Abstract
Pathophysiological conditions such as fibrosis, inflammation, and tumor progression are associated with modification of the extracellular matrix (ECM). These modifications create ligands that differentially interact with cells to promote responses that drive pathological processes. Within the tumor stroma, fibroblasts are activated and increase the expression of type I collagen. In addition, activated fibroblasts specifically express fibroblast activation protein-α (FAP), a post-prolyl peptidase. Although FAP reportedly cleaves type I collagen and contributes to tumor progression, the specific pathophysiologic role of FAP is not clear. In this study, the possibility that FAP-mediated cleavage of type I collagen modulates macrophage interaction with collagen was examined using macrophage adhesion assays. Our results demonstrate that FAP selectively cleaves type I collagen resulting in increased macrophage adhesion. Increased macrophage adhesion to FAP-cleaved collagen was not affected by inhibiting integrin-mediated interactions, but was abolished in macrophages lacking the class A scavenger receptor (SR-A/CD204). Further, SR-A expressing macrophages localize with activated fibroblasts in breast tumors of MMTV-PyMT mice. Together, these results demonstrate that FAP-cleaved collagen is a substrate for SR-A-dependent macrophage adhesion, and suggest that by modifying the ECM, FAP plays a novel role in mediating communication between activated fibroblasts and macrophages.
Introduction
Solid epithelial tumors evoke a reactive stromal response that is critical for growth and progression of the tumor. A reactive stroma is complex and consists of activated fibroblasts, newly formed vasculature, infiltrating immune cells, and extracellular matrix (ECM). Soluble signaling molecules such as cytokines and growth factors are well-documented mediators of interactions between cells in the tumor microenvironment. The tumor ECM also mediates communication between various cell types, in part by providing migration and adhesion signals [1]. Although increased deposition and cross-linking of collagen in the tumor stroma is associated with increased tumor growth [1–4], little is known about how specific ECM adhesion signals are created and regulated. It has been suggested that proteolytic cleavage of collagen increases cancer growth, invasion, and angiogenesis [5, 6]. Thus is likely that signals induced by changes in the ECM act in conjunction with soluble cytokines produced by other stromal components to modulate tumor growth.
Tumor associated fibroblasts (TAFs) are a key element of the reactive stroma. TAFs are fibroblasts that have undergone a major phenotypic change to an activated state characterized by increased proliferation, secretion of type I collagen, and expression of ECM-degrading proteases [7]. TAF-derived proteases present in the tumor microenvironment play a pivotal role in remodeling the ECM to make it permissive for tumor cell invasion and infiltration by normal endothelial cells and immune cells such as macrophages. Fibroblast Activation Protein-α (FAP) is a membrane-bound, serine protease that is expressed by activated fibroblasts including TAFs, but is absent from normal healthy adult tissues [8–10]. Although FAP reportedly cleaves type I collagen in vitro [11–14], the pathophysiological significance of FAP-generated collagen cleavage products is unclear. One possibility is that FAP participates in modifying ECM molecules to create and regulate cell adhesion in the tumor microenvironment.
Macrophages are another major cellular component of the tumor stroma and their infiltration and accumulation in the tumor microenvironment is correlated with tumor progression, invasion, and poor patient prognosis [15–18]. Tumor associated macrophages (TAMs) typically exhibit an M2 phenotype and are associated with secretion of a wide variety of growth factors, cytokines, and chemokines that suppress an anti-tumor immune response and promote tumor growth [16, 17]. Macrophages infiltrate and are retained in the tumor microenvironment through expression of specific adhesion proteins, such as integrins and scavenger receptors, that bind and mediate the adhesion of cells to ECM components of the tumor stroma. Interestingly, macrophages do not adhere well to native type I collagen [19, 20], which is the most abundant ECM protein in a tumor stroma [21]. This suggests that modifications of collagen are necessary for macrophage adhesion. Based on this background, we speculated that FAP, which is expressed by TAFs, cleaves collagen and converts it into an adhesive substrate for macrophages.
Materials and Methods
Animals
Animals used in this study include C57Bl/6 mice and SRA-/- mice in a C57Bl/6 background purchased from Jackson Laboratories (Bar Harbor, ME, USA); and MMTV-PyMT obtained from Dr. S. Gendler (Mayo Clinic, Scottsdale, AZ). All animals were maintained as colonies at the University of Arkansas for Medical Sciences and housed on a 12-hour light-dark cycle. To generate a breast tumor model, male MMTV-PyMT mice and C57Bl6 female mice were bred, and the offspring genotyped for the presence of MMTV-PyMT transgene. Breast tumors from female offspring that were heterozygous for MMTV-PyMT were isolated and used for tumor immunohistochemistry. All animals were provided food (Teklan Global 16% protein rodent diet; Harlan Laboratories, Indianapolis, IN, USA) and water ad libitum. Animal care and use were performed according to protocols reviewed and approved by the Institutional Animal Care and Use Committee at the University for Arkansas for Medical Sciences.
Protease activity assays
Recombinant human FAP (R&D Systems, Minneapolis, MN, USA) was incubated with dye quenched (DQ) type I collagen or type IV collagen (100 μg/ml; Life Technologies, Carlsbad, CA, USA) in assay buffer according to manufacturer’s instructions. Proteolytic cleavage of each DQ substrate was assessed by measuring fluorescence in a Synergy-2 plate reader (Biotek, Winooski, VT, USA) using excitation/emission wavelengths of 485/528 nm. Baseline fluorescence was determined by incubation of DQ substrates in assay buffer in the absence of FAP. As a control for proteolytic activity, FAP was incubated with its synthetic substrate Z-Gly-Pro-AMC (Bachem, Bubendorf, Switzerland) according to manufacturer’s instructions.
Cell isolation and treatment
Mouse peritoneal macrophages (MPMs) were isolated from C57BL/6J and SR-A-/- mice via peritoneal lavage with sterile saline from non-injected mice (for spreading assays) or from mice injected intraperitoneally with 4% thioglycollate 4 days prior to isolation (for attachment assays). For each assay, qualitatively similar results were obtained in preliminary experiments using non-elicited and elicited macrophages (data not shown). Isolated cells were immediately resuspended in DMEM GlutaMax (Life Technologies, Carlsbad, CA, USA) supplemented with FBS (10% vol/vol, Atlanta Biologicals, Flowery Branch, GA, USA), and penicillin/streptomycin (1%). Cell number and viability were assessed prior to use in experiments.
