Abstract
Biomarkers in exhaled breath are useful for respiratory disease diagnosis in human volunteers. Conventional methods that collect non-volatile biomarkers, however, necessitate an extensive dilution and sanitation processes that lowers collection efficiencies and convenience of use. Electret filter emerged in recent decade to collect virus biomarkers in exhaled breath given its simplicity and effectiveness. To investigate the capability of electret filters to collect protein biomarkers, a model that consists of an atomizer that produces protein aerosol and an electret filter that collects albumin and carcinoembryonic antigen-a typical biomarker in lung cancer development- from the atomizer is developed. A device using electret filter as the collecting medium is designed to collect human albumin from exhaled breath of 6 volunteers. Comparison of the collecting ability between the electret filter method and other 2 reported methods is finally performed based on the amounts of albumin collected from human exhaled breath. In conclusion, a decreasing collection efficiency ranging from 17.6% to 2.3% for atomized albumin aerosol and 42% to 12.5% for atomized carcinoembryonic antigen particles is found; moreover, an optimum volume of sampling human exhaled breath ranging from 100 L to 200 L is also observed; finally, the self-designed collecting device shows a significantly better performance in collecting albumin from human exhaled breath than the exhaled breath condensate method (p<0.05) but is not significantly more effective than reported 3-stage impactor method (p>0.05). In summary, electret filters are potential in collecting non-volatile biomarkers in human exhaled breath not only because it was simpler, cheaper and easier to use than traditional methods but also for its better collecting performance.
Introduction
Though volatile organic compounds (VOCs) in human breath have been studied and reviewed in depth in recent decades [1–10], increasing interests are seen in studies on non-volatile biomarkers in exhaled breath of human volunteers. Biomarker collection approaches have been abundant yet at different collection efficiencies [11, 12]. Among them is the exhaled breath condensate (EBC) method, where biomarkers, in the form of epithelial lining fluid (ELF) droplets from airway [13], are diluted approximately 20000-fold by condensed water vapor [14]. Unfortunately, the resulting concentration of biomarkers often falls below the detection limit of commercially available equipment, let alone the repeatability and verifiability [15–17]. It has been reported that inner coating of collection surface could make EBC method more efficiency, however, different coatings or even different condensing systems favor different biomarkers in breath [18]. Moreover, this method offers limited help in in vivo study [18]. The reasons include that the EBC method wastes more than 90% of the sub-micron particles in exhaled breath [19].
3-stage impactor is the second avenue to collect particles in exhaled breath based on inertia [20]. In particular, particles with greater inertia attach to the plate in the first stage, while those with less inertia flow through nozzles and enter into the following stages. Although such method have been reported to successfully collect protein particles [21, 22], the complexity of collection device and plate sanitation inhibit its widespread use.
The third and most recent means is electret filter that collects exhaled particles through electrostatic forces, Brownian diffusion, inertial impaction, and interception. The former two work on sub-micron particles (<1 μm) while the rest target bigger particles [11]. In human exhaled breath, most particles are charged (as their isoelectric points don’t equal to the pH of exhaled breath) or polarized by the electret filter with dimensions on the order of sub-microns [23–25], and thus allow for collection via electret filters using electrostatic forces. Collection of biomarkers such as influenza virus and human rhinovirus using electret filters has been attempted with success [26, 27], but studies on the collection of protein biomarkers using this method are rarely seen. One study compares cytokine particle collection using electret filter to that using a SKC Biosampler® and Omni 300TM but without consideration of collection capability changes (especially in samples contain liquid particles such as human exhaled breath) of the electret filter [11].
In this paper, a model that combines production and collection of liquid protein particles using electret filters is developed. Carcinoembryonic antigen (CEA)-a common biomarkers in lung cancer development [28, 29]and potential biomarker in early detection of lung cancer-from atomized aerosol and albumin-the indicative of early asthma deterioration [30]and a widely used reference marker of dilution in bronchoalveolar lavage fluid [31, 32]-both in atomized particles and human exhaled breath are studied. To our best knowledge, collection of these two proteins in either atomized aerosol or human exhaled breath is not reported.
A self-designed collection device using electret filter as collection medium is compared to two previously reported collecting methods in terms of the amounts of albumin in human exhaled breath collected. The results shows that electret filter has a better performance than EBC method though it is not more effective than 3-stage impactor method in collecting protein particles from human exhaled breath.
Materials and Methods
Model experiment
Experiment setup
A model was set up to produce aerosol particles containing proteins to mimic the “exhalation” scenario (Fig 1). The model used a medical atomizer (FOLEE, China) to produce aerosol particles and high purity nitrogen to carry the particles to the collection site.
Fig 1. Schematic diagram of experiment setup used in this study.
