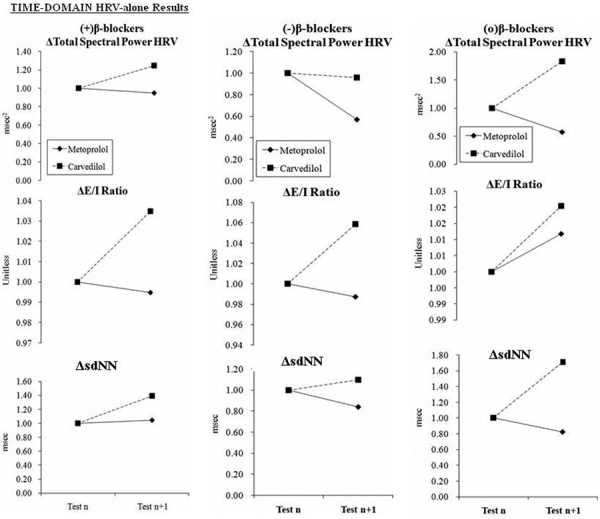
Total spectral power and time-domain HRV-alone responses to beta-blocker therapy at baseline and at follow-up. Therapy was changed as indicated after baseline testing. a) The naïve patient responses to the introduction of beta-blocker. b) Patients on stable beta-blocker therapy, which is then discontinued. c) Patients on stable beta-blocker therapy, which is then continued. (a) and (b) represent the experimental groups (1 and 2, respectively) and (c) represents the control group. (Please see text for details.)
The time-domain HRV-alone responses to the introduction of a beta-blocker (Fig. 4) were mixed and statistically significant in response to either agent (p<0.001). RangeHR (not shown graphically, see (24, Table 4)) and E/I ratio increase in response to Carvedilol and decrease in response to Metoprolol. SDNN increases significantly in response to either agent (Fig. 4a, bottom). The average response to introducing Carvedilol (p<0.001) is greater than that to Metoprolol (p<0.010). Responses to discontinuing a beta-blocker are varied. RangeHR decreases significantly in response to either agent (data not shown, see (24, Table 4); p<0.001), while the E/I ratio increase is statistically significant for Carvedilol (Fig. 4b, middle; p<0.001). The mixed sdNN responses (Fig. 4b, bottom) to discontinuing either agent are statistically significant (p<0.001 for both).
Discussion
Our study concurs with, and expands upon, previously reported effects of beta-blockers in restoration of autonomic balance (20). More specifically however, our study shows that Carvedilol, which is both an alpha- and beta-adrenergic blocker, demonstrates a greater response than Metoprolol, which is a pure beta-blocker, and demonstrates clear differences on the effects on sympathovagal balance (SB). This may be due to the fact that Carvedilol contains more adrenergic blockade as compared with Metoprolol.
Whether HRV-alone is in the form of time-domain measures or frequency-domain measures, HRV-alone is shown in this study to not be a reliable or consistent measure to differentiate parasympathetic activity independent from sympathetic activity (see the discussion in (24)).
The Effects after Continuation of Current Beta-Blocker Therapy
The subpopulation of patients who continued current beta-blocker therapy without any adjustment can be considered the control group. As shown in Figure 2c, no significant changes in average hemodynamic responses are demonstrated (note the scale of the ordinate). Similarly, as shown in Figure 3c, no significant changes in average parasympathetic or sympathetic responses are demonstrated. The changes in the P&S Method are consistent with the hemodynamic changes observed for the same group (p = 0.055). As seen in Figure 4c, the HRV-alone, spectral analysis method showed statistically significant responses to the continuation of beta-blockers. Carvedilol further decreased the LF measure (assumed to represent sympathetic tone) and increased the HF measure (assumed to represent parasympathetic tone), whereas Metoprolol decreased HF and produced no change to the LF measurements. The spectral analysis of HRV-alone changes are not consistent with the hemodynamic changes observed for the same group (p = 0.880).