Macrophage adhesion assays
Macrophage attachment was assessed as described previously [22, 23]. Briefly, 12-well tissue culture dishes were coated for 5 h at 37°C with 10 μg/cm2 of type I collagen (Stem Cell Technologies, Vancouver, Canada) or fibronectin (Sigma, St. Louis, MO, USA). Collagen coated wells were then treated (24 h; 37°C) with buffer (control), recombinant FAP (1 μg/ml), or FAP that was inhibited with 4 mM PMSF or heat inactivated at 80°C for 10 min. The treated wells were washed, and freshly isolated MPMs (106 cells/well) plated for 30 min at 37°C. Non-adhered cells were removed by washing with PBS, and the number of cells remaining attached were quantified using a hemocytometer.
Macrophage spreading was similarly examined [22, 23]. Tissue culture chamber slides (Nalge Nunc International, Naperville, IL, USA) were coated with type I collagen (10 μg/cm2; 4°C; 24 h), and then treated for 24 h at 37°C with buffer (control), active FAP, or heat inactivated FAP (1 μg/ml). Treated wells were washed, and freshly isolated MPMs (0.15 x 106 cells/chamber) plated for 2 hr at 37°C. Non-adherent cells were removed, and then adherent cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were stained with Alexa-Fluor 647-conjugated phalloidin (Life Technologies) and nuclei were stained with DAPI. Images (40x) were digitally captured with Olympus CKX41 microscope and the surface area of cells quantified using AxioVision software (Carl Zeiss, Jena, Germany). Five independent images with at least 75 cells total were quantified in each experiment.
Immunohistochemistry of MMTV-PyMT tumors
Tumor tissues were isolated from MMTV-PyMT females before individual tumors reached 1 cm in any dimension. Isolated tumors were immediately embedded in OCT medium, rapidly frozen in liquid nitrogen, and stored at -80°C. Sections (8 μm) were cut onto glass slides and tissues fixed by incubation in ice-cold acetone for 20 min. Endogenous peroxidases were blocked with a dual endogenous enzyme block (Dako North America, Carpinteria, CA, USA). Additional blocking was performed with serum-free protein block (Dako) and 2.5% horse serum (Vector, Burlingame, CA, USA). To detect collagen, tissues were incubated with an anti-collagen antibody (Rabbit Anti-Mouse, EMD Millipore, Billerica, MA, USA), followed by Vector ImmPress Anti-Rabbit Reagent for alkaline phosphatase, and Vector ImmPact Red Substrate Solution. A dual-staining approach was used to identify SR-A-expressing macrophages and FAP-expressing fibroblasts. Following the blocking steps outlined above, tissues were incubated with primary anti-SR-A antibody (Goat Anti-Mouse, R&D Systems), then treated with Vector ImmPress Anti-Goat Reagent for peroxidase and Vector Immpact DAB Solution to visualize SR-A staining in brown. A second blocking step was then performed with 2.5% horse serum, and tissue sections incubated with primary anti-FAP antibody (Rabbit Anti-Mouse, Millipore), followed by Vector Immpress Anti-Rabbit Reagent for alkaline phosphatase and Vector Immpact Red Substrate Solution to visualize FAP staining in red. Tumor sections were counterstained with hematoxylin, dehydrated and coverslipped.
Statistical analysis
As indicated in individual figure legends, experiments were repeated at least three times and data analyzed with GraphPad Prism software using a t-test for comparing 2 groups, or ANOVA followed by the appropriate post-hoc statistical test to compare multiple groups. Differences with p < 0.05 were considered statistically significant.
Results
FAP selectively cleaves type I collagen
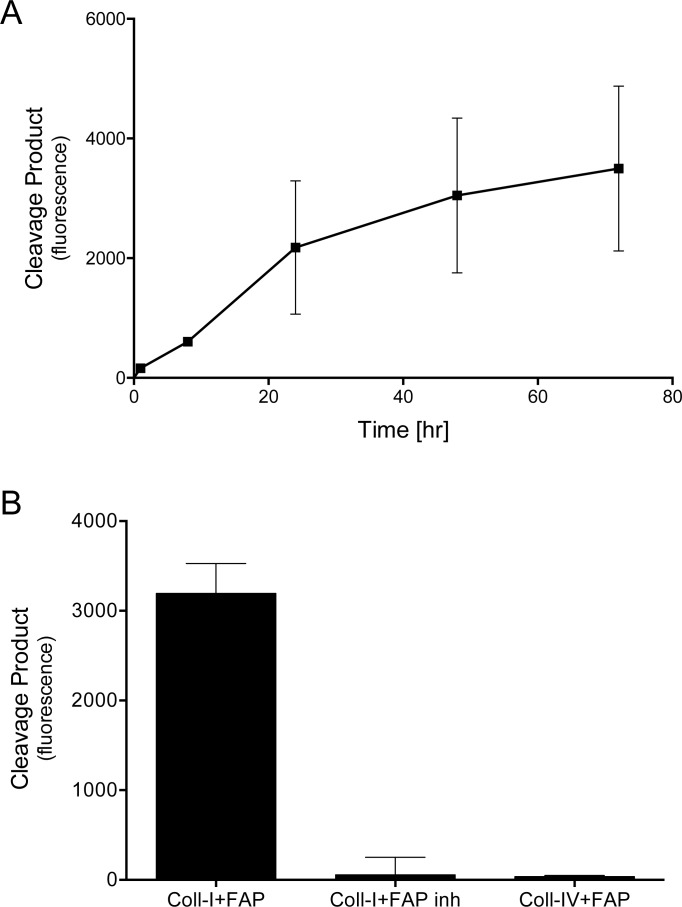
FAP has been reported to cleave type I collagen [11–14], which is the most abundant ECM protein in a tumor stroma [21]. This activity was confirmed using recombinant FAP and fluorescently quenched (DQ) collagen substrates that fluoresce when cleaved. As shown in Fig 1, incubating FAP (1 μg/ml) with DQ type I collagen resulted in a time-dependent increase in fluorescence that was most evident during the first 24 h (Fig 1A). Inhibiting FAP proteolytic activity with PMSF (Fig 1B) or by heat-inactivation (data not shown) prior to incubation with DQ type I collagen abolished the FAP dependent increase in fluorescence. In contrast to its effect on type I collagen, there was no increase in fluorescence when FAP was incubated with DQ type IV collagen (Fig 1B). Together these results demonstrate that FAP selectively cleaves type I collagen, and establish conditions (1 μg/ml, 24 h) that were used to examine the consequence of FAP-mediated collagen cleavage in the macrophage adhesion assays described below.
