Abstract
Background
Cardiovascular autonomic neuropathy (CAN) is recognized as a significant health risk. Specific and sensitive measures of CAN are needed for early identification and treatment to avoid complications, preferably in the preclinical state.
Objectives
In this first of two articles, the patient cohort is described and two measures of autonomic function are reviewed: the traditional heart rate variability (HRV)-alone method and the newer parasympathetic and sympathetic (P&S) Method. These systems are then evaluated against known effects of the alpha/beta-adrenergic blocker, Carvedilol, and the selective beta-adrenergic blocker, Metoprolol, on P&S activity.
Methods
Serial autonomic nervous system test data from 147 type 2 diabetes mellitus patients from eight ambulatory clinics were analyzed. Patients were grouped according to whether a beta-blocker was (1) introduced, (2) discontinued or (3) continued without adjustment. Group 3 served as the control. HRV-alone parameters are computed according to standards. The P&S Method, which is a time–frequency analyses of concurrent respiratory activity and HRV, is elucidated, as developed at MIT and Harvard Medical School (1981).
Results
The HRV-alone demonstrated that introducing either medication increased low frequency (msec2) and standard deviation of the beat-to-beat (N-N) interval (msec), as expected. The other HRV parameter responses were not consistent with expectations. Similar inconsistencies occurred when either medication was discontinued. The P&S Method demonstrated that introducing or discontinuing either agent decreased or increased sympathetic activity, respectively, according to expectations. With ongoing treatment, resting parasympathetic activity decreased with Metoprolol but increased with Carvedilol.
Conclusion
Autonomic assessment fidelity was significantly higher with the P&S Method as validated by comparison with previously known physiology of the cardiovascular system.
Keywords: Beta-blocker, Cardiac autonomic neuropathy, Heart rate variability, Patient outcomes, Respiratory analysis, Sympathovagal imbalance
Introduction
Autonomic neuropathy (AN) is recognized as a significant health risk and leads to reduced quality of life (QOL), increased mortality and morbidity and increased health care costs (1-2-3-4). Cardiovascular autonomic neuropathy (CAN) is an end stage of AN with severe repercussions. CAN is characterized by structural deficits due to loss of parasympathetic or sympathetic neurons that innervate the heart and blood vessels, resulting in abnormalities in heart rate (HR) control and vascular dynamics (4). We chose diabetic patients based on the well-defined, preclinical state to CAN known as diabetic autonomic neuropathy (DAN) (4). Early identification and treatment of CAN is known to slow the progression of autonomic dysfunction (AD).
The disease process of AN is poorly understood by many clinicians, and the early stages of the disease are often overlooked. AN is not a single entity. It is a failure of normal homeostasis of the parasympathetic and sympathetic (P&S) nervous systems. The P&S act together to maintain sympathovagal balance (SB) (5). AD marked by P&S imbalance prior to end-organ dysfunction is asymptomatic and precedes symptomatic CAN. Thus, AN can present in multiple ways. Until recently, the ability to break down AN into its two components (parasympathetic and sympathetic) was not available. New technology enables independent, simultaneous measures of P&S activity, specifying SB, providing a more precise target for treatment prior to end-stage symptom appearance. Establishing and maintaining appropriate SB slows the progression of AN, reducing morbidity and mortality, and improving outcomes (4, 6, 7). Therefore, diagnosis of AD prior to the onset of CAN and the advent of end-stage structural autonomic nerve damage is critical. Improved methods of detecting DAN, or advanced AD in nondiabetics, improves the chances of restoring and maintaining autonomic balance (SB (5)). A common means of treating AN (as characterized by sympathetic excess) is through sympatholytic therapy, including beta-blockers, such as Carvedilol or Metoprolol. Restoring SB is known to improve QOL, decrease mortality and morbidity and thereby decrease health care costs (7). We chose to evaluate the P&S Method because it enables independent, simultaneous measures of P&S activity and these more sensitive and specific indices of DAN and CAN (4).
Our hypothesis is that the P&S Method is more consistent with known hemodynamic and physiologic responses to beta-blockade, as represented by Carvedilol and Metoprolol, than heart rate variability (HRV) alone.
Methods
This is a retrospective study. Patients undergoing serial autonomic (P&S) testing (ANX-3.0; ANSAR Medical Technologies, Inc., Philadelphia, PA) based on best clinical judgment were asked to participate. Patients were free of diagnosis of arrhythmia at baseline and had no arrhythmia during testing. They were all diagnosed with noninsulin-dependent (type 2) diabetes mellitus and came from eight ambulatory clinics located in New York, Pennsylvania, New Jersey, Maryland and Virginia (demographics Tab. I). Patients were first screened for changes in medications other than beta-blockers. All patients who were stable on all other medications and did not have any other medication changed were enrolled and assigned to three prespecified groups. Experimental Group 1 are those patients who were beta-blocker naïve at the beginning of the study and were started on beta-blocker during the study. This group is labeled as “(+)beta-blocker.” Experimental Group 2 consists of patients who started the study period on beta-blocker and had beta-blocker discontinued during the study. This group is labeled “(−)beta-blocker.” A third, control, group are patients who were on stable doses of beta-blockers that did not change throughout the study. This group is labeled “(o)beta-blocker.” These patients were case-matched to the study population patients with respect to comorbid conditions such as hypertension (HTN) and cardiovascular disease (CVD), as well as age, gender, height and weight. Each experimental patient in the registry had a baseline P&S study (Test n) the day of the prescribed change to their beta-blocker. To remain in the registry, the patient was compliant with doctor's orders, according to grouping, for at least 3 months, after which the patient performed a follow-up P&S study (Test n+1). Clinical results are reported in a companion article (8). The final registry consists of 147 patients.
