Abstract
Background
Coronary heart disease (CHD) is a major health concern, affecting nearly half the middle-age population and responsible for nearly one-third of all deaths. Clinicians have several major responsibilities beyond diagnosing CHD, such as risk stratification of patients for major adverse cardiac events (MACE) and treating risks, as well as the patient. This second of a two-part review series discusses treating risk factors, including autonomic dysfunction, and expected outcomes.
Methods
Therapies for treating cardiac mortality risks including cardiovascular autonomic neuropathy (CAN), are discussed.
Results
While risk factors effectively target high-risk patients, a large number of individuals who will develop complications from heart disease are not identified by current scoring systems. Many patients with heart conditions, who appear to be well-managed by traditional therapies, experience MACE. Parasympathetic and Sympathetic (P&S) function testing provides more information and has the potential to further aid doctors in individualizing and titrating therapy to minimize risk. Advanced autonomic dysfunction (AAD) and its more severe form cardiovascular autonomic neuropathy have been strongly associated with an elevated risk of cardiac mortality and are diagnosable through autonomic testing. This additional information includes patient-specific physiologic measures, such as sympathovagal balance (SB). Studies have shown that establishing and maintaining proper SB minimizes morbidity and mortality risk.
Conclusions
P&S testing promotes primary prevention, treating subclinical disease states, as well as secondary prevention, thereby improving patient outcomes through (1) maintaining wellness, (2) preventing symptoms and disorder and (3) treating subclinical manifestations (autonomic dysfunction), as well as (4) disease and symptoms (autonomic neuropathy).
Keywords: Cardiac autonomic neuropathy, Cardiovascular risk factors, Heart disease, Mortality
Introduction
In the first article in this series, we briefly reviewed traditional, nontraditional, modifiable and nonmodifiable risk factors. We also reviewed (1) the failings of heart beat interval (HBI) alone (1-2-3) and noninvasive autonomic measures based solely on measures of HBI signals (e.g., heart rate variability (HRV) alone and beat-to-beat blood pressure (BP) (4-5-6-7)) and (2) the benefits of specific parasympathetic and sympathetic (P&S) monitoring or testing (8-9-10-11-12-13-14-15-16).
Based on the need to improve on the risk factors available, cardiovascular autonomic neuropathy (CAN) risk and its association with current risk factors was discussed, including (1) the association of CAN with cardiac mortality risk, (2) stratifying CAN risk, (3) CAN and diabetes risk, (4) CAN and nontraditional risk factors and (5) sudden cardiac death (SCD). In this article, we will discuss the treatment of CAN, specifically how treating autonomic balance (aka, sympathovagal balance (SB) (17)) modifies cardiovascular risk, and expected outcomes.
Background
Treating heart disease carries several important responsibilities beyond diagnosing coronary artery disease (CAD), including risk-stratifying for an adverse cardiac event and treating the individual risk factors pharmacologically. For the latter, exact dose, class and type of agent to use is often not clearly defined. For example, beta-blockers may be indicated in the postinfarction patient or in a patient with heart failure, but the optimal dose to titrate, or which type to use, is not known with certainty. The same applies to angiotensin antagonists, other antihypertensives and diuretics, as well as direct and indirect anticholinergics (e.g., antidepressants and anxiolytics). Antiplatelet therapy efficacy is very difficult to predict without genetic testing or in vitro laboratory testing. P&S testing, including the patient-specific physiologic measure of SB, provides more information. Studies have shown that establishing and maintaining proper SB minimizes morbidity and mortality risk (8, 18-19-20-21-22-23). As these studies have shown, more information through P&S testing promotes primary prevention, treating subclinical disease states, and secondary prevention, thereby improving patient outcomes through (1) maintaining wellness, (2) preventing symptoms and disorder and (3) treating subclinical manifestations (autonomic dysfunction), as well as (4) disease and symptoms (autonomic neuropathy) (8, 18, 20, 21).
Treating risk factors in heart disease
As discussed in the companion article, establishing a risk factor may also guide therapy. Demonstrating that therapy actually lowers risk is still needed. For example, it was well established in the 1970s and 1980s that elevated serum cholesterol levels significantly contributed to heart attacks and heart-related deaths (24). This was termed the “lipid hypothesis” (25) since it was not established at that time that lowering cholesterol reduced heart attacks and heart deaths. Eventually, well-designed trials did demonstrate that lowering cholesterol with pharmacological agents reduced cardiac mortality and coronary heart disease (CHD) complications (26-27-28-29-30-31-32-33-34). Findings included that atherosclerosis progression may be halted or reversed (35), with formulae developed to potentially reduce and reverse coronary plaque (24). The influence of statin therapy on plasma-oxidized low-density lipoprotein (LDL) biomarkers and high-sensitivity C-reactive protein (CRP) was demonstrated (see Fig. 1) (36). Subsequently, Dr. Nissen demonstrated that LDL-lowering statins could slow or halt the progression of atherosclerosis (37). Recently, it has been demonstrated that very low levels of serum LDL, down to 50 mg/dL, reduce mortality risk (38).