Fig 1. FAP selectively cleaves type I collagen.
(A) DQ type I collagen was incubated with FAP (1 μg/ml) for increasing times and the extent of substrate cleavage quantified by measurement of increasing fluorescence. (B) DQ type I collagen was incubated with active or PMSF inhibited FAP (1 μg/ml) and DQ type IV collagen was incubated with active FAP at the same concentration for 24 hr. Degradation of DQ collagens was quantified by measurement increasing fluorescence. Shown are the means ± SD of at least 3 experiments.
FAP-mediated cleavage of type I collagen increases macrophage adhesion
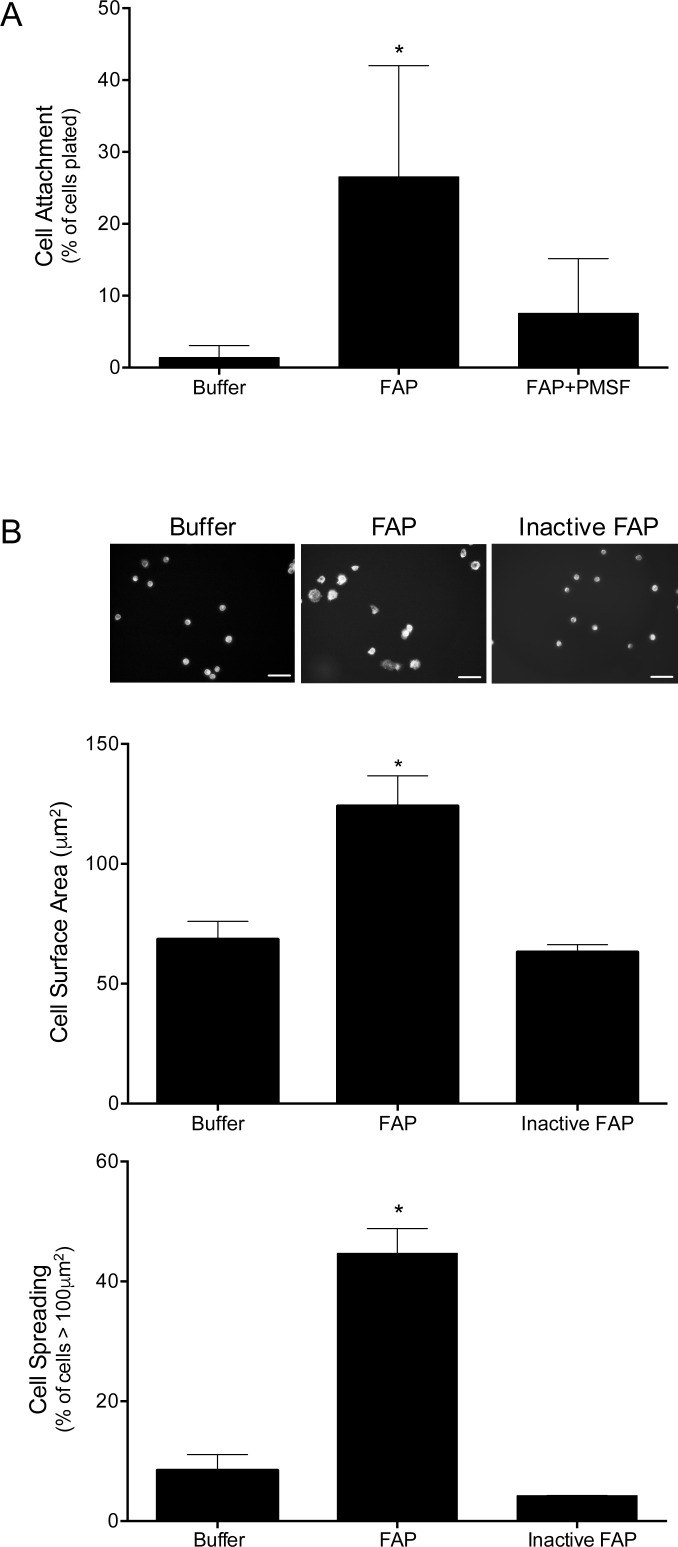
Specific modifications of collagen have been shown to enhance macrophage adhesion [19, 20, 24, 25]. We therefore tested whether macrophage adhesion to type I collagen was increased following FAP-mediated cleavage (Fig 2). Primary MPMs were plated for 30 min (to assess attachment) or 2 h (to assess spreading) on type I collagen-coated tissue culture dishes that were untreated, treated with catalytically active FAP, or treated with FAP that was inhibited with PMSF (FAP + PMSF) or heat inactivation (inactive FAP). Macrophages attached poorly (≤ 1% of plated cells) to untreated (native) type I collagen (Fig 2A). In contrast, the number of attached macrophages was significantly increased (> 30% of plated cells) when plated on collagen treated with active FAP, but not when plated on collagen treated with PMSF-inhibited FAP. Similarly, relative to macrophages plated for 2 h on native type I collagen, macrophages that were plated on type I collagen that was treated with active FAP exhibited enhanced spreading as evidenced by a significant increase in surface area with a high percentage of cells having a surface area > 100 μm2 (Fig 2B). Macrophage spreading on collagen treated with inactive FAP was similar to that on native collagen. Overall, these results demonstrate that macrophage adhesion to type I collagen is substantially enhanced by FAP-mediated cleavage.
Fig 2. FAP-mediated cleavage of type I collagen enhances macrophage adhesion.
(A) MPMs were adhered to type I collagen that was pretreated with buffer, FAP, and FAP inhibited with PMSF. Non-adhered cells were removed by washing, and the number of attached cells quantified and expressed as a percentage of total cells plated. Shown are the means ± SD of 4 experiments. Results were compared by one-way ANOVA with Tukey’s post-hoc test. (B) MPMs were adhered to type I collagen pretreated with buffer or FAP. Non-adhered cells were removed by washing, and attached cells were fixed and stained with fluorescent phalloidin and DAPI. Representative images were digitally captured and the surface area of cells quantified. Scale bars = 30 μm. Shown are the means ± SD of cell surface area and percentage of cells displaying a surface area > 100 μm2 from 3 experiments. Results were log-transformed and compared by one-way ANOVA with Dunnett’s post-hoc test. * indicates significant (p<0.05) difference from cells plated on buffer treated collagen.