Table I -.
Patient cohort demographics
| Demographics | Cohort | Group 1 (+) | Group 2 (−) | Controls (o) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Carvedilol | Metoprolol | Carvedilol | Metoprolol | Carvedilol | Metoprolol | Carvedilol | Metoprolol | ||
| The patients are diagnosed with noninsulin-dependent diabetes, and are arrhythmia free. Three subgroups include patients for whom a single beta-blocker was introduced (+), discontinued (−) or not changed (o) immediately following a baseline study. The patients were assessed again an average of 4.1 months later upon follow-up. The beta-blockers studied were Carvedilol or Metoprolol. Mean HR (bpm) changes are abbreviated as “ΔmHR.” Systolic and diastolic BP (mmHg) changes are abbreviated as “ΔsBP” and “ΔdBP,” respectively. Average values are included with standard deviations, indicated with the “±” symbol. See text for discussion. | ||||||||||
| Total N | 147 | 58 | 89 | 30 | 39 | 12 | 36 | 16 | 14 | 0.1519 |
| #Female | 89 60.5% | 29 50.0% | 58 65.2% | 19 63.3% | 26 66.7% | 8 66.7% | 27 75.0% | 9 55.0% | 8 58.6% | 0.0037 |
| Mean age (yrs) | 61.8 ± 12.6 | 57.6 ± 13.0 | 66.7 ± 14.0 | 51.7 ± 7.4 | 67.3 ± 12.2 | 57.5 ± 8.0 | 68.3 ± 11.3 | 63.7 ± 8.5 | 64.4 ± 13.1 | 0.0882 |
| Height (in) | 64.8 ± 6.4 | 64.3 ± 11.7 | 65.3 ± 12.1 | 65.8 ± 9.2 | 67.4 ± 10.8 | 63.6 ± 12.3 | 64.6 ±12.3 | 63.3 ± 13.1 | 64.0 ± 14.8 | 0.0288 |
| Weight (#) | 166.7 ± 27.9 | 173.3 ± 26.6 | 160.0 ± 21.8 | 176.8 ± 17.9 | 177.9 ± 21.6 | 172.9 ± 16.9 | 152.4 ± 25.9 | 170.3 ± 18.0 | 149.8 ± 23.6 | 0.1031 |
| HTN | 38 25.9% | 12 20.7% | 25 28.1% | 10 33.3% | 13 33.3% | 2 16.7% | 10 27.8% | 5 31.3% | 6 42.9% | 0.0973 |
| CVD | 29 19.7% | 15 25.9% | 22 24.7% | 5 16.7% | 7 17.9% | 6 50.0% | 9 25.0% | 4 25.0% | 6 42.9% | 0.0099 |
| Arrhythmics | 36 24.5% | 15 25.9% | 25 28.1% | 8 26.7% | 11 28.2% | 4 33.3% | 10 27.8% | 5 31.3% | 6 42.9% | 0.0594 |
| sBP (mmHg) | 125.2 ± 6.9 | 125.5 ± 19.1 | 123.2 ± 24.7 | 129.3 ± 16.7 | 125.8 ± 20.7 | 122.3 ± 19.9 | 120.7 ± 26.3 | 125.0 ± 20.6 | 123.3 ± 22.3 | 0.0337 |
| dBP (mmHg) | 69.4 ± 13.4 | 71.2 ± 12.4 | 68.4 ± 14.5 | 74.0 ± 9.9 | 73.3 ± 17.9 | 69.8 ± 4.6 | 67.3 ± 16.7 | 70.0 ± 19.7 | 64.5 ± 10.5 | 0.0893 |
| mHR (bpm) | 73.3 ± 4.7 | 72.1 ± 9.0 | 67.4 ± 10.0 | 75.4 ± 11.7 | 71.9 ± 13.4 | 73.9 ± 13.9 | 68.1 ± 9.3 | 67.0 ± 12.6 | 62.0 ± 13.4 | 0.0090 |
Statistical analysis was performed with SPSS v14.0. All graphs consist of two curves: the broken lines represent the responses to Carvedilol and the solid lines represent the responses to Metoprolol. Also in all figures, the responses are normalized to 1.0 in the baseline test to highlight the changes (Δ) in response to beta-blocker titration. The table and figure data present the average change (Δ) in a parameter from baseline to follow-up (Test n+1).