Fig. 1 -.
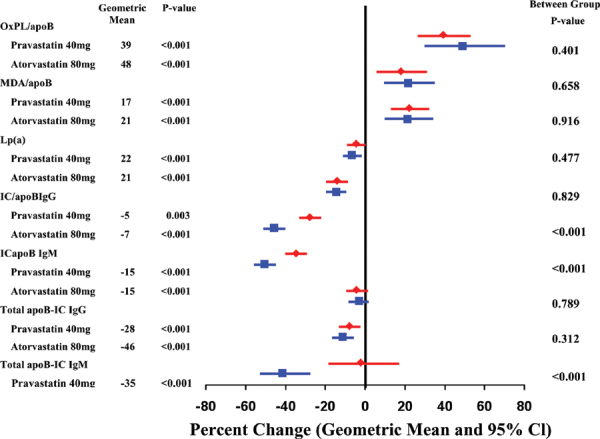
The influence of statin therapy on plasma-oxidized low-density lipoprotein (OxLDL) biomarkers and high-sensitivity C-reactive protein (CRP). apoB-IC = apolipoprotein B-100 immune complexes; CI = confidence interval; IC/apoB = immune complexes per apolipoprotein B-100; Ig = immunoglobin; Lp(a) = lipoprotein (a); MDA = malondialdehyde; MDA/apoB = malondialdehyde epitopes per apolipoprotein B-100; OxPL/apoB = oxidized phospholipid epitopes per apolipoprotein B-100. From The New England Journal of Medicine, Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. Vol. 345, No. 23, pp. 1667-1675. Copyright © 2001 Massachusetts Medical Society (83). Adapted with permission from Massachusetts Medical Society. See text for details.
Examples of risk factors that are still in need of treatment standardization include BP and blood glucose. Attaining normotensive systolic BP is important. It is known that treating hypertension reduces stroke, heart attack and heart failure. However, an absolute target level has not been clearly demonstrated (39). Optimal target blood sugar (hemoglobin A1c) in diabetics is also not known. Initial hypotheses that intensive control of blood sugar would lower cardiac heart disease events have not been proven. Results from recent studies involving various subsets of patients appear to contradict the initial hypotheses (9).
Furthermore, while specific therapies for heart disease have been recommended, optimal dosing recommendations have not been standardized (e.g., beta-blocker therapy). Evidence for beta-blocker use in CHD is derived from relatively old studies. It has subsequently been widely extrapolated to patients with CAD and even to patients at high risk for, but without established, CAD. It is not known if these extrapolations are justified. Moreover, the long-term efficacy of beta-blockers in patients treated with contemporary medical therapies is not known, even in patients with prior myocardial infarction (MI). At issue is that beta-blockers are not without adverse effects and their tolerability is not ideal. Therefore, the benefit of beta-blocker use is unclear. Recently published in JAMA, the REACH study assessed the association of beta-blocker use in stable patients with known risk for cardiovascular events. REACH concluded that the use of beta-blockers is not associated with a lower risk of composite cardiovascular events (40).
Risks associated with cardiovascular autonomic neuropathy
Treating cardiovascular autonomic neuropathy associated with cardiac mortality risk
Decreased HRV, specifically decreased resting parasympathetic activity, defines CAN (8, 19, 41, 42). Because of the higher mortality with CAN (43), investigators have suggested that individuals with abnormal autonomic testing should be candidates for closer surveillance and more aggressive pharmacological therapy. Suggested therapy targets values that achieve autonomic balance, even if the patient is asymptomatic or subclinical (8, 20). Using the quantitative measures of P&S activity (14, 15) and P&S balance as targets for treatment decisions, pharmacological agents (e.g., sympatholytics if too much sympathetic activity or anticholinergics if too much parasympathetic activity) may be appropriately titrated and utilized with more precise selection of class and dosing for the individual patient (21).
While many researchers in many subpopulations of heart disease patients have documented reduced autonomic activity with increased mortality (43-44-45-46-47), increased sympathetic activity and decreased parasympathetic activity often require different treatment modalities. Curtis and O’Keefe state:
“Any factor that leads to inappropriate activation of the sympathetic nervous system can be expected to have an adverse effect on … patient outcomes, while any factor that augments vagal tone tends to improve outcomes. Insulin resistance, sympathomimetics medications, and negative psychosocial factors all have the potential to affect autonomic function adversely and thus cardiovascular prognosis. Congestive heart failure and hypertension also provide important lessons about the adverse effects of sympathetic predominance, as well as illustrate the benefits of β-blockers and angiotensin-converting enzyme inhibitors, two classes of drugs that reduce adrenergic tone. Other interventions, such as exercise, improve cardiovascular outcomes partially by increasing vagal activity and attenuating sympathetic hyperactivity” (20).