Electret filters (North, Honeywell Inc., Morristown, NJ) with the same lot number were cut into circular shapes with diameters of 2 cm. When collecting, the circular electret filters were fixed in the collection site of the pipe (Fig 1). To make the atomization solutions, CEA and albumin (Cloud-Clone Corp., Houston, TX) were dissolved in phosphate buffer saline (PBS, 0.01 M and pH 7.4). The final concentrations of CEA and albumin were 2.5 ng/ml and 50 ng/ml, respectively. The protein solutions were then atomized by the atomizer to produce aerosol particles in diameters ranging from 0.5μm to 10μm. The produced particles were sprayed into the collection pipe by the atomizer at a flow rate of 5 L/min. Then the high purity nitrogen delivered the aerosol particles to the electret filter in the collection site. Flow rate of the carrier gas was kept at 10 L/min by a control valve and was monitored by a gas flowmeter (Siargo Inc., Santa Clara, CA). The overall flow rate was maintained at 15 L/min which was measured using gas flow meter (Siargo Inc., Santa Clara, CA).
Study method
To check atomizer stability during the experiment, the atomizing rate was firstly assessed by measuring amounts of atomized PBS (0.01 M, pH 7.4) solution in different atomizing durations. In the model experiment, 7 circular electret filters were then used to collect aerosol particles atomized using prepared CEA solution for 7 different periods (collecting particles for 1 min, 2 min, …, 7 min, respectively) according to the collection method introduced above. Collecting albumin particles produced by atomizing the albumin solution was also performed to validate the collection effectiveness of the electret filter for protein particles. In the control group, only PBS (0.01 M, pH 7.4) solution containing no protein was used instead of protein solutions to produce aerosol particles. Other treatments in the control group were the same as in the normal group.
The weights of the filters and the atomizing cup of the atomizer containing the protein solution were measured before and after collection with a high precision electronic scale (Sartorius AG, Gottingen, Germany).
Collecting human exhaled breath particles
Device design
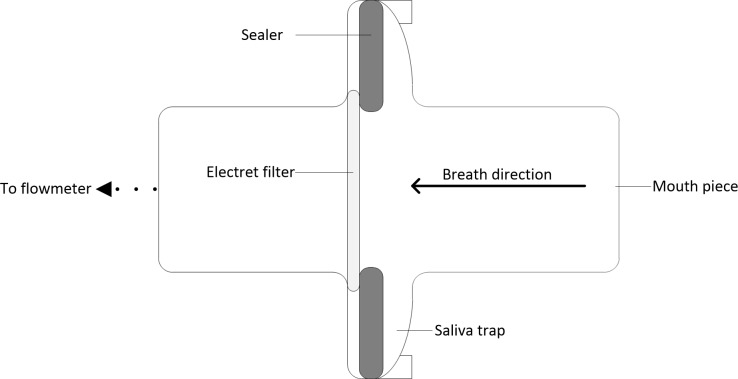
Based on the effectiveness of collecting protein in atomized particles, collecting protein in human exhaled breath particles was also studied. A device simply consisted of a mouth piece, a saliva trap and a rubber sealer was designed for this purpose (Fig 2). The electret filter (diameter 2 cm) was also used as the collecting medium fixed in the collecting site of the device. When collecting, a gas flow meter (Siargo Inc., Santa Clara, CA) was connected at the outlet end of the device to measure the volume of exhaled breath.
Fig 2. Schematic diagram of the designed collecting device in vertical section.
Study population
Six healthy volunteers recruited from Chongqing, China, participated in this study. Three men (S-2 and S-5 were smokers with no clinical overt disease) and three women were involved. Protocols of this study was approved by Medical Ethics Committee of Chongqing. All volunteers signed the informed consent after the procedure of the study was explained in detail. Additional information about the volunteers is provided in Table 1.
Table 1. Study population.
| M/Fa | Age (years) Median (range) | Smokers |
|---|---|---|
| 3/3 | 35 (24,54) | 2/6 |
aM/F: number of males and females
Study design
According to previous reports, particle concentration increases 10-70-folds in exhaled breath after taking a deep breath and exhaling to residual volume comparing to tidal breathing [25]. Thus a similar breathing maneuver was employed in this experiment. All volunteers were asked to wear a nose clip throughout the collecting procedure (Fig 3). The detailed breathing maneuver was described as follows:
Fig 3. Collecting exhaled breath particles using a self-designed collecting device.
Before collecting, all volunteers rinsed their mouth for 3 minutes with purified water and breathed deeply for 1 minute.
Volunteers inhaled the ambient air to their vital capacities.
Volunteers exhaled the breath into the designed collection device horizontally at a rate of 50 L/min until reaching their residual volumes.
Volunteers repeat step 2 and step 3 to give desired volumes of breath.
During the course of collection, volunteers were asked to swallow their accumulated saliva to avoid contamination. Particles from 7 different breath volumes (50 L breath, 100 L breath, …, 350 L breath, respectively) obtained from each volunteer were collected. In the control group, 7 designed collecting devices were implanted to the inlet port of an air pump that intake ambient air at the flow rate of 50 L/min. Volumes of air passed though the filters were the same as the volumes of human exhaled breath.