AD, P&S Method, and HRV-alone measurements
In this study, three noninvasive methods were employed to measure autonomic nerve function: traditional frequency-domain HRV (9), traditional time-domain HRV (9) and the P&S Method. The P&S Method includes time–frequency analysis of respiratory activity (RA) simultaneous with time–frequency analysis of concurrent HRV. The RA signal and the heart beat interval (HBI, underlying HRV analysis) signal are the two-component, independent variables of respiratory and cardiac function. In this way, the P&S Method provides two independent measures, quantifying both P&S contributors to autonomic function (9-10-11-12-13-14-15-16). The traditional HRV-alone measures include only an analysis of the HBI signal. This is only one independent measure quantifying only gross autonomic activity, and requiring assumptions to approximate P&S function.
The frequency-domain components are dependent parameters, computed via fast Fourier transform (FFT) of the time domain data, and produce two primary measures: low frequency (LF in msec2) and high frequency (HF in msec2). From these, two other parameters are computed: LF/HF ratio (unitless) and total spectral power (TSP=LF+HF, in msec2). LF is a mix of both sympathetic and parasympathetic activity (9). HF is modulated almost exclusively by parasympathetic activity, assuming adequate respiratory frequency (9). The LF/HF ratio is considered a better measure of changes in sympathetic activity, such as during postural change (9). The time-domain indices are the exhalation to inhalation (E/I) ratio (unitless), the standard deviation of the beat-to-beat interval (sdNN, in msec) and rangeHR (= greatest HBI – least HBI, in msec) (9). The E/I ratio is computed as the ratio of the HBI at peak exhalation divided by the HBI at peak inhalation (9), where higher variability indicates more parasympathetic activity. The sdNN is computed as the standard deviation of all of the HBI from the electrocardiogram (EKG) over the time period of observation, and represents sympathetic activity as modulated by parasympathetic activity (9).
RA is a measure of autonomic activity that provides an independent indication of vagal nerve (parasympathetic) activation as it affects HR, in the form of respiratory sinus arrhythmia. By adding independent analysis of RA to independent analysis of HRV, two distinct (mathematically independent) measures of autonomic activity are included. This satisfies the fundamental mathematical requirement of two independent measures to fully characterize a system with two distinct parameters. The ANX-3.0 (ANSAR Medical Technologies, Inc., Philadelphia, PA) has US Food and Drug Administration market clearance. It includes software that computes both P&S activity using the P&S Method.
Results
Table I presents the cohort at baseline testing. The average age, height and weight of the cohort are 61.8 ± 12 yrs, 166.7 ± 27 lb and 64.8 ± 6 in, respectively. Females accounted for 60.5% of the cohort. The prominent comorbidities are HTN (25.9%), CVD (19.7%) and arrhythmia (primarily atrial fibrillation, 24.5%). Serial tests are separated by 4.1 (±1.2) months (Tab. I).
Figure 1 displays the normalized, average hemodynamic changes for the subpopulations in response to change in beta-blocker therapy. In response to increasing either agent, the average mean HR (mHR) and systolic BP (sBP) decrease. In response to (+)Metoprolol, the average mHR decreases from 78 to 72 bpm. The average mHR response to (+)Carvedilol decreases from 76 to 70 bpm (both are statistically significant, p<0.001). The average changes in sBP were not significant in response to increasing either agent, staying at 125 mmHg for Metoprolol and changing from 122 to 121 mmHg for Carvedilol. The diastolic BP (dBP) responses to either agent are not consistent. The Metoprolol-naïve subpopulation demonstrates an average increase in dBP from 73 to 74 mmHg, and the Carvedilol-naïve subpopulation demonstrates no change. None of these values are clinically significant.
Fig. 1 -.
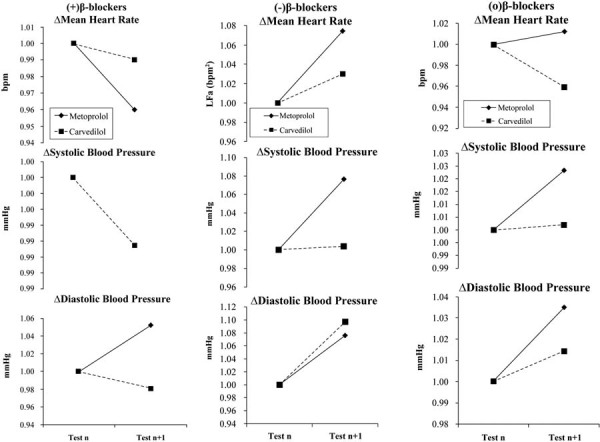
Hemodynamic responses to beta-blocker therapy at baseline and at follow-up. Therapy was changed as indicated after baseline testing. a) The naïve patient responses to the introduction of beta-blocker. b) Patients on stable beta-blocker therapy which was then discontinued. c) Patients on stable beta-blocker therapy which was then continued. (a) and (b) represent the experimental groups (1 and 2, respectively) and (c) represents the control group. (Please see text for details.)