HRV-alone or beat-to-beat BP may not clearly differentiate low parasympathetic from high sympathetic activity. Independent, simultaneous P&S information is required (8, 41). Tsuji found that his patients appeared to be free of any significant underlying CHD, suggesting that reduced autonomic activity may simply reflect a subclinical cardiac disease state (42).
Barthel and coworkers (48), based on years of follow-up, demonstrated that autonomic dysfunction is a significant risk predictor for poor outcome status after MI, history of previous MI, arrhythmia on Holter monitoring, poor glucose control and left ventricular ejection fraction (LVEF) less than 30%. This highlights the importance, even in low-risk patients, of performing P&S testing to risk-stratify for future cardiac events, including cardiac death. Prospective work in CHD and newly developing CAD by Liao and coworkers (49) expand the application of monitoring autonomic dysfunction beyond post-MI to a much larger patient base and the general population. Liao demonstrates that identifying autonomic dysfunction and CAN is important for secondary prevention, as well as primary prevention. Once identified, autonomic dysfunction should be treated to restore and maintain proper P&S balance.
Autonomic dysfunction also has been correlated with progression of CAD (50) and with silent ischemia. The latter leads to SCD and unexpected MI. Umetani et al found that autonomic activity declines normally with aging to below levels associated with increased risk of mortality (18). Wackers and coworkers (51) found that traditional cardiac risk factors, including inflammatory and prothrombotic markers, were not predictive, and emerging risk factors were not associated with abnormal stress tests or computed tomography imaging. By contrast, CAN was a strong predictor of ischemia. This offers more reason to test for P&S activity and treat autonomic dysfunction by restoring and maintaining balance to slow progression of autonomic dysfunction and neuropathy.
Minimizing cardiovascular autonomic neuropathy risk
CAN indicates very low parasympathetic activity, relative to sympathetic activity (42). CAN may be normal for geriatric and long-standing chronic disease patients. For example, based on Framingham risk factors, an 85-year-old has a greater mortality risk than a 45-year-old. More parasympathetic activity relative to sympathetic activity is known to be cardioprotective and reduces mortality risk (18). Chronic sympathetic activation is known to increase cardiovascular risk (20). Depression is known to elevate mortality risk in heart disease (52), and depression is associated with abnormally high levels of parasympathetic activity relative to sympathetic activity.
The relationship between P&S activity at rest is known as SB (17). CAN risk (the risk associated with very low parasympathetic activity with respect to sympathetic activity) may be stratified based on SB. High SB indicates high relative resting sympathetic excess (SE). CAN with high SB is considered high risk (53-54-55). In these cases, titrating higher sympatholytic therapy or lower anticholinergic therapy may normalize SB. In CAN cases where SB is persistently high with low HR, low BP and abnormal left ventricular function, consider an electrophysiology study to further document risk and the potential need for a cardiac device. Very low SB (<0.4) indicates a relative, resting parasympathetic excess. Very low SB, as it is associated with (subclinical) depression, elevates CAN risk (52). In these cases, titrating higher anticholinergic therapy or lower sympatholytic therapy may normalize SB. Normal SB, indicating a balanced autonomic nervous system, is associated with normal CAN risk (20). This may still be too much sympathetic activity, especially in patients with high HR or BP. In these cases, treat as if SB were high, indicating high risk. Low-normal SB, indicating more parasympathetic activity, is associated with minimal CAN risk (18). This is the recommended level of balance for geriatric cardiology patients.
Diabetes risk and autonomic neuropathy
While we have been discussing CAN, a late-stage autonomic neuropathy, earlier stages of autonomic dysfunction have been identified, including diabetic autonomic neuropathy (DAN). DAN is defined, using P&S monitoring, as low parasympathetic or sympathetic activity at rest, but not yet critically low resting parasympathetic activity as occurs in CAN. DAN is a very serious and common complication in diabetes (8). Identifying and treating DAN may stay progression of autonomic decline to the more serious condition known as CAN. Symptoms of DAN include (1) resting tachycardia, (2) exercise intolerance and (3) orthostatic hypotension and may also include (4) a glycemic autonomic failure (abnormal compensatory reflexes to hypoglycemia episodes). Several of these symptoms are also typical in nondiabetic chronic disease patients (e.g., chronic obstructive pulmonary disease, Parkinson’s disease, sleep apnea, and hypertensive cardiovascular disease). For these patients, we use the terminology AAD, and it likewise has low resting P or S activity, but not yet critically low resting P levels. Therefore, DAN and AAD have the same P&S measurements; the only difference is whether or not diabetes is present. These symptoms are often not associated with DAN, and DAN is misperceived as asymptomatic. DAN may impose a burden on an individual whose cardiac reserve may be compromised by underlying atherosclerosis or left ventricular abnormalities. Due to the potential for autonomic neuropathy, the American Diabetes Association (ADA) (56) recommends cardiac investigation before beginning physical activity that is more intense than usual. The ADA states that “hypoglycemia associated with autonomic failure can severely compromise stringent diabetes control and quality of life.” It is known that both hypoglycemia and CAN are associated with increased mortality risk. Therefore, prior to treating diabetics with physical exercise and more stringent glucose control, consider P&S testing for DAN or CAN.
