Abstract
Ecological associations where one species enhances habitat for another nearby species (facilitations) shape fundamental community dynamics and can promote niche expansion, thereby influencing how and where species persist and coexist. For the many breeding birds facing high nest-predation pressure, enemy-free space can be gained by nesting near more formidable animals for physical protection. While the benefits to protected species seem well documented, very few studies have explored whether and how protector species are affected by nest protection associations. Long-legged wading birds (Pelecaniformes and Ciconiiformes) actively choose nesting sites above resident American alligators (Alligator mississippiensis), apparently to take advantage of the protection from mammalian nest predators that alligator presence offers. Previous research has shown that wading bird nesting colonies could provide substantial food for alligators in the form of dropped chicks. We compared alligator body condition in similar habitat with and without wading bird nesting colonies present. Alligator morphometric body condition indices were significantly higher in colony than in non-colony locations, an effect that was statistically independent of a range of environmental variables. Since colonially nesting birds and crocodilians co-occur in many tropical and subtropical wetlands, our results highlight a potentially widespread keystone process between two ecologically important species-groups. These findings suggest the interaction is highly beneficial for both groups of actors, and illustrate how selective pressures may have acted to form and reinforce a strongly positive ecological interaction.
Introduction
Facilitation is a positive ecological exchange in which one species enhances habitat for another nearby species (sensu [1]). Identifying and assessing the strength of facilitative interactions has enriched our understanding of species coexistence/persistence (e.g., [2–6]), and of the factors shaping populations and communities (e.g., [7–9]). The potential for ecological facilitation to expand niche boundaries also challenges the long-held notion of species interactions necessarily causing niche shrinkage [10–12].
Creation of enemy-free space is one common currency of facilitative exchange, and theory predicts that this form of facilitation will occur most frequently in communities where members experience high consumer pressure (e.g., predation, herbivory) [13,14]. For many bird species nest predation is the greatest threat to reproductive success [15–19], so breeding birds may nest near more-formidable animals for physical protection (e.g., [20–23]). Despite the wealth of literature on nest protection associations (reviewed in [24–26]), only six papers assess costs/benefits to the protective species (“protectors” hereafter; Table 1). This research bias in avian nest protection associations mirrors that in facilitations more generally, as most facilitation research focuses on fitness effects to the partner ostensibly receiving benefits (but see [27]). In both cases a largely unilateral approach limits our understanding of how these interactions evolve and persist [1,26].
Table 1. Published results regarding fitness effects on protectors in avian nest protection associations.
| Protector | Protectee | Effect on protector | |||
|---|---|---|---|---|---|
| Order | Species | Species | Direction | Form | Source |
| Charadriiformes | Whimbrel (Numenius phaeopus) | Bar-tailed godwit (Limosa lapponica) | None | N/A | [28] |
| 3 species (family: Laridae) | Sand-colored nighthawk (Chordeiles rupestris) | (−) | Nest defense (higher cost) | [29] | |
| Passeriformes | Rufous-fronted thornbird (Phacellodomus rufifrons) | > 10 species (orders: Passeriformes, Galliformes) | (+) a | Nest defense (higher efficacy) | [30] |
| (−) a | Aggression / nest predation | ||||
| Falconiformes | Merlin (Falco columbarius) | Fieldfare (Turdus pilaris) | (+) | Nest defense (higher efficacy) | [31] |
| Lesser kestrel (Falco naumanni) | Jackdaw (Corvus monedula) | (+) | Nest defense (lower cost) | [32] | |
| Hymenoptera | Polistine wasp (Ropalidia cincta) | Red-cheeked cordonbleu (Uraeginthus bengalus) | None | N/A | [22] |
a The author could not draw definitive conclusions for effects of individual species due to small sample sizes.
Nutritional benefits to protectors have not been explored [26]. This is despite (1) many protectors commonly consuming young and eggs of the protected species (“protectees” hereafter) [26] and (2) aggregations of breeding birds often increasing local primary and secondary productivity, which could provide nutritive benefits to protectors [33–35]. Further, many colonially nesting birds lay more eggs than they can raise, and adjust brood size to fit available food resources through several processes of brood reduction (reviewed in [36]). This often amounts to 1–2 chicks being ejected alive or dead from each nest, providing a potentially substantial source of food for protectors, especially in concentrated nesting associations [37]. Thus colonies of breeding birds offer multiple avenues through which they could nutritionally benefit protectors that do not necessitate exploitation by either partner.
In this study we report on benefits for American alligators (Alligator mississippiensis) that associate with nesting colonies of long-legged wading birds (orders Ciconiiformes and Pelecaniformes: herons, egrets, ibises, storks, and spoonbills; “wading birds” hereafter). In mixed-species wading bird nesting colonies in the southeastern United States, medium-sized, arboreal, semiaquatic mammals such as North American raccoons (Procyon lotor) and Virginia opossums (Didelphis virginiana) present the greatest nest predation threat, and these birds have no evolved defences against such nest predators [38,39]. Recent research suggests that wading birds actively choose nesting sites above alligators, and that in wetlands, there is a mutually exclusive distribution of alligators and mammalian predators [40]. Together with evidence that alligators readily consume mammals [41–44], there is reasonably strong evidence that alligators deter mammalian nest predators, thereby greatly increasing reproductive success for nesting wading birds.