The Effects after Introducing Beta-Blockers
It is known that the sympathetic nervous system exerts major effects on mHR and the baroreceptor reflex response, which in turn contributes to the regulation of BP. While beta-blockers cause decreases in mHR, sBP and sympathetic activity (see Figs. 2 and 3), the net desired effect is an overall reduction in relative sympathetic activity. The data demonstrate that, on average, Metoprolol has less of a sympatholytic effect that Carvedilol (see Fig. 2a, top). These results are corroborated by the SB responses (SB = S/P from the P&S Method) seen in Figure 3a, bottom. Although LF, HF and LF/HF ratio (Fig. 3a) responses to the introduction of Metoprolol are greater than that to Carvedilol due to overall reduction in relative sympathetic activity, a significantly greater increase in TSP (Fig. 4a, top) in response to Carvedilol with respect to Metoprolol is demonstrated. Since TSP is based on the arithmetic sum of LF and HF (25), and since the LF measure decreases, and the HF measure increases, the change in TSP indicates a relatively greater increase in the HF with Carvedilol than with Metoprolol. Traditionally, the HF measure is associated with parasympathetic activity (25). Therefore, the change in TSP would indicate a net, relative increase in parasympathetic activity. Similarly, increase in E/I ratio, rangeHR and HRV, and the greater sdNN increase in response to Carvedilol over Metoprolol is significant. These results suggest that Carvedilol not only reduces overall sympathetic dominance, but concomitantly increases parasympathetic activity, while Metoprolol only reduces relative sympathetic dominance.
The Effects after Discontinuing Beta-Blockers
After weaning from beta blockers, an increase in sympathetic activity and decrease in parasympathetic activity is expected. Increased sympathetic activity is known to reduce HRV. Decreased parasympathetic activity is known to further reduce HRV. Increases in mHR, BP and sympathetic activity increase significantly more after weaning from Carvedilol than weaning from Metoprolol. Significant increases in the SB response to withdrawing Carvedilol indicate a shift in the autonomic balance toward a more sympathetic dominant state, as our results reflect. The spectral domain HRV-alone (LF and HF) responses to discontinuing beta-blockers are mixed. HF decreases in response to discontinuing either agent (Fig. 4b, middle). This is consistent with the respective decrease in parasympathetic activity. LF increases in response to discontinuing Carvedilol, which is consistent with a significant increase in sympathetic activity as compared with the decrease in parasympathetic activity. However, LF decreases in response to discontinuing Metoprolol. This dichotomy may also be due to the dual alpha- and beta-adrenergic activity of Carvedilol having greater sympathetic affect. Given that LF is defined as a measure of sympathetic activity as modulated by parasympathetic activity (25), the effect of discontinuing Metoprolol on the LF measure may indicate a weaker sympathetic rebound and a relatively stronger decrease in parasympathetic activity. In other words, SB (the measure of relative sympathetic to parasympathetic activity) increases when Carvedilol is discontinued and decreases when Metoprolol is discontinued. This theory is supported by the absolute P&S values of these changes (see (24), Table 2).
Conclusion
Noninvasive measures of hemodynamic and autonomic responses to changes in beta-blocker therapy were collected. Overall, the hemodynamic results are consistent with the known effects of changes in specific beta-blocker vs alpha- and beta-blocker therapy (21-22-23). The combination of alpha- and beta-blocker decreases sympathetic tone and increases parasympathetic tone, whereas a pure beta-blocker precipitates a reflex increase in alpha-adrenergic activity, leading to less of an increase in parasympathetic tone. The P&S Method results are consistent with the hemodynamic results and with the expected autonomic effects of changes in beta-blocker therapy known to underlie the hemodynamic responses (13). As shown, HRV-alone measures are not always consistent with the hemodynamic responses, and are only consistent with expected variations in HR and perhaps total autonomic activity.
Adrenergic-blockade is shown to reduce sympathetic activity. By providing both alpha- and beta-blockade, Carvedilol has a more profound effect in reducing sympathetic activity as compared with the pure beta-blockade of Metoprolol. Carvedilol also blocks a reflexive increase in alpha-adrenergic activity (see Fig. 5). Our results also suggest that Carvedilol not only reduces overall sympathetic dominance, but concomitantly increases parasympathetic activity. Adrenergic antagonists may restore autonomic balance and decrease functional disturbances that can lead to early AN (26), which leads to increased morbidity and earlier mortality (3, 27). A simple measure of balance (SB) done prospectively may add significantly to the ability to identify and predict people at risk, and the adoption of multifactorial preventive measures.
Fig. 5 -.
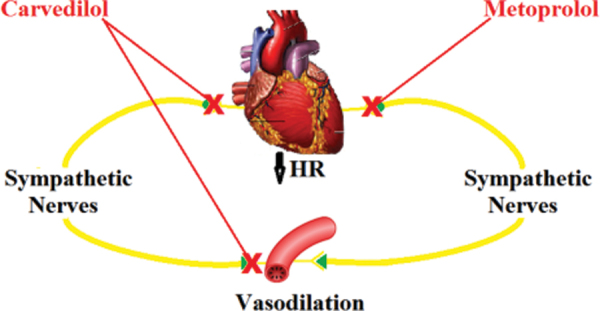
A diagram illustrating the effects of Carvedilol and Metoprolol. Both agents block sympathetic input to the heart, reducing cardiac sympathetic tone, thereby lowering HR. Carvedilol also blocks sympathetic input to the vasculature, reducing sympathetic tone in the blood vessels, leading to vasodilation.