In their discussion of CAN under “Neuropathy screening and treatment” ((pS37), 56), the ADA states that “special testing is rarely needed and may not affect management or outcomes.” This, of course refers to the symptomatic nature of CAN, implying that once symptoms present, management is already in place and outcomes are known without special testing. However, they recommend testing “at least annually” for diabetic polyneuropathy (DPN), for the autonomic aspect of DPN is largely asymptomatic until autonomic neuropathy is evident, and even then it is (silently) progressive and continues to affect morbidity and mortality. The majority of the recommendations for autonomic dysfunction are for early testing to specify and customize autonomic therapy to delay autonomic neuropathy onset and reduce morbidity and mortality risk. “Medications for the relief of specific symptoms related to autonomic neuropathy are recommended, including tri-cyclic drug recommendations and other therapy dosing ((table 16, pS38), 57)), as they improve the quality of life of the patient …. The early recognition and appropriate management of neuropathy in the patient with diabetes is important for a number of reasons[.] … [A]utonomic neuropathy may involve every system in the body, and CAN causes substantial morbidity and mortality” ((pS37), 57). The therapy recommendations are known to affect SB. Normalizing autonomic dysfunction (balance, including SB) is known to reduce morbidity and mortality risk (8, 18-19-20-21, 58-59-60-61-62-63).
Nontraditional risk factors and autonomic neuropathy
CRP is a useful biomarker of increased long-term risk of SCD (64). CRP is associated with decreased autonomic function, even after controlling for traditional risk factors that decrease CAD (65). It is postulated that autonomic dysregulation may represent one pathway leading to CAD, even with treatment of risk factors to prevent the development of CAD. Inflammation is a significant contributor toward atherosclerosis and is a nontraditional risk factor with incremental value (65). The association of diminished autonomic function with elevated CRP levels is potentially significant. Restoration of autonomic balance is possible and has been shown with therapeutic lifestyle changes, increased physical activity, beta-blockers, aldose reductase inhibitors, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and potent antioxidants such as alpha-lipoic acid. There are also exciting new prospects for pathogenesis-oriented intervention (63).
Microalbuminuria has been associated with an increased risk of cardiovascular mortality independently of other known coronary artery risk factors (66,67). The Hoorn study (68) supports the fact that it may be useful to treat both microalbuminuria and CAD in populations at a high risk for cardiovascular mortality.
Treating autonomic balance modifies autonomic neuropathy risk
Identifying CAN early (specifically parasympathetic or sympathetic dysfunction) and treating it aggressively (based, at least in part, on the autonomic findings) may reduce the emergence of CHD and the ancillary complications. More prospective studies are needed in this area, as the majority of the data are hypothesis generating. However, treatment to establish and maintain proper P&S balance has been known to minimize mortality risk (18). It therefore makes empiric sense to attempt to normalize autonomic dysfunction. Treatment to modulate one autonomic branch or the other (e.g., with sympatholytics, such as beta-blockers or antihypertensives (20), or anticholinergics, such as low-dose antidepressants or anxiolytics (52)) has been shown to reduce mortality as well as morbidity risk in some studies. This evidence suggests that treating in an attempt to normalize P&S balance may reduce CAN risk (21).
In many cases, P&S assessment may provide more information where required. For example, LVEF between 35% and 40% is considered moderately depressed and a borderline indication for implantable cardioverter defibrillator (ICD) placement. CHD patients who present moderately depressed LVEF with high BP or HR (including arrhythmia) may be treated pharmacologically with more sympatholytics (20), as confirmed by documenting SE. However, for CHD patients who present moderately depressed LVEF with SE and low BP or HR, more sympatholytics may not be appropriate. Typically, these patients demonstrate parasympathetic insufficiency, indicating a potential inability to prevent a sympathetically mediated ventricular tachyrhythm from becoming fibrillation or worse. Parasympathetic insufficient patients with low HR and BP may require a lower threshold for the clinician to implant a defibrillator device or undertake more sophisticated electrophysiology studies in an individual patient.
Atorvastatin and other statins have been shown to be most effective in treating dyslipidemias, especially in patients with risk factors for coronary atherosclerosis or those with underlying coronary atherosclerosis. Atorvastatin has both anti-inflammatory and lipid-lowering effects, reducing CRP and LDL cholesterol (69). This study involving 20 patients with stable CAD and 20 patients without CAD demonstrated that atorvastatin improved autonomic function. Landmark survival studies with statins have shown significant benefit with their institution, plausible mechanisms for reduction of clinical events and that primary and secondary CAD prevention may include not only lowering LDL cholesterol and inflammatory CRP (70), but also possibly normalizing autonomic dysfunction, as demonstrated by Gentlesket al (58). Again, increased parasympathetic activity is known to be cardioprotective (18). Therefore, for individuals with abnormal autonomic function, aggressive lipid-lowering treatment with statins may be indicated based on these findings.