In response to discontinuing either agent, all hemodynamic measures increase (Fig. 1, normalized data). However, these increases are not clinically significant. There is a statistically significant increase in mHR in response to removing (−)Carvedilol (73 to 77 bpm; p<0.001) as compared to removing (−)Metoprolol (68 to 69 bpm; p<0.001). The average sBP increase to discontinuing either agent (from 115 to 116 mmHg and 122 to 123 mmHg for Metoprolol and Carvedilol, respectively) is also statistically significant (p<0.001), but not clinically significant. The average dBP increases in response to discontinuing either agent are not significant. In response to continuing either agent, none of the hemodynamic responses are significant.
From Table 2, the P&S Method results show that when either beta-blocker is introduced, there is a decrease in sympathetic activity (ΔS): (+)Carvedilol = −3.67 bpm2 and (+)Metoprolol = −0.04 bpm2. Only the decrease for Carvedilol is significant (p<0.001). A significant increase in parasympathetic activity (ΔP: (+)Carvedilol = 0.99 bpm2 and (+)Metoprolol = 0.29 bpm2) is measured (p<0.001) in response to either agent, with the average parasympathetic increase in response to Carvedilol greater than that for Metoprolol. A significant decrease in SB, (+)Carvedilol = −0.40 and (+)Metoprolol = −0.20 (p<0.010), is recorded in response to introducing either agent. When discontinuing a beta-blocker, the opposite responses were documented. Sympathetic responses to discontinuing either agent increase significantly: (−)Carvedilol = 2.07 bpm2 (p<0.001) and (−)Metoprolol = 0.28 bpm2 (p<0.010). Parasympathetic responses decrease significantly (p<0.001 for both (−)Carvedilol = −0.76 bpm2 and (−)Metoprolol = −0.47 bpm2). For both P&S, the change in response to Carvedilol is greater than that for Metoprolol. The average SB responses are mixed and both are statistically significant (p<0.010 for both (−)Carvedilol = 0.77 and (−)Metoprolol = −1.15). The average SB response to (−)Carvedilol increases and that to (−)Metoprolol decreases. For those patients continuing beta-blocker therapy without any adjustment, there is very little change in P&S Method results: p = 0.747 for sympathetic activity, p = 0.301 for parasympathetic activity and p = 0.275 for SB, overall (see Tab. II).
Table II -.
Average changes in P&S measures, sympathetic (S, in bpm2), parasympathetic (P, in bpm2) and sympathovagal balance (SB, unitless) responses, for each subgroup and agent
| Change | ΔS (bpm2) | ΔP (bpm2) | ΔSB (unitless) |
|---|---|---|---|
| Significance of changes are marked (*p<0.010 or **p<0.001). See Table I for more details. See text for discussion. | |||
| From Table II, the results of the P&S M. | |||
| (+)Carvedilol | −3.67** | 0.99** | −0.40* |
| (+)Metoprolol | −0.04 | 0.29** | −0.20* |
| (−)Carvedilol | 2.07** | −0.76** | 0.77* |
| (−)Metoprolol | 0.28* | −0.47** | −1.15* |
| (o)Carvedilol | 0.05 | 0.00 | −0.28 |
| (o)Metoprolol | −0.04 | 0.09 | 0.15 |
LF (Tab. III) decreases in response to (+)Carvedilol (−1.01 msec2) and (+)Metoprolol (−2.27 msec2). Neither are significant. HF increases in response to (+)Carvedilol (1.27 msec2) and (+)Metoprolol (2.11 msec2). Neither are significant. LF/HF decreases in response to (+)Carvedilol (−0.06 msec2) and (+)Metoprolol (−1.61 msec2). Only the change in LF/HF to Metoprolol is significant: p<0.010. Changes in TSP are significant: (+)Carvedilol = 240.9 msec2 (p<0.001) and (+)Metoprolol = −42.2 msec2 (p<0.010). LF increases in response to (−)Carvedilol (2.80 msec2, not significant), and decreases in response to (−)Metoprolol (−0.80 msec2, not significant). HF decreases (−1.20 msec2; p<0.010) in response to (−)Carvedilol, and increases in response to (−)Metoprolol (−1.20 msec2; not significant). LF/HF decreases in response to (−) Carvedilol (−0.55, p<0.010), and increases in response to (−)Metoprolol (0.58; p<0.010). TSP decreases in response to (−)Carvedilol (−32.6 msec2) and (−)Metoprolol (−658.7 msec2). Only the TSP response to (−)Metoprolol is significant (p<0.010). For patients who continued beta-blocker without adjustment, (o)Carvedilol further decreases the LF measure (−0.34 msec2, p<0.010) and (o)Metoprolol increases LFnu (0.40 msec2, not significant). (o)Carvedilol increases the HF measure (0.77 msec2, p<0.010), whereas (o)Metoprolol decreases the HF measure (0.68, p<0.010). There is no significant change in LF/HF ratio in response to continuing either (o)Carvedilol (−0.08) or (o)Metoprolol (0.16). The TSP responses to (o)Carvedilol (116.5 msec2) and (o)Metoprolol (−141.5 msec2) are inconsistent. Neither are significant.