Importance of Restoration of Sympathovagal Balance on Morbidity and Mortality
The importance of autonomic assessment is emphasized by the current reports from the DIAD study (28) and the ACCORD study (29, 30). DIAD demonstrates that abnormal HRV is associated with a prediction of greater increased risk of major adverse cardiovascular events (MACEs). The risk is greater than all traditional risk factors, including perfusion scanning (28). The development of AD is a multifactorial process, and maintaining SB may improve longevity and decrease morbidity and mortality. Thus, it is likely that combination therapies directed at the various components of the pathogenic pathway will be needed to reduce the development of autonomic nerve fiber dysfunction. With intensification of glycemic control, ACCORD demonstrates a 22% increase in sudden deaths associated with AD. Perhaps autonomic screening before embarking on intensive glycemic control is warranted. Establishing and maintaining normal parasympathetic and sympathetic balance may reduce morbidity risk and minimize mortality risk.
Abbreviations
- Δ
"Delta,” designates a change in the parameter it precedes
- (+)beta-blocker
indicates a beta-blocker was introduced
- (−)beta-blocker
indicates a beta-blocker was discontinued
- (o)beta-blocker
indicates no change in beta-blocker dosing from baseline
- AD
Autonomic dysfunction
- AN
Autonomic neuropathy
- ANS
Autonomic nervous system
- BP
Blood pressure (mmHg)
- bpm
beats per minute
- bpm2
beats per minute squared
- CAN
Cardiovascular autonomic neuropathy
- CVD
Cardiovascular disease
- dBP
diastolic blood pressure (mmHg).
- E/I ratio
Exhalation – inhalation ratio (unitless)
- FFT
Fast Fourier transform
- HF
High frequency (ms2)
- HFnu
normalized high frequency (normalized to TSP, unitless)
- HR
Heart rate (bpm)
- HRV
Heart rate variability
- HTN
Hypertension
- LF
Low frequency (ms2)
- LFa
Low frequency area (bpm2)
- LFnu
normalized low frequency (normalized to TSP, unitless)
- MACE
Major adverse cardiovascular events
- mHR
mean heart rate (bpm)
- mmHg
millimeters of mercury
- ms2
milliseconds squared
- P&S
Parasympathetic and sympathetic
- QOL
Quality of life
- rangeHR
range heart rate (bpm)
- RFa
Respiratory frequency area (bpm2)
- SB
Sympathovagal balance (= S/P, unitless)
- sBP
systolic BP (mmHg)
- sdNN
standard deviation of the beat-to-beat intervals (NN, ms)
- S/P
Sympathetic/parasympathetic
- Test n
the baseline test
- Test n+1
the follow-up test
- TSP
Total spectral power (= LF+HF, ms2)
Disclosures
Financial support: None.
Conflict of interest: Drs. Bloom and Vinik are unpaid advisors to ANSAR. Dr. Colombo is Medical Director of ANSAR.