Macrophage adhesion to FAP-cleaved collagen is integrin-independent
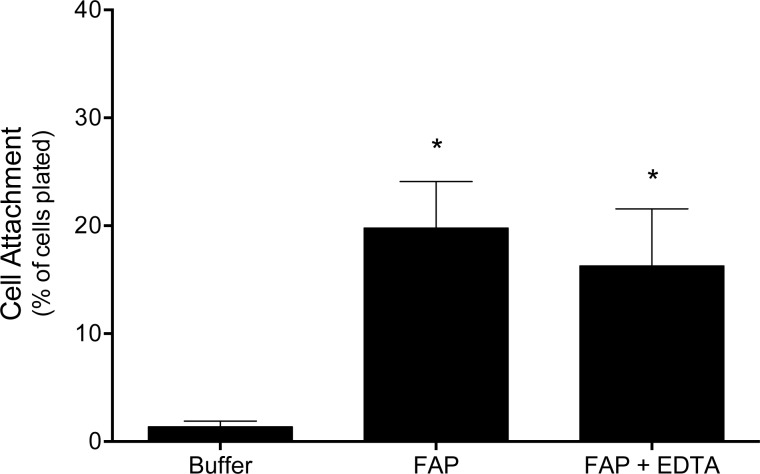
Integrins are widely expressed surface proteins that mediate Ca2+-dependent cell adhesion to ECM, thus sequestering extracellular cations can disrupt this interaction [26–29]. To determine whether increased macrophage adhesion to FAP-cleaved type I collagen is mediated by integrins, macrophage attachment was examined following chelation of divalent cations with EDTA. Primary MPMs were adhered to untreated (control) and FAP-cleaved type I collagen for 30 min in the presence or absence of EDTA (5 mM). Results depicted in Fig 3 show that macrophage attachment to type I collagen was substantially increased by FAP-mediated cleavage, and this increased attachment was not impaired by EDTA treatment. To confirm that integrin-mediated adhesion could be inhibited with this approach, MPM adhesion to fibronectin was tested in the presence and absence of EDTA. Over 80% of plated macrophages adhered to fibronectin in the absence of EDTA; however <10% adhered in the presence of EDTA confirming disruption of integrin-mediated adhesion (data not shown). The divalent cation independence of the increased macrophage adhesion to FAP-cleaved type I collagen indicates that this adhesion is not mediated by integrins.
Fig 3. Macrophage adhesion to FAP modified collagen is integrin independent.
Type I collagen-coated plates were pretreated with buffer or FAP, and then MPMs were adhered in the presence or absence of EDTA. Non-adhered cells were removed by washing, and the number of remaining attached cells quantified and expressed as a percentage of total cells plated. Shown are the means ± SD of 4 experiments. Results were compared with one-way ANOVA with Tukey’s post hoc test. * indicates significant (p<0.05) difference from cells plated on buffer treated collagen.
Macrophage adhesion to FAP-cleaved collagen is mediated by SR-A
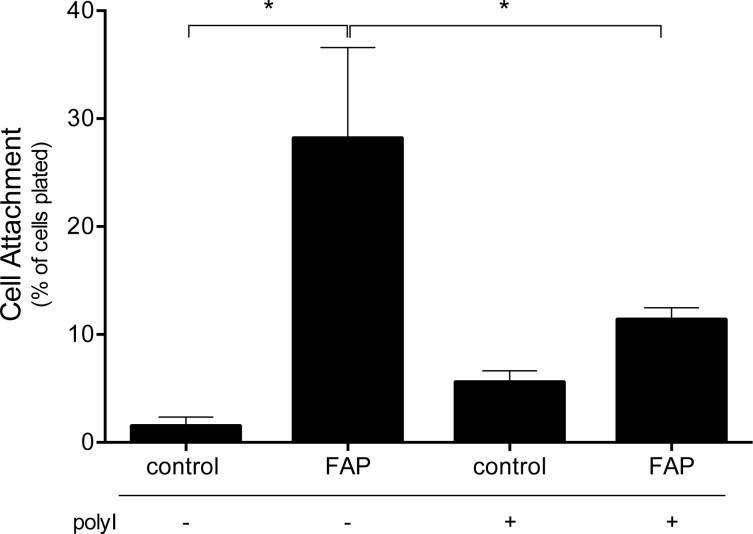
Macrophages express a variety of receptors that recognize modified ECM components. In particular, SR-A mediates the divalent cation independent macrophage adhesion to a variety of substrates including glucose-modified collagen, denatured collagen, and collagenase-cleaved type I collagen [20, 25, 30]. Therefore, we determined whether SR-A mediates macrophage adhesion to FAP-cleaved type I collagen. MPMs were adhered to tissue culture plates that were coated with type I collagen and left untreated (control) or treated with active FAP in the presence or absence of polyinosine, which is used to antagonize SR-A binding [31]. As shown in Fig 4, increased adhesion of MPMs to FAP-cleaved collagen was significantly inhibited in the presence of polyinosine. This result is consistent with a role for SR-A in mediating macrophage adhesion to FAP-cleaved collagen.
Fig 4. Polyinosine inhibits macrophage adhesion to FAP-modified type I collagen.
(A) Type I collagen-coated plates were pretreated with buffer or FAP, and then MPMs were adhered in the presence or absence of polyinosine. Non-adhered cells were removed by washing, and the number of attached cells quantified and expressed as a percentage of total cells plated. Shown are the means ± SD of 4 experiments. Results were compared by t-test. * indicates significant (p<0.05) differences between groups.