Because albumin is widely used as a reference marker of dilution in bronchoalveolar lavage fluid [31, 32], and thus indicates albumin is relatively constant in the epithelial lining fluid (ELF) of healthy volunteers. Only the concentration of exhaled albumin was measured for its relatively stable origin.
Filter treatment after collecting
After collecting particles, all filters were cut into pieces and transferred to 5 ml centrifuge tubes (Eppendorf, Hamburg, Germany). 2 ml of eluent (0.01 M PBS containing 0.13% Tween-20) was added to the tubes. Filter pieces were flushed repeatedly using the eluent until all pieces were fully wet. The tubes containing filter pieces were then transferred into an ultrasonic cleaner (cleaning for 5 minutes) to wash out proteins collected by the filters. After that, 1 ml of the eluate was transferred to a 1.5 ml centrifuge tube (Eppendorf, Hamburg, Germany) and centrifuged under 1000 r/min for 2 minutes (Centrifuge 5804R, Eppendorf, Germany). At last, 0.5 ml supernatant of the eluate was used to measure protein concentrations.
Protein concentration measurement
Salivary amylase was firstly measured to ensure that samples were not contaminated by saliva. Concentrations of CEA and albumin in the samples were measured using commercial SEAl50Hu and HEB028Hu ELISA kit (Cloud-Clone Corp., Houston, TX) respectively according to the manufacturer’s instructions and analyzed using the same ELISA plate. A microplate reader (Tecan, Mannedorf, Switzerland) was used to read plate absorbance at 450 nm (the reference wavelength was set at 620 nm). Protein concentrations were calculated from a four-parametric standard curve fitted using OriginPro V9.0.0 (OriginLab Corporation, Northampton, MA).
Calculations
Generally, weights of electret filters increased after collection. We calculated the weight increase of the filter as follows:
| (1) |
where Δm (g) is weight increase of an electret filter, mFB (g) is the weight of the filter before collecting, and mFA (g) is the weight of the filter after collecting.
Because protein concentrations in a nebulizer solution (and in exhaled breath of human) were very low, the weight increase of the filter after collecting was mainly caused by water. The amount of protein collected using an electret filter can be calculated as follows:
| (2) |
where MC (ng or pg) is the amount of protein collected using the electret filter, Δm (g) is the amount of liquid particles that the filter collected, CC (ng/ml or pg/ml) is the protein concentration measured by ELISA (CC below the limit of detection was assigned equal to LOD / √2) [22], ρ (1 g/ml) is the density of water, and VE (2 ml) is the volume of eluent used.
Mean collecting efficiencies of protein aerosol particles using an electret filter were calculated as follows:
| (3) |
where EC (%) is the collecting efficiency of the electret filter method, CpB and CpA (both in pg/ml or ng/ml) are the concentrations of proteins in the nebulizer measured using ELISA before and after atomization, respectively. And VcB (ml) and VcA (ml) are the volumes of the protein solution in the nebulizer cup before and after atomization respectively. These volumes were calculated by weight changes of the protein solutions in the atomization cup.
Statistics
In the model experiment, Levene’s test was firstly used to assess the equality of variances of the obtained data if the variances in the population differed significantly (p<0.05), and Kruskal-Wallis ANOVA was performed if the amount of atomized protein solution significantly influenced the weight increase of the filters and the amounts of proteins collected.
In the experiment collecting human exhaled breath particles, Kruskal-Wallis ANOVA was also performed to analyze whether breath volumes of each volunteer significantly affected the weight increase of the filter and the amount of albumin collected. Finally, Kruskal-Wallis ANOVA was performed again to test if the collecting efficiencies of 3 different methods were significantly different from each other.
All statistical analyses were performed using Origin Pro 9.0.0 (OriginLab Corporation, MA, USA).
Results
The mean percentage change of the atomizing rate for different collecting durations was 3.85%±2.88% (mean±SD). This little change of the atomizing rate would guarantee a stable origin of aerosol particles for collection experiment.