TABLE III -.
Average changes in spectral-domain HRV-alone measures, including low frequency (LF, in msec2), high frequency (HF, in msec2), ratio (LF/HF, unitless), and total spectral power (TSP=LF+HF, in msec2), for each subgroup and agent
| Change | ΔLFnu (msec2) | ΔHFnu (msec2) | ΔLF/HF (unitless) | ΔTSP (msec2) |
|---|---|---|---|---|
| The LF and HF are normalized values indicated by “nu.” Significance of changes are marked (*p<0.010 or **p<0.001). See Table I for more details. See text for discussion. | ||||
| (+)Carvedilol | −1.01 | 1.27 | −0.06 | 240.9** |
| (+)Metoprolol | −2.27 | 2.11 | −1.61* | −42.2* |
| (−)Carvedilol | 2.80 | −1.20* | −0.55* | −32.6 |
| (−)Metoprolol | −0.80 | 0.46 | 0.58* | −658.7* |
| (o)Carvedilol | −0.34* | 0.77* | −0.08 | 116.5 |
| (o)Metoprolol | 0.40 | −0.68* | 0.16 | −141.5 |
RangeHR (Tab. IV) increases in response to (+)Carvedilol (1.92 bpm) and decreases in response to (+)Metoprolol (−2.55 bpm). Both are significant at p<0.001. E/I ratio increases in response to (+)Carvedilol (0.04, p<0.001) and decreases in response to (+)Metoprolol (−0.01, not significant). SdNN increases in response to (+)Carvedilol (12.66 msec2) and (+)Metoprolol (1.54 msec2). Both are significant at p<0.001 and p<0.010, respectively. RangeHR decreases in response to (−)Carvedilol (−0.20 bpm, p<0.001), and (−)Metoprolol (−3.49 bpm, p<0.001). E/I ratio increases in response to (−)Carvedilol (0.07; p<0.001) and decreases in response to (−)Metoprolol (−0.01; not significant). SdNN increases in response to (−)Carvedilol (0.76 msec, p<0.001), and decreases in response to (−)Metoprolol (−0.60 msec; p<0.001). For patients who continued beta-blocker without adjustment, both agents further decrease rangeHR: (o)Carvedilol = −0.35 bpm and (o)Metoprolol = −0.75 bpm. Neither are significant. Continuing beta-blocker increases E/I ratio, but not significantly: (o)Carvedilol = 0.02 and (o)Metoprolol = 0.01. SdNN increase with continued Carvedilol therapy (12.25 msec, p<0.010) and decreases with continued Metoprolol therapy (−4.50 msec, not significant).
TABLE IV -.
Average changes in time-domain HRV-alone measures, including range of HRV (rangeHR, in msec), exhalation over inhalation ratio (E/I ratio, unitless) and standard deviation of the beat-to-beat heart rate (sdNN, in msec), for each subgroup and agent
| Change | Δrange HR (bpm) | ΔE/I ratio (unitless) | ΔsdNN (msec) |
|---|---|---|---|
| Significance of changes are marked (*p<0.010 or **<0.001). See Table I for more details. See text for discussion. | |||
| (+)Carvedilol | 1.92** | 0.04** | 12.66** |
| (+)Metoprolol | −2.55** | −0.01 | 1.54* |
| (−)Carvedilol | −0.20** | 0.07** | 0.76** |
| (−)Metoprolol | −3.49** | −0.01 | −0.60** |
| (o)Carvedilol | −0.35 | 0.02 | 12.25* |
| (o)Metoprolol | −0.75 | 0.01 | −4.50 |
Discussion
Carvedilol and Metoprolol, as adrenergic antagonists, are sympatholytics. Thus, they are expected to reduce HR and BP by reducing sympathetic activity. They may also increase parasympathetic activity. The P&S Method claims direct measures of sympathetic and parasympathetic activity, with SB a precise measure of the relative amounts of P&S activity. The HRV-alone measures in chronic disease patients (such as heart failure patients or patients with diabetes or HTN) assume the parasympathetics are negligible as compared with the sympathetics (in other words, sympathetic activity is significantly greater than parasympathetic activity); thus, LF, LF/HF ratio, TSP, rangeHR and sdNN are sympathetic measures, and HF and E/I ratio are parasympathetic measures.