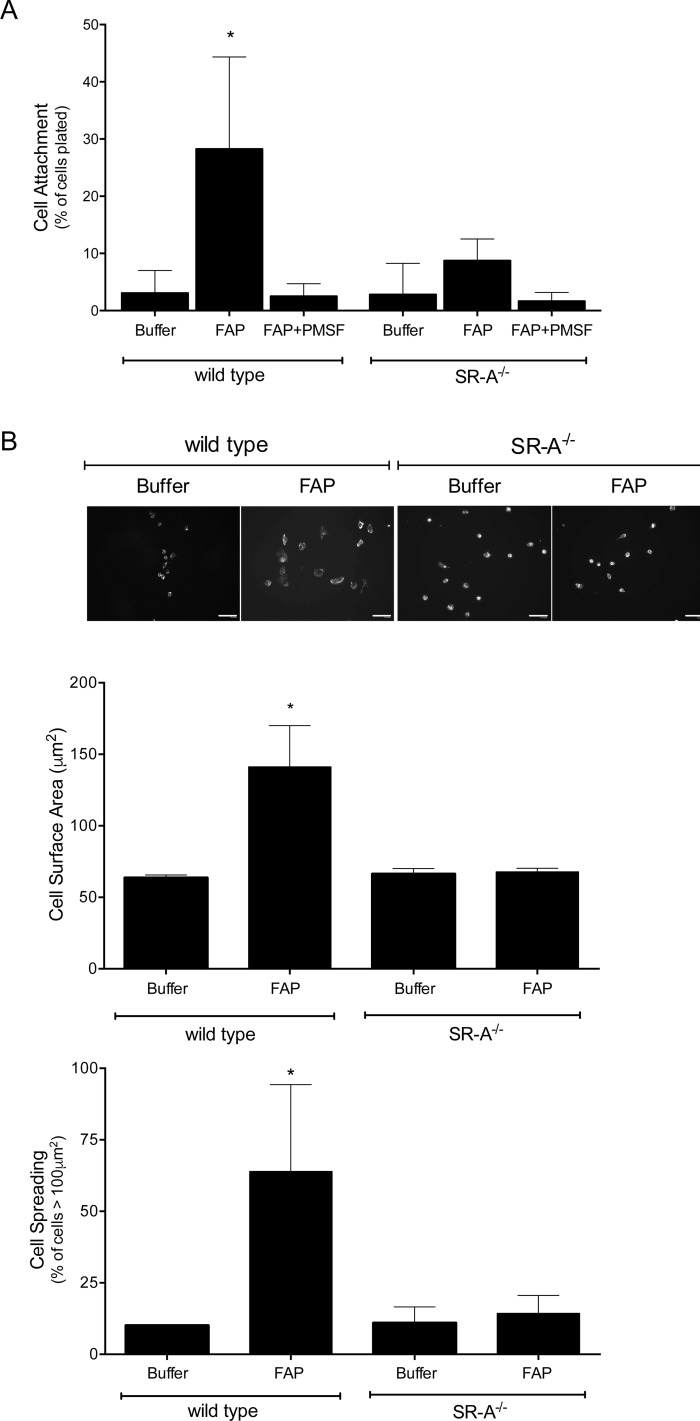
Although polyinosine binds SR-A, it is not specific for SR-A. Therefore, we compared macrophage adhesion to FAP-cleaved type I collagen using MPMs isolated from wild type (SR-A+/+) and SR-A knock out (SR-A-/-) mice. Primary MPMs were plated for 30 min (to assess attachment) or 2 h (to assess spreading) on type I collagen-coated tissue culture dishes that were untreated, treated with active FAP, or treated with inhibited FAP. As shown in Fig 5A, both wild type and SR-A-/- macrophages attached poorly to native type I collagen (buffer treated). Treating collagen with active FAP, but not with PMSF-inhibited FAP, significantly increased the attachment of wild-type, but not SR-A-/- macrophages. In parallel, the majority of wild-type and SR-A-/- macrophages remained rounded with a surface area < 100 μm2 when plated for 2 h on native type I collagen. In contrast, compared to SR-A-/- macrophages, significantly more wild-type macrophages adopted a spread morphology with a surface area >100 μm2 when plated on type I collagen that was treated with active FAP, but not inactive FAP (Fig 5B). These results identify SR-A as the macrophage receptor responsible for increased adhesion to FAP-cleaved type I collagen.
Fig 5. SR-A mediates macrophage adhesion to FAP-modified type I collagen.
(A) MPMs isolated from wild-type (SR-A+/+) or SR-A-/- mice were adhered to type I collagen pretreated with buffer, FAP, or PMSF-inhibited FAP. Non-adherent cells were removed by washing, and the number of attached cells quantified and expressed as a percentage of total cells plated. Shown are the means ± SD of 4 experiments. Results were compared by one-way ANOVA with Tukey’s post-hoc test. (B) MPMs were adhered to type I collagen pretreated with buffer or FAP. Non-adhered cells were removed by washing, and attached cells were fixed and stained with fluorescent phalloidin and DAPI. Representative images were digitally captured and the surface area of cells quantified. Scale bars = 30 μm. Shown are the means ± SD of cell surface area and percentage of cells displaying a surface area > 100 μm2 from 3 experiments. Results were compared by t-test. * indicates significant (p<0.05) difference from wild-type macrophages plated on buffer treated collagen.
SR-A expressing TAMs localize to the tumor stroma
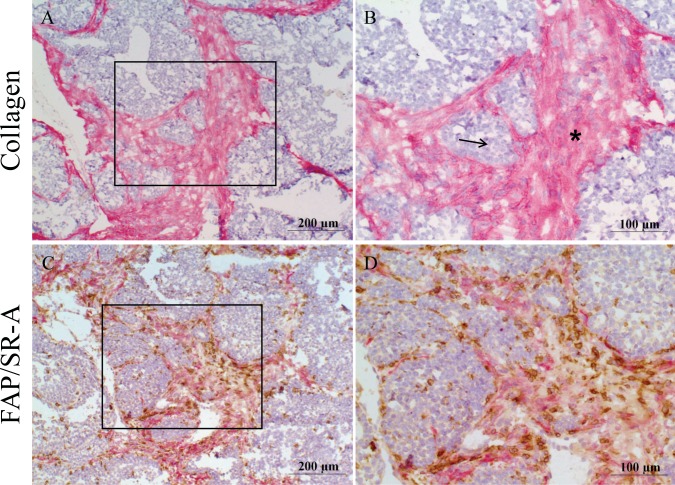
The finding that FAP-cleaved collagen enhances SR-A-mediated macrophage adhesion suggests that SR-A-expressing TAMs should be enriched in areas of tumor where TAFs are abundant. To assess this prediction, we isolated mammary tumors from female MMTV-PyMT mice and performed immunostaining for collagen, and dual staining for FAP, SR-A that would indicate co-localization of TAFs and TAMs, respectively. As shown in Fig 6, the breast tumors are characterized by a distinct collagen-rich stromal compartment surrounding the acini of malignant epithelial cells. Importantly, dual staining for FAP-expressing TAFs and SR-A-expressing TAMs showed an abundant macrophage infiltrate into these tumors and that FAP-positive fibroblasts and SR-A-positive macrophages are localized to the same stromal areas in the tumors. (Fig 6). This co-localization of FAP-expressing TAFs and SR-A-expressing TAMs in the collagen-rich stroma supports the mechanism for localized stromal cell communication mediated by FAP and SR-A via the ECM modifications.