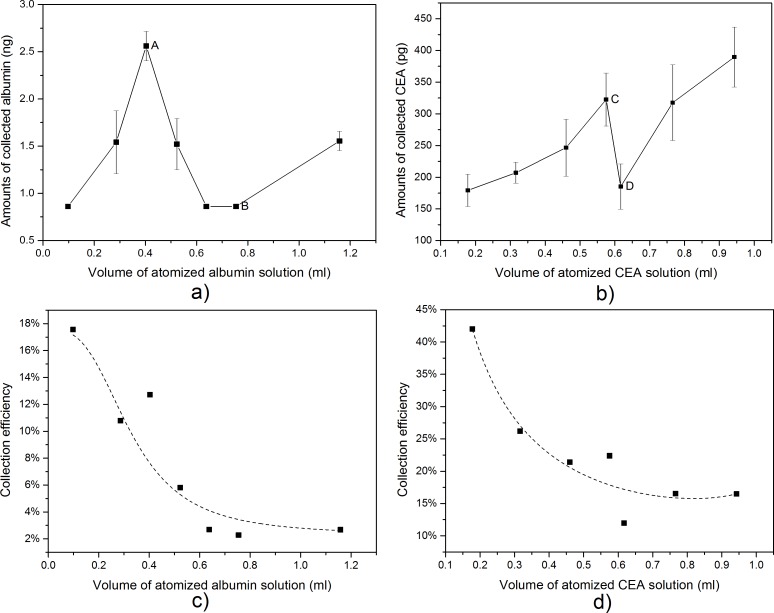
In the model experiment, the amounts of collected proteins (both CEA and albumin) in the atomized particles had a similar trend with the volume of the atomized protein solution increased (Fig 4). At first, the amounts of collected protein increased with the volume of atomized protein solution, then deceased, and finally increased again. Nevertheless, our results showed that the volume of atomized protein solution had no significant effect on the amounts of collected protein (Kruskal-Wallis ANOVA, p>0.05). In addition, decreased collecting efficiencies of the electret filters in this experiment were observed. As shown in Fig 4, a decrease of collection efficiency for atomized albumin particles from 17.6% to 2.3% and a decrease of collection efficiency for atomized CEA particles from 42% to 12.5% were found.
Fig 4. Collecting ability of electret filters in collecting atomized protein particles.
(a) Amounts of collected albumin when the volume of atomized albumin solution increased. Amounts of collected albumin increased as the volume of atomized albumin solution increased before point A, then decreased, and finally increased again after point B. (b) Amounts of collected CEA when the volume of atomized CEA solution increased. Amounts of collected CEA increased as the volume of atomized CEA solution increased before point C, then decreased, and finally increased again after point D. (c) Mean collecting efficiency of electret filters when the volume of atomized albumin solution increased. (d) Mean collecting efficiency of electret filters when the volume of atomized CEA solution increased. Amounts of collected proteins were calculated by Eq 2. Collecting efficiencies were calculated by Eq 3. Error bars shown in a) and b) were standard deviation of triplicate experiments.
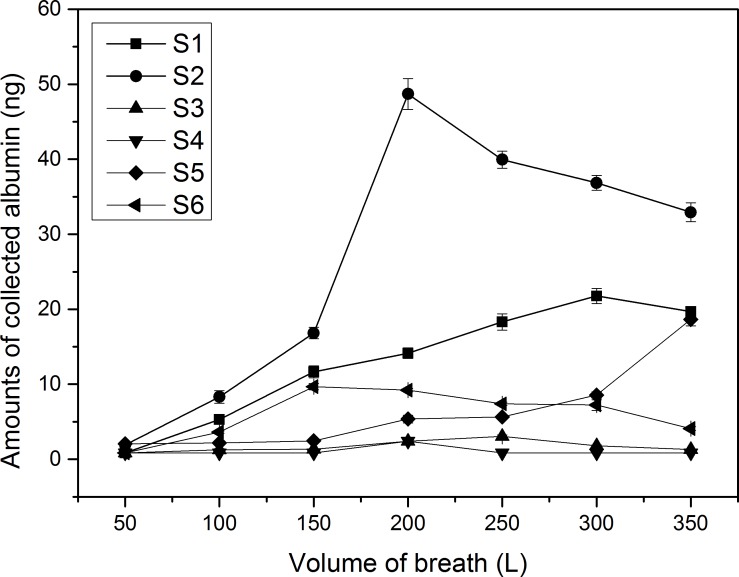
In the experiment collecting human exhaled breath particles, amounts of collected albumin had a similar trend as the volume of breath increased comparing to the model experiment (except volunteer S-5). However, amounts of collected albumin did not increase in the final stage when comparing to the model experiment (Fig 5). In addition, different volunteers and different breath volumes of the same volunteer significantly affected the collected albumin (Kruskal-Wallis ANOVA, p<0.05). However, an optimum breath volume range of 100 L-200 L for collecting human exhaled breath particles using the electret filter method can be determined from the obtained data. A smaller breath volume may not be able to collect enough target biomarkers to be detected, and a higher breath volume may face the same problem and, at the same time, increase the discomfort of volunteers.
Fig 5. Amounts of albumin in human exhaled breath collected using self-designed collecting devices.
The amounts of collected albumin were calculated by Eq 2. Error bars shown in the figure were standard deviation of triplicate measurements of each sample.
In both experiments, the weight of all of the electret filters increased after collection (Table A and Table B in S1 File). In the model experiment, increased weights of the filters were not significantly affected by the volume of atomized protein solution (Kruskal-Wallis ANOVA, p>0.05). However, exhaled breath volume was found significantly affected the weight increase of filters when collecting human exhaled breath particles (Kruskal-Wallis ANOVA, p<0.05).