Once the patient responds to a sympatholytic, however, this assumption is no longer valid (because sympatholytics reduce sympathetic activity and it may no longer be significantly greater than parasympathetic activity). Therefore, there is no accurate evaluation for sympathetic activity with HRV-alone measures, especially from follow-up testing. This is a major reason for the poor correlation with expected outcomes using HRV-alone indices as a follow-up measure of an individual patient’s response to adrenergic blockade. Our clinical experience demonstrates the same is true for cholinergic antagonists. It is known that the LF, sdNN and other assumed sympathetic measures of HRV are dependent measures of HRV (dependent in the mathematical sense) and represent sympathetic activity as modulated by parasympathetic activity (9). The HF is a dependent measure of HRV. HF is a broadband measure, including parasympathetic activity, assuming the respiratory frequency is high enough (9). The LF/HF ratio is a dependent measure of HRV. The ratio is considered to be a more specific measure of the change in sympathetic activity when the ANS is challenged. All other frequency-domain HRV-alone measures are dependent on the LF and HF components. The (mathematical) dependency forces assumption and approximation and is not a reliable or consistent method of differentiating parasympathetic activity independent from sympathetic activity (9, 17-18-19-20-21-22-23). The reason is a fundamental principle of mathematics. The EKG underlying HRV-alone methods is only one independent measure of the two autonomic branches. To fully characterize the P&S systems, two independent measures of P&S are required. The (second) independent signal, continuous RA, in the P&S Method provides the second independent measure (17-18-19-20-21-22-23).
Our study concurs and expands upon previously reported effects of beta-blockers in restoration of autonomic balance (24). More specifically, our study demonstrates (i) clear differential effects on SB, and (ii) that Carvedilol (an alpha- and beta-adrenergic blocker) elicits a greater response than Metoprolol (a pure beta-blocker). This may be due to the fact that Carvedilol contains more adrenergic blockade as compared with Metoprolol. From Figure 1, introducing, or titrating higher, beta-blocker therapy decreases mean HR and systolic BP, as expected (Fig. 1). Discontinuing either agent caused all hemodynamic measures to increase, as expected. For those patients continuing beta-blocker therapy without any adjustment, no hemodynamic response is significant, again as expected. From Table II, the P&S Method results demonstrate a decrease in sympathetic activity, an increase in parasympathetic activity and a decrease in SB in response to introducing a beta-blocker. This is as expected, especially the decrease in SB, indicating a net sympathetic decrease, which is the goal of beta-blockade. For patients in whom a beta-blocker is discontinued, the opposite occurred for P&S measures. This is as expected. The average SB responses, however, are mixed. The average SB response to discontinuing Carvedilol increases, indicating a relative increase in sympathetic activity, suggesting a “rebound” effect. The average SB response to discontinuing Metoprolol decreases, indicating a relative increase in parasympathetic activity, suggesting a weaker sympathetic effect. This is seen in the absolute measures of sympathetic responses to discontinuing these agents: (−) Carvedilol = 2.07 bpm2 (p<0.001) and (−)Metoprolol=0.28 bpm2 (p<0.010). For those patients continuing beta-blocker therapy without any adjustment, there is very little change in P&S Method results. No change in therapy should, in stable patients, elicit no change in P&S response.
Since HRV-alone parameters are mixed measures of P&S activity, the results of HRV-alone analyses (Tabs. III and IV) are also mixed, and do not concur with expected results from changes in beta-adrenergic antagonist therapy. In fact, the P&S Method results may provide additional information, elucidating the HRV-alone responses. From Table III, average, normalized LF (LFnu) decreases in response to adding beta-blocker, as expected; however, responses to neither agent were significant. From Table II, it is documented that the parasympathetic responses to increasing beta-blockade also elicit a significant parasympathetic response. Given that the LF term is known to be a mix of P&S activity (9), the lack of significance in the LFnu decrease is not surprising. The changes in HF are also not significant (see Tab. III), perhaps due to the nature of the measure. A fixed, broadband, frequency domain measure of a narrow band parameter permits noise to enter the system and be analyzed as part of the signal of interest. The noise, which includes harmonics from the lower, sympathetic activity, adds a random nature to the responses, reducing the significance of the HF responses. LF/HF ratio decreases in response to adding beta-blockade, which is expected; however, only the response to Metoprolol is significant. This is unexpected, given that Carvedilol blocks more adrenergic receptors. This may also be explained by the P&S results. The smaller sympatholytic effect (ΔS) of (+)Metoprolol (Tab. II) and the relatively greater parasympathetic response (ΔP) mathematically cause a greater decrease in the ratio. Changes in TSP are significant, as expected, since TSP is overtly recognized as the combination of both P and S activity. However, TSP increases in response to (+)Carvedilol and decreases in response to (+)Metoprolol. If the assumption in chronic disease patients (such as those with diabetes, CV disease and HTN) is that sympathetic activity is significantly greater than parasympathetic activity, then the TSP response to (+)Carvedilol is unexpected.