Sudden cardiac death
Lastly, one cannot discuss diagnosis and treatment of cardiovascular diseases without addressing SCD (71). Approximately 67% of symptoms of SCD are related to CHD (72-73-74). Approximately 450,000 individuals per year have SCD in the United States (75), and this is probably an underestimate of the frequency. The risk is three times greater in men than in women, based on the Framingham Study data (76). People at high risk for SCD may be treated with ICDs or have other precipitating factors corrected so as to prevent further episodes.
Important risk factors for SCD are underlying CAD, heart failure, left ventricular dysfunction and prior MI. The risk factors for CAD are the same risk factors for SCD. Heart failure is also a significant risk factor for SCD. Significant genetic factors for SCD (77) showed that parental SCD is an independent risk factor for sudden death in a middle-aged man. The existence of familial risk factors for SCD may help us better explain subjects at a high risk and enable us to prevent SCD early on (78-79-80). Patients with left ventricular dysfunction are at high risk for SCD. This risk is used as an index for aggressive treatment for devices such as defibrillators. A community-wide study showed that only one-third of the evaluated SCD patients having severe left ventricular dysfunction met the criteria for prophylactic cardioverter defibrillator implantations (81). Prophylactically implanted cardiac device trials may represent a minority of SCD population (82). Therefore, screening patients for SCD based on left ventricular dysfunction is not a very sensitive technique and will miss approximately two thirds of SCD patients.
In a review article, Myerburg (75) states that SCD is an unresolved problem despite more insight into the mechanisms and therapeutic advances. Prediction and prevention of SCD should not be restricted to assessing an individual for the presence of CAD, coronary ischemia, left ventricular dysfunction or heart failure. This is a much more complicated issue underlying various diseases and risk factors. It is anticipated that independent, simultaneous P&S testing for cardiac autonomic dysfunction will provide additional information to understand these issues, to guide therapy and treatment and affect improved outcomes. P&S testing allows for the risk assessment of patients for major adverse cardiac events, even when they are asymptomatic and have no clinical CAD. Subclinical CAD is associated with CAN. Therefore, testing for CAN and SB may be extremely productive in identifying and treating patients at high risk for cardiac events (21, 62, 63).
Conclusion
CAN is associated with increased cardiac morbidity and mortality. Identifying and addressing CAN early, especially in a subclinical cardiac patient, will further differentiate which asymptomatic patients require more aggressive therapy. The results from P&S testing documenting CAN may be used as a baseline. One should view these test results as a guide toward more individualized treatment. A more specific selection of medications and dosing based on these results is possible. P&S test results represent objective data which are useful in guiding pharmacological and lifestyle changes. In addition to normalization and improvement of CAN (21), independent and simultaneous P&S testing, providing objective P&S activity levels, may guide the physician toward the type and dosing of pharmacological agents necessary to achieve an objective clinical target or outcome. The pharmacopeia includes adrenergic (beta-blockers, antihypertensives, bronchodilators and vasopressors) and cholinergic (antidepressants, anxiolytics and antipsychotics) agents. This would eliminate arbitrarily dosing medications without a clear target outside of HR and BP. Also, the threshold for the implanting of prophylactic devices such as cardiac defibrillators may be better defined by assessing and following P&S dysfunction. While further studies are indicated, the clinical and epidemiological data are too compelling not to test for, diagnose and aggressively treat CAN with abnormal SB to guard the patient’s well-being, not only in diabetics (62, 63), but in all patients with risk factors for heart disease.
Disclosures
Financial support: None.
Conflict of interest: Dr. DePace, Ms. Mears, and Mr. Yayac have no conflict of interest. Dr. Colombo is Medical Director, Executive Vice President, Board Member and part owner of ANSAR Medical Technologies, Inc., Philadelphia, PA, USA, a researcher, developer, manufacturer and distributor of autonomic function testing technology.