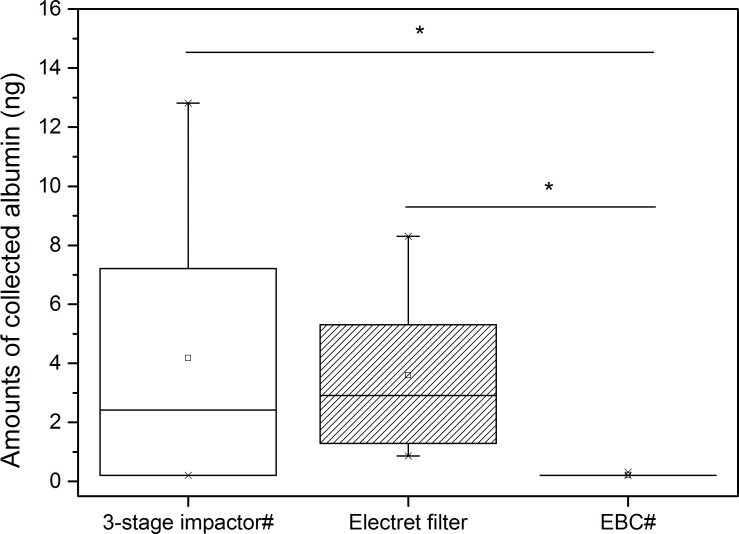
A comparison of collecting ability of the electret filter method with other 2 reported methods (3-stage compactor and EBC method) was performed based on the amounts of albumin collected from exhaled breath particles (Fig 6). The breathing maneuvers of volunteers and the volume of exhaled breath were the same as in the 2 reported methods. The results showed that our self-designed collection device based on the electret filter method was significantly more effective than the EBC method in collecting albumin from human exhaled breath (Kruskal-Wallis ANOVA, p<0.05) but was not more effective than the 3-stage impactor (Kruskal-Wallis ANOVA, p>0.05).
Fig 6. Comparison of collecting ability of 3 different collecting methods.
*indicates significance at p<0.05. # indicates boxplot was drawn by the author from data in [22].
In the model experiment, electret filters that collected particles produced using PBS contained no detectable levels of albumin and CEA. In the experiment collecting human exhaled breath particles, electret filters that collected particles in ambient air contained no detectable levels of albumin.
Discussion
Increases and subsequent decreases in the effectiveness of collecting protein using an electret filter in both the model and human volunteer experiments were observed in this study. The reasons were listed as follows:
At the beginning of collection, sub-micron particles (<1μm) in atomized aerosol (or in exhaled breath) were collected because of electrostatic forces between the particles and the electret fiber [11, 23]. Therefore, collected proteins increased as the volume of atomized protein solution (or exhaled breath) increased. Increased protein concentration in the atomized droplets may also play a role in increasing the amounts of collected protein in the model experiment [33].
As the collecting procedure progressed, more and more liquid particles were collected by the electret fiber, and a water layer was formed around the fiber. This layer can weaken the electric field around the fibers [34]. On the other hand, the opposite particle charge of the particles can also neutralize the electret charge to decay the electrostatic forces [34]. Both factors contributed to the subsequent decrease of collecting effectiveness of the electret filters.
In the model experiment, amounts of collected protein both increased after point B and point D in the collection curve (Fig 4). However, a similar trend was not observed in the experiment of human volunteers (Fig 5). The difference may be partly caused by different particle size distribution in these two experiments. The atomized aerosol contained more large particles (>0.5μm) while exhaled breath contained fewer [24, 25, 35]. Because the electret fibers were covered by a water layer, large particles were more easily captured as a result of inertial impaction. An increasing concentration of protein particles in atomized droplets may also have contributed to the increase of collected protein in the model experiment [33].
As discussed above, the electrostatic forces of the electret filter were weakened during the collection of liquid particles. As introduced earlier, electrostatic forces were main mechanisms of electret filters in collecting sub-micron particles (<1μm), thus a decay in electrostatic forces caused a decrease of collection efficiency of the electret filter, as seen in Fig 4C and Fig 4D. This finding agreed with the results obtained by Ji et al who collected atomized NaCl and dioctyl sebacate particles using electret filters [36]. These results may indicate that, in future experiments, an effective method for obtaining relatively higher concentrations of collected protein in exhaled breath particles is to dry the exhaled breath or use more non-wettable electret filters.
Contrary to the results obtained from human volunteers, the weight of the electret filters and the amounts of collected protein were not significantly affected by an increase of the volume of the atomized solution. This difference may be also caused by bigger liquid particles in our model experiment, in which inertial impaction played a role. Nevertheless, weight changes (mainly caused by water) of the electret filter in both the model and human volunteer experiments were negligible (<0.015 g) when comparing to 2 ml of eluent added in the subsequent experiment.
Rosias et al reported EBC method obtained 69.4% of albumin recovery in in vitro study and 7/13 albumin positive detection in in vivo study (asthmatic children as volunteers) using silicone coating in the collection surface [18]. However, the electret filters method showed a better performance. The albumin recovery rate in our model experiment before elution process reached more than 2500% (though the final albumin recovery rate drop to less than 2.5% because of more than 1000-folds dilution in subsequent elution process in the study, data calculated based on Table B in S2 File). And our human study (healthy adults as volunteers) showed 6/6 of albumin positive detection. The results indicated that electret filter method had a better collection ability than the EBC method in collecting albumin in exhaled breath. It was consistent with comparison results demonstrated in Fig 6.