References
- 1.Low P. Assessment: clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46(3):873–880. [PubMed] [Google Scholar]
- 2.Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Semin Neurol. 2003;23(4):365–372. doi: 10.1055/s-2004-817720. [DOI] [PubMed] [Google Scholar]
- 3.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 4.Bullinga JR, Alharethi R, Schram MS, Bristow MR, Gilbert EM. Changes in heart rate variability are correlated to hemodynamic improvement with chronic CARVEDILOL therapy in heart failure. J Card Fail. 2005;11(9):693–699. doi: 10.1016/j.cardfail.2005.06.435. [DOI] [PubMed] [Google Scholar]
- 5.Fantoni C, Raffa S, Regoli F et al. Cardiac resynchronization therapy improves heart rate profile and heart rate variability of patients with moderate to severe heart failure. J Am Coll Cardiol. 2005;46(10):1875–1882. doi: 10.1016/j.jacc.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 6.Chen PS, Chou CC, Tan AY et al. The mechanisms of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(s3 Suppl 3):S2–S7. doi: 10.1111/j.1540-8167.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 7.Copie X, Lamaison D, Salvador M et al.; VALID Investigators. Heart rate variability before ventricular arrhythmias in patients with coronary artery disease and an implantable cardioverter defibrillator. Ann Noninvasive Electrocardiol. 2003;8(3):179–184. doi: 10.1046/j.1542-474X.2003.08302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alter P, Grimm W, Vollrath A, Czerny F, Maisch B. Heart rate variability in patients with cardiac hypertrophy—relation to left ventricular mass and etiology. Am Heart J. 2006;151(4):829–836. doi: 10.1016/j.ahj.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Debono M, Cachia E. The impact of cardiovascular autonomic neuropathy in diabetes: is it associated with left ventricular dysfunction? Auton Neurosci. 2007;132(1-2):1–7. doi: 10.1016/j.autneu.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Just H. Peripheral adaptations in congestive heart failure: a review. Am J Med. 1991;90(5 5B):23S–26S. doi: 10.1016/0002-9343(91)90269-4. [DOI] [PubMed] [Google Scholar]
- 11.Manfrini O, Morgagni G, Pizzi C, Fontana F, Bugiardini R. Changes in autonomic nervous system activity: spontaneous versus balloon-induced myocardial ischaemia. Eur Heart J. 2004;25(17):1502–1508. doi: 10.1016/j.ehj.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 12.DePace NL, Mears JP, Yayac M, Colombo J. Cardiac autonomic testing and diagnosing heart disease, A clinical perspective. Accepted, Heart Int. 2014 doi: 10.5301/heartint.5000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8(5):491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 14.Low PA, Engstrom JW. Harrison’s principles of internal medicine. 16th ed. McGraw-Hill, USA: Disorders of the autonomic nervous system. In: 2006:2428-2434. [Google Scholar]
- 15.Goldsmith RL, Bloomfield DM, Rosenwinkel ET. Exercise and autonomic function. Coron Artery Dis. 2000;11(2):129–135. doi: 10.1097/00019501-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31(3):593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 17.Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA. 2002;287(7):883–889. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 18.Waheed A, Ali MA, Jurivich DA et al. Geriatric Medicine Society Meeting. Chicago, IL: Gender differences in longevity and autonomic function. 2006. [Google Scholar]
- 19.Ebbehøj E, Poulsen PL, Hansen KW, Knudsen ST, Mølgaard H, Mogensen CE. Effects on heart rate variability of metoprolol supplementary to ongoing ACE-inhibitor treatment in type I diabetic patients with abnormal albuminuria. Diabetologia. 2002;45(7):965–975. doi: 10.1007/s00125-002-0869-7. [DOI] [PubMed] [Google Scholar]
- 20.Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. Am J Cardiol. 2003;91(2):137–142. doi: 10.1016/s0002-9149(02)03098-9. [DOI] [PubMed] [Google Scholar]
- 21.Kveiborg B, Major-Petersen A, Christiansen B, Torp-Pedersen C. Carvedilol in the treatment of chronic heart failure: lessons from the Carvedilol Or Metoprolol European Trial. Vasc Health Risk Manag. 2007;3(1):31–37. [PMC free article] [PubMed] [Google Scholar]
- 22.Arumanayagam M, Chan S, Tong S, Sanderson JE. Antioxidant properties of carvedilol and metoprolol in heart failure: a double-blind randomized controlled trial. J Cardiovasc Pharmacol. 2001;37(1):48–54. doi: 10.1097/00005344-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Remme WJ, Cleland JG, Erhardt L et al. Effect of carvedilol and metoprolol on the mode of death in patients with heart failure. Eur J Heart Fail. 2007;9(11):1128–1135. doi: 10.1016/j.ejheart.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Vinik AI, Bloom HL, Colombo J. Differential effects of adrenergic antagonists (Carvedilol vs. Metoprolol) on parasympathetic and sympathetic activity: a comparison of measures. Accepted, Heart Int. 2014 [PMC free article] [PubMed] [Google Scholar]
- 25.Malik M. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 26.Pfeifer MA, Schumer MP. Clinical trials of diabetic neuropathy: past, present, and future. Diabetes. 1995;44(12):1355–1361. doi: 10.2337/diab.44.12.1355. [DOI] [PubMed] [Google Scholar]
- 27.Stevens MJ, Raffel DM, Allman KC et al. Cardiac sympathetic dysinnervation in diabetes: implications for enhanced cardiovascular risk. Circulation. 1998;98(10):961–968. doi: 10.1161/01.cir.98.10.961. [DOI] [PubMed] [Google Scholar]
- 28.Young LH, Wackers FJ, Chyun DA et al.; DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301(15):1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pop-Busui R, Evans GW, Gerstein HC et al.; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(7):1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calles-Escandón J, Lovato LC, Simons-Morton DG et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(4):721–727. doi: 10.2337/dc09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]