Similar explanations may be made for the other HRV-alone responses to discontinuing or not altering beta-blockade. For example, LFnu increases in response to (−)Carvedilol as expected, and decreases in response to (−)Metoprolol – unexpected (Tab. III). From the P&S responses, (Tab. II) this may be due to the significantly smaller sympathetic increase to (−)Metoprolol as compared to (−)Carvedilol, and the comparable parasympathetic decrease to both. This may also explain the LF/HF ratio responses. As an example from the patients who continued beta-blocker without adjustment, (o)Carvedilol increases HF and (o)Metoprolol decreases HF (Tab. III). From Table II, since the P&S responses are small, consider SB (the net or relative effect of a change in therapy). SB decreases in response to (o)Carvedilol, indicating a relative parasympathetic increase supporting the (o)Carvedilol HF response. SB increases in response to (o)Metoprolol, indicating a relative sympathetic increase supporting the (o)Metoprolol HF response. RangeHR and E/I ratio (Tab. IV) increase in response to (+)Carvedilol and decrease in response to (+)Metoprolol. Both are measures of HR variability. More or less HRV correlates to more or less parasympathetic activity. Only parasympathetic activity, the high-frequency component, contributes significantly to variability. Therefore, the opposing responses to the two agents (Tab. IV) are explained by the differing effects on parasympathetic activity (Tab. II); (+)Carvedilol elicits a nearly fourfold parasympathetic response than does Metoprolol.
Conclusion
Noninvasive measures of hemodynamic and autonomic responses to changes in beta-blocker therapy were collected. Overall, the hemodynamic results are consistent with the known effects of changes in specific beta-blocker vs alpha- and beta-blocker therapy (25-26-27). The combination of alpha- and beta-blocker decreases sympathetic tone and increases parasympathetic tone, whereas a pure beta-blocker precipitates a reflex increase in alpha-adrenergic activity, leading to less of an increase in parasympathetic tone. The P&S Method results are consistent with the hemodynamic results and with the expected autonomic effects of changes in beta-blocker therapy known to underlie the hemodynamic responses (28). As shown, HRV-alone measures poorly correlate with the hemodynamic responses and only weakly correlate with expected variations in HR, and total autonomic activity.
Adrenergic-blockade is shown to reduce sympathetic activity. By providing both alpha- and beta-blockade, Carvedilol has a more profound effect in reducing sympathetic activity as compared to the pure beta-blockade of Metoprolol. Our results also suggest that Carvedilol not only reduces overall sympathetic dominance, but concomitantly increases parasympathetic activity, which is known to be cardioprotective (7). Adrenergic antagonists may restore autonomic balance and decrease functional disturbances that can lead to early AN (29), which leads to increased morbidity and earlier mortality (4, 30). A simple measure of balance (SB) done prospectively may add significantly to the ability to identify and predict people at risk and to the adoption of multifactorial preventive measures.
Abbreviations
- Δ
“Delta,” designates a change in the parameter it precedes
- (+)beta-blocker
indicates a beta-blocker was introduced
- (−)beta-blocker
indicates a beta-blocker was discontinued
- (o)beta-blocker
indicates no change in beta-blocker dosing from baseline
- AD
Autonomic dysfunction
- AN
Autonomic neuropathy
- ANS
Autonomic nervous system
- BP
Blood pressure (mmHg)
- bpm
beats per minute
- bpm2
beats per minute squared
- CAN
Cardiovascular autonomic neuropathy
- CVD
Cardiovascular disease
- dBP
diastolic blood pressure (mmHg)
- E/I
ratio Exhalation – inhalation ratio (unitless)
- FFT
Fast Fourier transform
- HBI
Heart beat interval
- HF
High frequency (msec2)
- HFnu
normalized high frequency (normalized to TSP, unitless)
- HR
Heart rate (bpm)
- HRV
Heart rate variability
- HTN
Hypertension
- LF
Low frequency (msec2)
- LFa
Low frequency area (bpm2)
- LFnu
normalized low frequency (normalized to TSP, unitless)
- MACE
Major adverse cardiovascular events
- mHR
mean heart rate (bpm)
- mmHg
millimeters of mercury
- msec2
milliseconds squared
- P&S
Parasympathetic and sympathetic
- QOL
Quality of life
- rangeHR
range heart rate (bpm)
- RFa
Respiratory frequency area (bpm2)
- SB
Sympathovagal balance (= S/P, unitless)
- sBP
systolic BP (mmHg)
- sdNN
standard deviation of the beat-to-beat (N-N) intervals (msec)
- S/P
Sympathetic/parasympathetic
- Test n
the baseline test
- Test n+1
the follow-up test
- TSP
Total spectral power (= LF + HF, ms2)
Disclosures
Financial support: None.
Conflict of interest: Drs. Bloom and Vinik are unpaid advisors to ANSAR. Dr. Colombo is Medical Director of ANSAR.