Fig 6. SR-A expressing macrophages localize in the tumor stroma.
Consecutive frozen tumor sections were prepared from MMTV-PyMT mice and immunostained using A-B) a collagen antibody to detect stromal regions (red stain); C-D) a dual-stain technique with a FAP antibody to detect fibroblasts (red stain) and a SR-A antibody to visualize SR-A-positive macrophages (brown stain). Tissues were counterstained with hematoxylin and images digitally captured. B and D represent high power magnification (40x) of inset regions from A and C respectively. Arrows indicate acini of epithelial cells and asterisks indicate collagen-rich stroma.
Discussion
Our results demonstrate that FAP-mediated cleavage of collagen creates an adhesion substrate for macrophages that is recognized by SR-A. This conclusion is based on the increased attachment and spreading of macrophages on collagen treated with enzymatically active FAP, but not to collagen exposed to FAP that was enzymatically inactivated. The cation-independence of this adhesion and the disruption of this adhesive interaction by polyinosine, a compound that disrupts scavenger receptor interactions, suggested that SR-A mediates macrophage attachment to FAP-cleaved collagen. The specific importance of SR-A in mediating macrophage adhesion to FAP-cleaved collagen was confirmed by comparing the adhesion of wild type macrophages to those that lack SR-A. Together, these results identify FAP-cleaved type I collagen as a ligand for SR-A-mediated macrophage adhesion.
FAP is an activated fibroblast-specific protease that is implicated in modifications of tumor stroma [12, 14, 32, 33] and correlated with higher histological grades of malignancies, metastasis, and poor patient prognosis [34–36]. Importantly, FAP is not normally found in adult healthy tissues but occurs at sites of inflammation [12, 33, 37, 38]. Although the biological significance of specific FAP cleavage products is not known, there is increasing evidence that FAP activity plays an important role in modifying collagen matrices, particularly in tumors and atherosclerosis [13, 32, 39]. For example, the expression and proteolytic activity of FAP was correlated with the presence of macrophages in human atheroma [13]. In mouse tumor models, FAP-/- and FAP enzymatic inhibition slowed tumor growth, increased stromal collagen content, and decreased collagen fibril organization in the tumor stroma compared to tumor tissues isolated from FAP+/+ mice [32]. It was further shown that FAP-targeted elimination of TAFs in the 4T1 mouse model of breast cancer reduced infiltration of TAMs and other immune cells to the tumor site [40]. Our results demonstrating that FAP-cleaved collagen increases macrophage adhesion suggest a novel mechanism by which FAP regulates inflammatory responses within tumors by modulating the behavior of macrophages.
Macrophages are key contributors to many chronic inflammatory conditions [41–44]. Targeted influx and accumulation of macrophages are important determinants of macrophage function, and critical to the regulation of inflammatory, immune, and repair processes [45–47]. In cancer, macrophage accumulation and interaction with other cells in the tumor microenvironment are linked to poor patient prognosis [15–18]. Thus, an important implication of our findings is the possibility that increased FAP expression by TAFs in the tumor stroma results in the cleavage of type I collagen to promote the localized accumulation of macrophages.
A key feature of the model described above is that the adhesion of macrophages in tumors and/or chronic inflammatory sites is modulated by ECM modification. Macrophages express multiple receptors that recognize ECM components, including integrins and pattern recognition receptors (PRRs) such as SR-A [48–50]. SR-A mediates the cation-independent adhesion of macrophages to several modified ECM components including heat-denatured collagen and collagenase-treated type I collagen, but does not bind to native collagen [20, 24, 25, 51]. Our findings suggest that a novel biological function of FAP proteolysis of type I collagen is to expose previously masked adhesion sites for SR-A on the collagen. Although MMPs such as collagenase are known to aggressively degrade and convert native type I collagen into an adhesive substrate for SR-A, FAP is a serine protease that cleaves collagen differently than MMPs creating products that are not the same as those generated by collagenases [52–54]. Thus, one cannot assume a priori that previous findings using collagenase to degrade collagen [e.g., [20]] apply to FAP-cleaved collagen. Nonetheless, our results showing that macrophage adhesion to FAP-cleaved collagen is cation-independent, inhibited by polyinosine, and absent in SR-A-/- macrophages identify SR-A as a macrophage receptor that mediates adhesion to FAP-cleaved collagen.
The role of SR-A in infection, atherosclerosis and Alzheimer’s disease are well studied [reviewed in [55, 56]]. However, little is known about the contributions of SR-A to the formation and progression of tumors. Recent reports suggest that SR-A may be a marker for macrophages present in aggressive tumors [57, 58]. Importantly, Neyen et al. demonstrated that SR-A deficiency in vivo protects mice from tumor progression and metastasis [59]. In addition, we previously reported that SR-A-mediated adhesion activates multiple signaling cascades including Lyn-PI3K and PLA2-12/15LOX pathways [22, 23]. We further showed that SR-A-dependent macrophage adhesion induces the release of PGE2 which acts in an autocrine/paracrine mechanism to inhibit TNFα production and increase secretion of IL-10 [60]. Other studies examining the role of SR-A in mouse models of inflammatory disease demonstrated a similar association of SR-A with a tumor supportive M2 macrophage phenotype [61–63]. These reports suggest that in addition to promoting macrophage retention, SR-A-mediated interaction with components of the tumor microenvironment may contribute to the regulation of TAM phenotype.
Conclusion
Our results show that FAP cleavage of type I collagen promotes macrophage adhesion (attachment and spreading), and that these interactions are specifically mediated by macrophage class A scavenger receptors (SR-A/CD204). We further show that FAP positive TAFs and SR-A positive TAMs co-localize in the stroma of breast tumors from MMTV-PyMT mice. Together our results implicate a novel biological role for FAP-cleaved type I collagen, and define a mechanism for dialog between fibroblasts and macrophages via a FAP-ECM-SR-A axis, that could increase macrophage retention and function in tumors and potentially other inflammatory sites.
Acknowledgments
The authors would like to thank Beixiang He and Jessica Webber for technical assistance. We would also like to thank Dr. Ginell Post for critical reading of the manuscript. Support was provided by the Dept. of Pathology, the UAMS College of Medicine Research Council, and an NIH award to TK and SRP (R21CA185691).
Data Availability
All relevant data are within the paper.