References
- 1.Cammann H, Michel J. How to avoid misinterpretation of heart rate variability power spectra? Comput Methods Programs Biomed. 2002;68(1):15–23. doi: 10.1016/s0169-2607(01)00154-7. [DOI] [PubMed] [Google Scholar]
- 2.Badra LJ, Cooke WH, Hoag JB et al. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280(6):H2674–H2688. doi: 10.1152/ajpheart.2001.280.6.H2674. [DOI] [PubMed] [Google Scholar]
- 3.Hayano J, Mukai S, Sakakibara M, Okada A, Takata K, Fujinami T. Effects of respiratory interval on vagal modulation of heart rate. Am J Physiol. 1994;267(1 Pt 2):H33–H40. doi: 10.1152/ajpheart.1994.267.1.H33. [DOI] [PubMed] [Google Scholar]
- 4.Eckberg DL. Physiological basis for human autonomic rhythms. Ann Med. 2000;32(5):341–349. doi: 10.3109/07853890008995937. [DOI] [PubMed] [Google Scholar]
- 5.Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol (1985) 1993;75(5):2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- 6.Novak V, Novak P, de Champlain J, Le Blanc AR, Martin R, Nadeau R. Influence of respiration on heart rate and blood pressure fluctuations. J Appl Physiol (1985) 1993;74(2):617–626. doi: 10.1152/jappl.1993.74.2.617. [DOI] [PubMed] [Google Scholar]
- 7.Parati G, Rizzoni D. Assessing the prognostic relevance of blood pressure variability: discrepant information from different indices. J Hypertens. 2005;23(3):483–486. doi: 10.1097/01.hjh.0000160200.51158.9a. [DOI] [PubMed] [Google Scholar]
- 8.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Miller ME, Genuth S et al.; ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 11.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249(4 Pt 2):H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 12.Akselrod S, Eliash S, Oz O, Cohen S. Hemodynamic regulation in SHR: investigation by spectral analysis. Am J Physiol. 1987;253(1 Pt 2):H176–H183. doi: 10.1152/ajpheart.1987.253.1.H176. [DOI] [PubMed] [Google Scholar]
- 13.Akselrod S. Spectral analysis of fluctuations in cardiovascular parameters: a quantitative tool for the investigation of autonomic control. Trends Pharmacol Sci. 1988;9(1):6–9. doi: 10.1016/0165-6147(88)90230-1. [DOI] [PubMed] [Google Scholar]
- 14.Aysin B, Aysin E. Effect of respiration in heart rate variability (HRV) analysis. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1776–1779. doi: 10.1109/IEMBS.2006.260773. [DOI] [PubMed] [Google Scholar]
- 15.Aysin B, Aysin E, Colombo J. Comparison of HRV analysis methods during orthostatic challenge: HRV with respiration or without?. IEEE Engineering in Medicine and Biology Conference; Lyons, France. 2007. [DOI] [PubMed] [Google Scholar]
- 16.Ewing DJ, Campbell IW, Clarke BF. Assessment of cardiovascular effects in diabetic autonomic neuropathy and prognostic implications. Ann Intern Med. 1980;92(2 Pt 2):308–311. doi: 10.7326/0003-4819-92-2-308. [DOI] [PubMed] [Google Scholar]
- 17.Low P ed. 2nd ed. Philadelphia, PA: Lippincott-Raven Publishers; Clinical autonomic disorders: evaluation and management. 1997. [Google Scholar]
- 18.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31(3):593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 19.Vinik AI, Maser RE, Nakave AA. Diabetic cardiovascular autonomic nerve dysfunction. US Endocrine Disease. 2007 Dec;:2–9. [Google Scholar]
- 20.Curtis BM, O’Keefe JH., Jr Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc. 2002;77(1):45–54. doi: 10.4065/77.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Vinik AI, Murray GL. Autonomic neuropathy is treatable. US Endocrinol. 2008;2:82–84. [Google Scholar]
- 22.Nanavati SH, Bulgarelli RJ, Vazquez-Tanus J, Ghosh-Dastidar S, Colombo J, Arora RR. Altered autonomic activity with atrial fibrillation as demonstrated by non-invasive autonomic monitoring. US Cardiology. 2010;7(1):47–50. [Google Scholar]
- 23.Tobias H, Vinitsky A, Bulgarelli RJ, Ghosh-Dastidar S, Colombo J. Autonomic nervous system monitoring of patients with excess parasympathetic responses to sympathetic challenges—clinical observations. US Neurology. 2010;5(2):62–66. [Google Scholar]
- 24.DePace NL, Dowinsky SK. New York, NY: W. W. Norton & Company; The heart repair manual: The Philadelphia formula program for preventing and reversing atherosclerosis. 1993. [Google Scholar]
- 25.Ahrens EH., Jr The management of hyperlipidemia: whether, rather than how. Ann Intern Med. 1976;85(1):87–93. doi: 10.7326/0003-4819-85-1-87. [DOI] [PubMed] [Google Scholar]
- 26.Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis. 2007;49(3):373–382. doi: 10.1053/j.ajkd.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 27.Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46(7):1225–1228. doi: 10.1016/j.jacc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Pyörälä K, Ballantyne CM, Gumbiner B et al.; Scandinavian Simvastatin Survival Study (4S). Reduction of cardiovascular events by simvastatin in nondiabetic coronary heart disease patients with and without the metabolic syndrome: subgroup analyses of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 2004;27(7):1735–1740. doi: 10.2337/diacare.27.7.1735. [DOI] [PubMed] [Google Scholar]