References
- 1.Low P. Assessment: clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46(3):873–880. [PubMed] [Google Scholar]
- 2.Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Semin Neurol. 2003;23(4):365–372. doi: 10.1055/s-2004-817720. [DOI] [PubMed] [Google Scholar]
- 3.Boulton AJ, Vinik AI, Arezzo JC et al.; American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 4.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 5.Low PA, Engstrom JW. In: Harrison’s principles of internal medicine. 16th ed. McGraw-Hill: “Disorders of the autonomic nervous system.”. 2006: 2428-2434. [Google Scholar]
- 6.Goldsmith RL, Bloomfield DM, Rosenwinkel ET. Exercise and autonomic function. Coron Artery Dis. 2000;11(2):129–135. doi: 10.1097/00019501-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31(3):593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 8.Vinik AI, Bloom HL, Colombo J. Differential effects of adrenergic antagonists (carvedilol vs. metoprolol) on parasympathetic and sympathetic activity: a comparison of clinical results. In press, 2014. [PMC free article] [PubMed] [Google Scholar]
- 9.Malik M. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 10.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 11.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249(4 Pt 2):H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 12.Akselrod S, Eliash S, Oz O, Cohen S. Hemodynamic regulation in SHR: investigation by spectral analysis. Am J Physiol. 1987;253(1 Pt 2):H176–H183. doi: 10.1152/ajpheart.1987.253.1.H176. [DOI] [PubMed] [Google Scholar]
- 13.Akselrod S. Spectral analysis of fluctuations in cardiovascular parameters: a quantitative tool for the investigation of autonomic control. Trends Pharmacol Sci. 1988;9(1):6–9. doi: 10.1016/0165-6147(88)90230-1. [DOI] [PubMed] [Google Scholar]
- 14.Aysin B, Aysin E. IEEE Engineering in Medicine and Biology Society Conference. New York, NY: Effect of respiration in heart rate variability (HRV) analysis. 2006. [DOI] [PubMed] [Google Scholar]
- 15.Aysin B, Aysin E, Colombo J. IEEE Engineering in Medicine and Biology Conference. Lyons, France: Comparison of HRV analysis methods during orthostatic challenge: HRV with respiration or without? 2007. [DOI] [PubMed] [Google Scholar]
- 16.Arora RR, Bulgarelli RJ, Ghosh-Dastidar S, Colombo J. Autonomic mechanisms and therapeutic implications of postural diabetic cardiovascular abnormalities. J Diabetes Sci Tech. 2008;2(4):645–657. doi: 10.1177/193229680800200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol. 1991;261(4 Pt 2):H1231–H1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- 18.Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol (1985) 1993;75(5):2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- 19.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96(9):3224–3232. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 20.Badra LJ, Cooke WH, Hoag JB et al. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280(6):H2674–H2688. doi: 10.1152/ajpheart.2001.280.6.H2674. [DOI] [PubMed] [Google Scholar]
- 21.Cammann H, Michel J. How to avoid misinterpretation of heart rate variability power spectra? Comput Methods Programs Biomed. 2002;68(1):15–23. doi: 10.1016/s0169-2607(01)00154-7. [DOI] [PubMed] [Google Scholar]
- 22.Freeman R. Assessment of cardiovascular autonomic function. Clin Neurophysiol. 2006;117(4):716–730. doi: 10.1016/j.clinph.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Pinna GD, Maestri R, La Rovere MT, Gobbi E, Fanfulla F. Effect of paced breathing on ventilatory and cardiovascular variability parameters during short-term investigations of autonomic function. Am J Physiol Heart Circ Physiol. 2006;290(1):H424–H433. doi: 10.1152/ajpheart.00438.2005. [DOI] [PubMed] [Google Scholar]
- 24.Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. Am J Cardiol. 2003;91(2):137–142. doi: 10.1016/s0002-9149(02)03098-9. [DOI] [PubMed] [Google Scholar]
- 25.Kveiborg B, Major-Petersen A, Christiansen B, Torp-Pedersen C. Carvedilol in the treatment of chronic heart failure: lessons from the Carvedilol Or Metoprolol European Trial. Vasc Health Risk Manag. 2007;3(1):31–37. [PMC free article] [PubMed] [Google Scholar]
- 26.Arumanayagam M, Chan S, Tong S, Sanderson JE. Antioxidant properties of carvedilol and metoprolol in heart failure: a double-blind randomized controlled trial. J Cardiovasc Pharmacol. 2001;37(1):48–54. doi: 10.1097/00005344-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Remme WJ, Cleland JG, Erhardt L et al. Effect of carvedilol and metoprolol on the mode of death in patients with heart failure. Eur J Heart Fail. 2007;9(11):1128–1135. doi: 10.1016/j.ejheart.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8(5):491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifer MA, Schumer MP. Clinical trials of diabetic neuropathy: past, present, and future. Diabetes. 1995;44(12):1355–1361. doi: 10.2337/diab.44.12.1355. [DOI] [PubMed] [Google Scholar]
- 30.Stevens MJ, Raffel DM, Allman KC et al. Cardiac sympathetic dysinnervation in diabetes: implications for enhanced cardiovascular risk. Circulation. 1998;98(10):961–968. doi: 10.1161/01.cir.98.10.961. [DOI] [PubMed] [Google Scholar]