Even so, EBC method may collect some volatile biomarkers (such as NH4+ and HCO3- in condensate) that electret filters cannot collect effectively. Although our self-designed collecting device was not more effective than the 3-stage impactor in collecting albumin in human breath particles, it was much cheaper, simpler and easier to use. The device can be even used as a disposable collection accessory to collect particle biomarkers when using other respiratory equipment (e.g., a spirometer). In addition, the electret filter collecting method has the potential to obtain higher protein concentrations by, for example, drying the breath samples, using more non-wettable electret filters and reducing consumption of eluent. Moreover, charging the exhaled breath particles may make the electret filter more effective in collecting them [37, 38].
Limitations of study
Firstly, we did not study the collecting efficiency of electret filters in different particle concentrations. Different concentrations of the liquid particles may decay the electrostatic force of the electret filter differently and thus influence their collecting efficiency. The fact is, to our knowledge, particle concentration in different people varies significantly [39, 40].
Secondly, we did not consider the charging state of the particles (both in atomizer and exhaled breath). Actually, according to the formula obtained by Chikao Kanaoka et al [41], the collecting efficiency of electret filter would change as the charging state of the particles. To address this problem, exhaled samples should be charged or uncharged uniformly in future studies.
Moreover, electret filters can only collect non-volatile biomarkers in aerosol particles of breath, volatile biomarkers (volatile organic compounds, for example) in exhaled breath cannot be collected by this method.
Finally, only 6 volunteers and two model proteins were included in our study. To obtain more conclusive results, more volunteers and biomarkers should be involved.
Conclusions
Our study found successful collection of proteins in both atomized aerosol and exhaled breath particles using electret filters, though with a decreasing collection efficiency. In addition, the amounts of collected albumin in exhaled breath vary with volunteers and the volume of exhaled breath. Furthermore, an optimum breath volume ranging from 100L to 200 L for collecting proteins in exhaled breath particles using the electret filter method was found.
We also found that our self-designed collecting device using an electret filter as the collecting medium had a much better collection ability than traditional EBC method in collecting non-volatile biomarkers in exhaled breath. Although it was not more effective in collecting albumin in human exhaled breath particles than the 3-stage impactor, it was much simpler, cheaper and easier to use, and had more potential to improve its performance. However, volatile biomarkers in exhaled breath cannot be collected by this method. Future research includes assessing the performance of collecting other particle biomarkers in exhaled breath using this method and improving the collection performance of the electret filter method by appropriate desiccation of exhaled breath sample, using more non-wettable electret filters and a better eluting method which could considerably reduce usage of eluent and charging biomarker particles in the sample uniformly.
Supporting Information
(DOCX)
(XLSX)
Acknowledgments
The authors wish to thank all the volunteers for their participation in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 81101172, http://www.nsfc.gov.cn/), the National Key Technologies R&D Program (No. 2012BAI19B03 and 2013BAI03B04, http://program.most.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gordon SM, Szidon JP, Krotoszynski BK, Gibbons RD, Oneill HJ. Volatile Organic-Compounds in Exhaled Air from Patients with Lung-Cancer. Clin Chem. 1985;31(8):1278–82. . [PubMed] [Google Scholar]
- 2.Phillips M. Method for the collection and assay of volatile organic compounds in breath. Anal Biochem. 1997;247(2):272–8. 10.1006/abio.1997.2069 . [DOI] [PubMed] [Google Scholar]
- 3.Phillips M, Gleeson K, Hughes JMB, Greenberg J, Cataneo RN, Baker L, et al. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet. 1999;353(9168):1930–3. 10.1016/S0140-6736(98)07552-7 . [DOI] [PubMed] [Google Scholar]