- 29.Crea F, Monaco C, Lanza GA et al. Inflammatory predictors of mortality in the Scandinavian Simvastatin Survival Study. Clin Cardiol. 2002;25(10):461–466. doi: 10.1002/clc.4960251005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robins S, Ballantyne CM, Olsson AG et al. Low high-density lipoprotein cholesterol and response to simvastatin therapy in Scandinavian Simvastatin Survival Study (4S). Circulation. 2002;106(2):e8. doi: 10.1161/01.cir.0000019970.99823.b2. author reply e8. [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Stafford RS, Ausiello JC, Chaisson CE. Randomized clinical trials and recent patterns in the use of statins. Am Heart J. 2001;141(6):957–963. doi: 10.1067/mhj.2001.115587. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen TR, Wilhelmsen L, Faergeman O et al. Follow-up study of patients randomized in the Scandinavian simvastatin survival study (4S) of cholesterol lowering. Am J Cardiol. 2000;86(3):257–262. doi: 10.1016/s0002-9149(00)00910-3. [DOI] [PubMed] [Google Scholar]
- 33.Haffner SM, Alexander CM, Cook TJ et al. Reduced coronary events in simvastatin-treated patients with coronary heart disease and diabetes or impaired fasting glucose levels: subgroup analyses in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1999;159(22):2661–2667. doi: 10.1001/archinte.159.22.2661. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen TR. Coronary artery disease: the Scandinavian Simvastatin Survival Study experience. Am J Cardiol. 1998;82(10 10B):53T–56T. doi: 10.1016/s0002-9149(98)00727-9. [DOI] [PubMed] [Google Scholar]
- 35.Nicholls SJ, Ballantyne CM, Barter PJ et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 36.Choi SH, Chae A, Miller E et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol. 2008;52(1):24–32. doi: 10.1016/j.jacc.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls SJ, Tuzcu EM, Sipahi I et al. Effects of obesity on lipid-lowering, anti-inflammatory, and antiatherosclerotic benefits of atorvastatin or pravastatin in patients with coronary artery disease (from the REVERSAL Study). Am J Cardiol. 2006;97(11):1553–1557. doi: 10.1016/j.amjcard.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 38.Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol. 2011;57(16):1666–1675. doi: 10.1016/j.jacc.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 39.Filippone EJ, Foy A, Newman E. Goal-directed antihypertensive therapy: lower may not always be better. Cleve Clin J Med. 2011;78(2):123–133. doi: 10.3949/ccjm.78a.10101. [DOI] [PubMed] [Google Scholar]
- 40.Bangalore S, Steg G, Deedwania P et al.; REACH Registry Investigators. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308(13):1340–1349. doi: 10.1001/jama.2012.12559. [DOI] [PubMed] [Google Scholar]
- 41.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 42.Tsuji H, Venditti FJ, Jr, Manders ES et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 43.DePace NL, Mears JP, Yayac M, Colombo J. Cardiac autonomic testing and diagnosing heart disease. “A clinical perspective.”. Heart Int. 2014 (In press). doi: 10.5301/heartint.5000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29(2):334–339. doi: 10.2337/diacare.29.02.06.dc05-1242. [DOI] [PubMed] [Google Scholar]
- 45.Pop-Busui R, Evans GW, Gerstein HC et al.; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(7):1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Istenes I, Keresztes K, Hermányi Z et al. Relationship between autonomic neuropathy and hypertension—are we underestimating the problem? Diabet Med. 2008;25(7):863–866. doi: 10.1111/j.1464-5491.2008.02458.x. [DOI] [PubMed] [Google Scholar]
- 47.Astrup AS, Nielsen FS, Rossing P et al. Predictors of mortality in patients with type 2 diabetes with or without diabetic nephropathy: a follow-up study. J Hypertens. 2007;25(12):2479–2485. doi: 10.1097/HJH.0b013e3282f06428. [DOI] [PubMed] [Google Scholar]
- 48.Barthel P, Bauer A, Müller A et al. Reflex and tonic autonomic markers for risk stratification in patients with type 2 diabetes surviving acute myocardial infarction. Diabetes Care. 2011;34(8):1833–1837. doi: 10.2337/dc11-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao D, Cai J, Rosamond WD et al. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145(8):696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 50.Huikuri HV, Jokinen V, Syvänne M et al. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19(8):1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 51.Wackers FJT, Young LH, Inzucchi SE et al.; Detection of Ischemia in Asymptomatic Diabetics Investigators. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27(8):1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 52.Lichtman JH, Bigger JT, Jr, Blumenthal JA et al.; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 53.Bullinga JR, Alharethi R, Schram MS, Bristow MR, Gilbert EM. Changes in heart rate variability are correlated to hemodynamic improvement with chronic CARVEDILOL therapy in heart failure. J Card Fail. 2005;11(9):693–699. doi: 10.1016/j.cardfail.2005.06.435. [DOI] [PubMed] [Google Scholar]