Funding Statement
Support was provided by the Dept. of Pathology, the UAMS College of Medicine Research Council, and an NIH award to TK and SRP (R21CA185691). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196(4):395–406. 10.1083/jcb.201102147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73(6):1721–32. Epub 2013/01/25. 10.1158/0008-5472.CAN-12-2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng MR, Brugge JS. A stiff blow from the stroma: collagen crosslinking drives tumor progression. Cancer Cell. 2009;16(6):455–7. Epub 2009/12/08. 10.1016/j.ccr.2009.11.013 . [DOI] [PubMed] [Google Scholar]
- 4.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. Epub 2009/11/26. 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69(4):1517–26. Epub 2009/02/12. 10.1158/0008-5472.CAN-08-3255 . [DOI] [PubMed] [Google Scholar]
- 6.Taniwaki K, Fukamachi H, Komori K, Ohtake Y, Nonaka T, Sakamoto T, et al. Stroma-derived matrix metalloproteinase (MMP)-2 promotes membrane type 1-MMP-dependent tumor growth in mice. Cancer Res. 2007;67(9):4311–9. Epub 2007/05/08. 10.1158/0008-5472.CAN-06-4761 . [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. 10.1038/nrc1877 . [DOI] [PubMed] [Google Scholar]
- 8.Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, et al. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci U S A. 1994;91(12):5657–61. Epub 1994/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber MA, Kraut N, Park JE, Schubert RD, Rettig WJ, Peter RU, et al. Fibroblast activation protein: differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J Invest Dermatol. 2003;120(2):182–8. Epub 2003/01/25. 10.1046/j.1523-1747.2003.12035.x . [DOI] [PubMed] [Google Scholar]
- 10.Rosenblum JS, Kozarich JW. Prolyl peptidases: a serine protease subfamily with high potential for drug discovery. Current opinion in chemical biology. 2003;7(4):496–504. 10.1016/S1367-5931(03)00084-X . [DOI] [PubMed] [Google Scholar]
- 11.Kelly T. Evaluation of seprase activity. Clinical & experimental metastasis. 1999;17(1):57–62. Epub 1999/07/02. . [DOI] [PubMed] [Google Scholar]
- 12.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274(51):36505–12. . [DOI] [PubMed] [Google Scholar]
- 13.Brokopp CE, Schoenauer R, Richards P, Bauer S, Lohmann C, Emmert MY, et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur Heart J. 2011;32(21):2713–22. Epub 2011/02/05. 10.1093/eurheartj/ehq519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Muller E, et al. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology. 1999;29(6):1768–78. Epub 1999/05/29. 10.1002/hep.510290631 . [DOI] [PubMed] [Google Scholar]
- 15.Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33(19):2423–31. Epub 2013/06/04. 10.1038/onc.2013.191 . [DOI] [PubMed] [Google Scholar]
- 16.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clinical & developmental immunology. 2012;2012:948098 10.1155/2012/948098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer research. 2006;66(2):605–12. Epub 2006/01/21. 10.1158/0008-5472.CAN-05-4005 . [DOI] [PubMed] [Google Scholar]
- 18.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14(2):425–31. Epub 2005/07/14. . [PubMed] [Google Scholar]
- 19.Koyama Y, Norose-Toyoda K, Hirano S, Kobayashi M, Ebihara T, Someki I, et al. Type I collagen is a non-adhesive extracellular matrix for macrophages. Arch Histol Cytol. 2000;63(1):71–9. Epub 2000/04/19. . [DOI] [PubMed] [Google Scholar]
- 20.Gowen BB, Borg TK, Ghaffar A, Mayer EP. Selective adhesion of macrophages to denatured forms of type I collagen is mediated by scavenger receptors. Matrix Biology. 2000;19(1):61–71. [DOI] [PubMed] [Google Scholar]
- 21.Thomas DW, O'Neill ID, Harding KG, Shepherd JP. Cutaneous wound healing: a current perspective. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 1995;53(4):442–7. Epub 1995/04/01. . [DOI] [PubMed] [Google Scholar]
- 22.Nikolic DM, Cholewa J, Gass C, Gong MC, Post SR. Class A scavenger receptor-mediated cell adhesion requires the sequential activation of Lyn and PI3-kinase. Am J Physiol Cell Physiol. 2007;292(4):C1450–8. Epub 2006/12/29. 10.1152/ajpcell.00401.2006 . [DOI] [PubMed] [Google Scholar]
- 23.Nikolic DM, Gong MC, Turk J, Post SR. Class A scavenger receptor-mediated macrophage adhesion requires coupling of calcium-independent phospholipase A(2) and 12/15-lipoxygenase to Rac and Cdc42 activation. J Biol Chem. 2007;282(46):33405–11. Epub 2007/09/18. 10.1074/jbc.M704133200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkham PA, Spooner G, Ffoulkes-Jones C, Calvez R. Cigarette smoke triggers macrophage adhesion and activation: role of lipid peroxidation products and scavenger receptor. Free radical biology & medicine. 2003;35(7):697–710. Epub 2003/10/30. 10.1016/S0891-5849(03)00390-3 . [DOI] [PubMed] [Google Scholar]
- 25.El Khoury J, Thomas CA, Loike JD, Hickman SE, Cao L, Silverstein SC. Macrophages adhere to glucose-modified basement membrane collagen IV via their scavenger receptors. J Biol Chem. 1994;269(14):10197–200. [PubMed] [Google Scholar]
- 26.Qu A, Leahy DJ. The role of the divalent cation in the structure of the I domain from the CD11a/CD18 integrin. Structure. 1996;4(8):931–42. Epub 1996/08/15. . [DOI] [PubMed] [Google Scholar]
- 27.Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364(6435):343–6. [DOI] [PubMed] [Google Scholar]
- 28.Post SR, Gass C, Rice S, Nikolic D, Crump H, Post GR. Class A scavenger receptors mediate cell adhesion via activation of G(i/o) and formation of focal adhesion complexes. J Lipid Res. 2002;43(11):1829–36. Epub 2002/10/29. . [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, Chen J. The regulation of integrin function by divalent cations. Cell Adh Migr. 2012;6(1):20–9. 10.4161/cam.18702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santiago-Garcia J, Kodama T, Pitas RE. The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix. J Biol Chem. 2003;278(9):6942–6. Epub 2002/12/19. 10.1074/jbc.M208358200 . [DOI] [PubMed] [Google Scholar]
- 31.Lysko PG, Weinstock J, Webb CL, Brawner ME, Elshourbagy NA. Identification of a small-molecule, nonpeptide macrophage scavenger receptor antagonist. J Pharmacol Exp Ther. 1999;289(3):1277–85. Epub 1999/05/21. . [PubMed] [Google Scholar]