- 4.Miekisch W, Schubert JK, Noeldge-Schomburg GFE. Diagnostic potential of breath analysis—focus on volatile organic compounds. Clin Chim Acta. 2004;347(1–2):25–39. 10.1016/j.cccm.2004.04.023 . [DOI] [PubMed] [Google Scholar]
- 5.Mazzone PJ. Analysis of volatile organic compounds in the exhaled breath for the diagnosis of lung cancer. J Thorac Oncol. 2008;3(7):774–80. 10.1097/JTO.0b013e31817c7439 [DOI] [PubMed] [Google Scholar]
- 6.Broza YY, Haick H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine-Uk. 2013;8(5):785–806. 10.2217/Nnm.13.64 . [DOI] [PubMed] [Google Scholar]
- 7.Amann A, Mochalski P, Ruzsanyi V, Broza YY, Haick H. Assessment of the exhalation kinetics of volatile cancer biomarkers based on their physicochemical properties. J Breath Res. 2014;8(1). Artn 016003 10.1088/1752-7155/8/1/016003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolinska A, Klaassen EMM, Dallinga JW, van de Kant KDG, Jobsis Q. Profiling of Volatile Organic Compounds in Exhaled Breath As a Strategy to Find Early Predictive Signatures of Asthma in Children (vol 9, e95668, 2014). Plos One. 2014;9(8). ARTN e105447 10.1371/journal.pone.0105447 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broza YY, Mochalski P, Ruzsanyi V, Amann A, Haick H. Hybrid Volatolomics and Disease Detection. Angew Chem Int Edit. 2015;54(38):11036–48. 10.1002/anie.201500153 . [DOI] [PubMed] [Google Scholar]
- 10.Krilaviciute A, Heiss JA, Leja M, Kupcinskas J, Haick H, Brenner H. Detection of cancer through exhaled breath: a systematic review. Oncotarget. 2015;6(36):38643–57. 10.18632/oncotarget.5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenzie JH, McDevitt JJ, Fabian MP, Hwang GM, Milton DK. Collection of Aerosolized Human Cytokines Using Teflon (R) Filters. Plos One. 2012;7(5). ARTN e35814 10.1371/journal.pone.0035814 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Conrad DH, Chow S, Tran VH, Yates DH, Thomas PS. Collection devices influence the constituents of exhaled breath condensate. Eur Respir J. 2007;30(4):807–8. 10.1183/09031936.00080207 . [DOI] [PubMed] [Google Scholar]
- 13.Mutlu GM, Garey KW, Robbins RA, Danziger LH, Rubinstein I. Collection and analysis of exhaled breath condensate in humans. Am J Resp Crit Care. 2001;164(5):731–7. . [DOI] [PubMed] [Google Scholar]
- 14.Effros RM, Dunning MB, Biller J, Shaker R. The promise and perils of exhaled breath condensates. Am J Physiol-Lung C. 2004;287(6):L1073–L80. 10.1152/ajplung.00069.2004 . [DOI] [PubMed] [Google Scholar]
- 15.Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Balint B, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26(3):523–48. 10.1183/09031936.05.00029705 . [DOI] [PubMed] [Google Scholar]
- 16.Sapey E, Bayley D, Ahmad A, Stockley R. The validation of assays used to measure biomarkers in exhaled breath condensate. Eur Respir J. 2008;32(5):1408–9. 10.1183/09031936.00088608 . [DOI] [PubMed] [Google Scholar]
- 17.Koczulla AR, Noeske S, Herr C, Koepke J, Jorres RA, Nell C, et al. Alpha-1 antitrypsin is elevated in exhaled breath condensate and serum in exacerbated COPD patients. Resp Med. 2012;106(1):120–6. 10.1016/j.rmed.2011.06.015 . [DOI] [PubMed] [Google Scholar]
- 18.Rosias PP, Robroeks CM, Niemarkt HJ, Kester AD, Vernooy JH, Suykerbuyk J, et al. Breath condenser coatings affect measurement of biomarkers in exhaled breath condensate. Eur Respir J. 2006;28(5):1036–41. Epub 2006/07/28. 09031936.06.00110305 [pii] 10.1183/09031936.06.00110305 . [DOI] [PubMed] [Google Scholar]
- 19.Verreault D, Moineau S, Duchaine C. Methods for sampling of airborne viruses. Microbiol Mol Biol R. 2008;72(3):413–44. 10.1128/Mmbr.00002-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almstrand AC, Ljungstrom E, Lausmaa J, Bake B, Sjovall P, Olin AC. Airway Monitoring by Collection and Mass Spectrometric Analysis of Exhaled Particles. Analytical Chemistry. 2009;81(2):662–8. 10.1021/ac802055k . [DOI] [PubMed] [Google Scholar]
- 21.Bredberg A, Gobom J, Almstrand AC, Larsson P, Blennow K, Olin AC, et al. Exhaled Endogenous Particles Contain Lung Proteins. Clin Chem. 2012;58(2):431–40. 10.1373/clinchem.2011.169235 . [DOI] [PubMed] [Google Scholar]
- 22.Larsson P, Mirgorodskaya E, Samuelsson L, Bake B, Almstrand AC, Bredberg A, et al. Surfactant protein A and albumin in particles in exhaled air. Resp Med. 2012;106(2):197–204. 10.1016/j.rmed.2011.10.008 . [DOI] [PubMed] [Google Scholar]