- 54.Copie X, Lamaison D, Salvador M et al.; VALID Investigators. Heart rate variability before ventricular arrhythmias in patients with coronary artery disease and an implantable cardioverter defibrillator. Ann Noninvasive Electrocardiol. 2003;8(3):179–184. doi: 10.1046/j.1542-474X.2003.08302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alter P, Grimm W, Vollrath A, Czerny F, Maisch B. Heart rate variability in patients with cardiac hypertrophy—relation to left ventricular mass and etiology. Am Heart J. 2006;151(4):829–836. doi: 10.1016/j.ahj.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 56.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gentlesk PJ, Wiley T, Taylor AJ. A prospective evaluation of the effect of simvastatin on heart rate variability in non-ischemic cardiomyopathy. Am Heart J. 2005;150(3):478–483. doi: 10.1016/j.ahj.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 59.Arora RR, Bulgarelli RJ, Ghosh-Dastidar S, Colombo J. Autonomic mechanisms and therapeutic implications of postural diabetic cardiovascular abnormalities. J Diabetes Sci Tech. 2008;2(4):645–657. doi: 10.1177/193229680800200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vinik AI, Arora RR, Colombo J. Age matched attenuation of both autonomic branches in chronic disease: II. Diabetes mellitus. Cleveland Clinic Heart-Brain Summit, Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV. 23-24 September 2010. [Google Scholar]
- 61.Pereira E, Baker S, Bulgarelli RJ, Murray G, Arora RR, Colombo J. Gender differences in longevity and sympathovagal balance. Presented at the Cleveland Clinic Heart-Brain Summit, Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV. 23-24 September 2010. [Google Scholar]
- 62.Vinik AI, Maser RE, Ziegler D. Neuropathy: the crystal ball for cardiovascular disease? Diabetes Care. 2010;33(7):1688–1690. doi: 10.2337/dc10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabet Med. 2011;28(6):643–651. doi: 10.1111/j.1464-5491.2010.03184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105(22):2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 65.Su S, Lampert R, Zhao J et al. Pleiotropy of C-reactive protein gene polymorphisms with C-reactive protein levels and heart rate variability in healthy male twins. Am J Cardiol. 2009;104(12):1748–1754. doi: 10.1016/j.amjcard.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerstein HC, Mann JFE, Yi Q et al.; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 67.Hermans MMH, Henry R, Dekker JM et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol. 2007;18(6):1942–1952. doi: 10.1681/ASN.2006111217. [DOI] [PubMed] [Google Scholar]
- 68.Hermans MMH, Henry RMA, Dekker JM, Nijpels G, Heine RJ, Stehouwer CDA. Albuminuria, but not estimated glomerular filtration rate, is associated with maladaptive arterial remodeling: the Hoorn Study. J Hypertens. 2008;26(4):791–797. doi: 10.1097/HJH.0b013e3282f50066. [DOI] [PubMed] [Google Scholar]
- 69.Pehlivanidis AN, Athyros VG, Demitriadis DS, Papageorgiou AA, Bouloukos VJ, Kontopoulos AG. Heart rate variability after long-term treatment with atorvastatin in hypercholesterolaemic patients with or without coronary artery disease. Atherosclerosis. 2001;157(2):463–469. doi: 10.1016/s0021-9150(00)00746-2. [DOI] [PubMed] [Google Scholar]
- 70.Ridker PM, Danielson E, Fonseca FA et al; JUPITER Trial Study Group. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 71.Fauci AS, Braunwald E, Kasper DL et al. 17th ed. New York, NY: McGraw-Hill Professional; Harrison’s principles of internal medicine. 2008. [Google Scholar]
- 72.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 73.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97(21):2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention (CDC). Decline in deaths from heart disease and stroke—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48(30):649–656. [PubMed] [Google Scholar]
- 75.Myerburg RJ. Scientific gaps in the prediction and prevention of sudden cardiac death. J Cardiovasc Electrophysiol. 2002;13(7):709–723. doi: 10.1046/j.1540-8167.2002.00709.x. [DOI] [PubMed] [Google Scholar]
- 76.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110(5):522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 77.Jouven X, Desnos M, Guerot C, Ducimetière P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99(15):1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 78.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330(15):1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 79.Jouven X, Lemaître RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26(20):2142–2147. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 80.Kannel WB, Cupples LA, D’Agostino RB. Sudden death risk in overt coronary heart disease: the Framingham Study. Am Heart J. 1987;113(3):799–804. doi: 10.1016/0002-8703(87)90722-8. [DOI] [PubMed] [Google Scholar]
- 81.Stecker EC, Vickers C, Waltz J et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 82.Buxton AE, Ellison KE, Kirk MM et al. Primary prevention of sudden cardiac death: trials in patients with coronary artery disease. J Interv Card Electrophysiol. 2003;9(2):203–206. doi: 10.1023/a:1026236524273. [DOI] [PubMed] [Google Scholar]
- 83.Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]