- 32.Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119(12):3613–25. Epub 2009/11/19. 10.1172/JCI38988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly T, Huang Y, Simms AE, Mazur A. Fibroblast activation protein-alpha: a key modulator of the microenvironment in multiple pathologies. International review of cell and molecular biology. 2012;297:83–116. 10.1016/B978-0-12-394308-8.00003-0 . [DOI] [PubMed] [Google Scholar]
- 34.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37(2):154–8. Epub 2008/07/31. 10.1097/MPA.0b013e31816618ce . [DOI] [PubMed] [Google Scholar]
- 35.Iwasa S, Okada K, Chen WT, Jin X, Yamane T, Ooi A, et al. 'Increased expression of seprase, a membrane-type serine protease, is associated with lymph node metastasis in human colorectal cancer'. Cancer Lett. 2005;227(2):229–36. Epub 2005/10/01. . [DOI] [PubMed] [Google Scholar]
- 36.Mikheeva SA, Mikheev AM, Petit A, Beyer R, Oxford RG, Khorasani L, et al. TWIST1 promotes invasion through mesenchymal change in human glioblastoma. Mol Cancer. 2010;9:194 Epub 2010/07/22. 10.1186/1476-4598-9-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990;87(18):7235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niedermeyer J, Scanlan MJ, Garin-Chesa P, Daiber C, Fiebig HH, Old LJ, et al. Mouse fibroblast activation protein: molecular cloning, alternative splicing and expression in the reactive stroma of epithelial cancers. Int J Cancer. 1997;71(3):383–9. . [DOI] [PubMed] [Google Scholar]
- 39.Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC cancer. 2011;11:245 Epub 2011/06/15. 10.1186/1471-2407-11-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PloS one. 2009;4(11):e7965 Epub 2009/12/04. 10.1371/journal.pone.0007965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gown AM, Tsukada T, Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986;125(1):191–207. Epub 1986/10/01. [PMC free article] [PubMed] [Google Scholar]
- 42.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–9. Epub 1993/04/29. 10.1038/362801a0 . [DOI] [PubMed] [Google Scholar]
- 43.Hirata K, Shikata K, Matsuda M, Akiyama K, Sugimoto H, Kushiro M, et al. Increased expression of selectins in kidneys of patients with diabetic nephropathy. Diabetologia. 1998;41(2):185–92. Epub 1998/03/14. 10.1007/s001250050888 . [DOI] [PubMed] [Google Scholar]
- 44.Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kido Y, et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52(10):2586–93. Epub 2003/09/30. . [DOI] [PubMed] [Google Scholar]
- 45.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183(5):1352–63. 10.1016/j.ajpath.2013.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93(6):875–81. 10.1189/jlb.1012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weidenbusch M, Anders HJ. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. Journal of innate immunity. 2012;4(5–6):463–77. 10.1159/000336717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ammon C, Meyer SP, Schwarzfischer L, Krause SW, Andreesen R, Kreutz M. Comparative analysis of integrin expression on monocyte-derived macrophages and monocyte-derived dendritic cells. Immunology. 2000;100(3):364–9. Epub 2000/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111(7):927–30. Epub 2003/01/01. . [DOI] [PubMed] [Google Scholar]
- 50.Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem. 2000;275(31):23409–12. [DOI] [PubMed] [Google Scholar]
- 51.El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382(6593):716–9. Epub 1996/08/22. 10.1038/382716a0 . [DOI] [PubMed] [Google Scholar]
- 52.Overall CM. Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol. 2002;22(1):51–86. 10.1385/MB:22:1:051 . [DOI] [PubMed] [Google Scholar]
- 53.Eckhard U, Huesgen PF, Schilling O, Bellac CL, Butler GS, Cox JH, et al. Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix biology: journal of the International Society for Matrix Biology. 2015. 10.1016/j.matbio.2015.09.003 . [DOI] [PubMed] [Google Scholar]
- 54.Edosada CY, Quan C, Tran T, Pham V, Wiesmann C, Fairbrother W, et al. Peptide substrate profiling defines fibroblast activation protein as an endopeptidase of strict Gly(2)-Pro(1)-cleaving specificity. FEBS Lett. 2006;580(6):1581–6. 10.1016/j.febslet.2006.01.087 . [DOI] [PubMed] [Google Scholar]
- 55.Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res. 2009;50 Suppl:S282–6. Epub 2008/12/17. 10.1194/jlr.R800066-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plüddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43(3):207–17. 10.1016/j.ymeth.2007.06.004 . [DOI] [PubMed] [Google Scholar]
- 57.Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, et al. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2010;5(10):1507–15. Epub 2010/08/31. 10.1097/JTO.0b013e3181eba692 . [DOI] [PubMed] [Google Scholar]
- 58.Yang G, Addai J, Tian WH, Frolov A, Wheeler TM, Thompson TC. Reduced infiltration of class A scavenger receptor positive antigen-presenting cells is associated with prostate cancer progression. Cancer Res. 2004;64(6):2076–82. Epub 2004/03/18. . [DOI] [PubMed] [Google Scholar]
- 59.Neyen C, Pluddemann A, Mukhopadhyay S, Maniati E, Bossard M, Gordon S, et al. Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. J Immunol. 2013;190(7):3798–805. Epub 2013/03/01. 10.4049/jimmunol.1203194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nikolic DM, Vadali S, He B, Ware J, Kelly T, Post SR. Prostaglandins produced during class A scavenger receptor-mediated macrophage adhesion differentially regulate cytokine production. J Leukoc Biol. 2015;97(5):901–8. 10.1189/jlb.2A1014-471RR . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, et al. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186(9):1431–9. 10.1084/Jem.186.9.1431 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cotena A, Gordon S, Platt N. The class A macrophage scavenger receptor attenuates CXC chemokine production and the early infiltration of neutrophils in sterile peritonitis. J Immunol. 2004;173(10):6427–32. Epub 2004/11/06. . [DOI] [PubMed] [Google Scholar]
- 63.Tsujita K, Kaikita K, Hayasaki T, Honda T, Kobayashi H, Sakashita N, et al. Targeted deletion of class A macrophage scavenger receptor increases the risk of cardiac rupture after experimental myocardial infarction. Circulation. 2007;115(14):1904–11. Epub 2007/03/29. 10.1161/CIRCULATIONAHA.106.671198 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.