- 23.Wang CS, Otani Y. Removal of Nanoparticles from Gas Streams by Fibrous Filters: A Review. Ind Eng Chem Res. 2013;52(1):5–17. 10.1021/ie300574m . [DOI] [Google Scholar]
- 24.Holmgren H, Ljungstrom E, Almstrand AC, Bake B, Olin AC. Size distribution of exhaled particles in the range from 0.01 to 2.0 mu m. J Aerosol Sci. 2010;41(5):439–46. 10.1016/j.jaerosci.2010.02.011 . [DOI] [Google Scholar]
- 25.Fabian P, Brain J, Houseman EA, Gern J, Milton DK. Origin of Exhaled Breath Particles from Healthy and Human Rhinovirus-Infected Subjects. J Aerosol Med Pulm D. 2011;24(3):137–47. 10.1089/jamp.2010.0815 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabian P, McDevitt JJ, DeHaan WH, Fung ROP, Cowling BJ, Chan KH, et al. Influenza Virus in Human Exhaled Breath: An Observational Study. Plos One. 2008;3(7). ARTN e2691 10.1371/journal.pone.0002691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stelzer-Braid S, Oliver BG, Blazey AJ, Argent E, Newsome TP, Rawlinson WD, et al. Exhalation of Respiratory Viruses by Breathing, Coughing, and Talking. J Med Virol. 2009;81(9):1674–9. 10.1002/jmv.21556 . [DOI] [PubMed] [Google Scholar]
- 28.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76(2):138–43. 10.1016/j.lungcan.2011.11.012 . [DOI] [PubMed] [Google Scholar]
- 29.Zhu WY, He JY, Chen DD, Zhang BJ, Xu LY, Ma HJ, et al. Expression of miR-29c, miR-93, and miR-429 as Potential Biomarkers for Detection of Early Stage Non-Small Lung Cancer. Plos One. 2014;9(2). ARTN e87780 10.1371/journal.pone.0087780 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khor YH, Teoh AKY, Lam SM, Mo DCQ, Weston S, Reid DW, et al. Increased vascular permeability precedes cellular inflammation as asthma control deteriorates. Clin Exp Allergy. 2009;39(11):1659–67. 10.1111/j.1365-2222.2009.03349.x . [DOI] [PubMed] [Google Scholar]
- 31.Ward C, Duddridge M, Fenwick J, Gardiner PV, Fleetwood A, Hendrick DJ, et al. Evaluation of Albumin as a Reference Marker of Dilution in Bronchoalveolar Lavage Fluid from Asthmatic and Control Subjects. Thorax. 1993;48(5):518–22. 10.1136/Thx.48.5.518 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pocino K, Minucci A, Manieri R, Conti G, De Luca D, Capoluongo ED. Description of an Automated Method for Urea Nitrogen Determination in Bronchoalveolar Lavage Fluid (BALF) of Neonates and Infants. J Lab Autom. 2015. Epub 2015/01/15. 2211068214567147 [pii] 10.1177/2211068214567147 . [DOI] [PubMed] [Google Scholar]
- 33.Phipps PR, Gonda I. Droplets Produced by Medical Nebulizers—Some Factors Affecting Their Size and Solute Concentration. Chest. 1990;97(6):1327–32. 10.1378/chest.97.6.1327 . [DOI] [PubMed] [Google Scholar]
- 34.Otani Y, Emi H, Mori J. Initial Collection Efficiency of Electret Filter and Its Durability for Solid and Liquid Particles. Kagaku Kogaku Ronbun. 1992;18(2):240–7. . [Google Scholar]
- 35.Morawska L, Johnson GR, Ristovski ZD, Hargreaves M, Mengersen K, Corbett S, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40(3):256–69. 10.1016/j.jaerosci.2008.11.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji JH, Bae GN, Kang SH, Hwang J. Effect of particle loading on the collection performance of an electret cabin air filter for submicron aerosols. J Aerosol Sci. 2003;34(11):1493–504. 10.1016/S0021-8502(03)00103-4 . [DOI] [Google Scholar]
- 37.Park JH, Yoon KY, Kim YS, Byeon JH, Hwang J. Removal of submicron aerosol particles and bioaerosols using carbon fiber ionizer assisted fibrous medium filter media. J Mech Sci Technol. 2009;23(7):1846–51. 10.1007/s12206-009-0613-z . [DOI] [Google Scholar]
- 38.Park JH, Yoon KY, Hwang J. Removal of submicron particles using a carbon fiber ionizer-assisted medium air filter in a heating, ventilation, and air-conditioning (HVAC) system. Build Environ. 2011;46(8):1699–708. 10.1016/j.buildenv.2011.02.010 . [DOI] [Google Scholar]
- 39.Fairchild CI, Stampfer JF. Particle Concentration in Exhaled Breath—Summary Report. Am Ind Hyg Assoc J. 1987;48(11):948–9. . [DOI] [PubMed] [Google Scholar]
- 40.Wan GH, Wu CL, Chen YF, Huang SH, Wang YL, Chen CW. Particle Size Concentration Distribution and Influences on Exhaled Breath Particles in Mechanically Ventilated Patients. Plos One. 2014;9(1). ARTN e87088 10.1371/journal.pone.0087088 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanaoka C, Emi H, Otani Y, Iiyama T. Effect of Charging State of Particles on Electret Filtration. Aerosol Sci Tech. 1987;7(1):1–13. 10.1080/02786828708959142 . [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.