Wading bird nesting aggregations can substantially increase nearby nutrient deposition [45–47] and may enhance primary and secondary productivity as a result. Moreover, the quantity of food potentially available to scavengers from wading bird colonies via dead chicks is substantial, enough to theoretically support large populations of alligators [37]. Given the potential for significant energetic benefits to alligators in wading bird nesting colonies, we predicted that alligators associated with wading bird colonies will have higher body condition indices than alligators in similar habitat without colonies.
Materials and Methods
Ethics Statement
All animal use was approved by the University of Florida’s Institute of Food and Agricultural Sciences Animal Research Committee under Approval No. 007-13WEC. All field work and sample collection was performed under Florida Fish and Wildlife Conservation Commission Scientific Collecting Permit No. SPGS-13-58 and United States Fish and Wildlife Service Arthur R. Marshall Loxahatchee National Wildlife Refuge Special Use Permit No. B14-006. All efforts were made to minimize stress to animals during measurements and tissue sampling, and study animals were released at the point of capture immediately after processing (within ~1 hour).
Study Sites
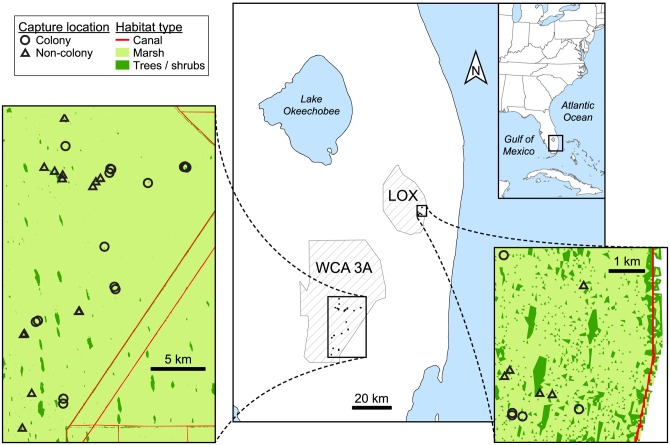
This study took place in the Everglades of Florida, USA: Water Conservation Area 3A (WCA 3A; 25.961°, −80.701°) in Miami-Dade and Broward Counties, and Arthur R. Marshall Loxahatchee National Wildlife Refuge (LOX; 26.489°, −80.337°) in Palm Beach County (Fig 1). These freshwater marshes are a mosaic of habitats including deeper-water sloughs, wet prairies, sawgrass (Cladium jamaicense) strands, and elevated tree islands. Hydrologic conditions fluctuate seasonally (by ~40 to >100 cm, depending on the area and year) with lowest water depths during the November to May dry season. Wading bird nesting colonies are predominantly located in inundated, lower-elevation islands with the longest hydroperiods [39]. In WCA 3A these islands are typically dominated by coastalplain willows (Salix caroliniana), while in LOX most are comprised of swamp bay (Persea palustris), dahoon holly (Ilex cassine), and other trees/shrubs.
Fig 1. Map of the study area.
Locations of Water Conservation Area 3A (WCA 3A) and Arthur R. Marshall Loxahatchee National Wildlife Refuge (LOX) in the Everglades of Florida, USA, and inset maps of adult female alligator capture sites. In capture-site maps, habitat type shows the densities of tree islands (clusters of trees / shrubs) and locations of canals near capture sites; white areas in the LOX inset are non-habitat. Open-source data for habitat type were obtained from the Ecological Modeling Team at Everglades National Park: http://simglades.org/.
Alligators were captured in sloughs surrounding tree islands that were designated as either colony or non-colony sites as follows. We used data from systematic, 100% coverage aerial and ground surveys conducted during the nesting season to locate active wading bird nesting colonies (see [48] for details). Approximately half of the alligators were caught within 200 m of islands with 20–800 nests of wading birds (colony sites); the other half were caught near islands that (1) were > 1 km from the nearest active colony and (2) had not been occupied by nesting birds in the previous 5 years (non-colony sites). Both colony and non-colony sites were > 1 km from the nearest canal because these unnatural areas affect alligator size-distributions [49] and fish abundances [50]. The distances of 1 km and 200 m were derived from radio telemetry studies that estimated daily linear movements and home range sizes for alligators in our study area [51]. For non-colony sites in WCA 3A, we used Google Earth [52] to identify tree islands bordered by the same canals, of similar size and vegetation composition, and within 5–10 km of each colony site. In LOX, the ubiquity of tree islands and their relatively similar size allowed us to use any location > 1 km away from the nearest nesting colony as a non-colony site.
Alligator Sampling
We captured adult and subadult (≥ 125 cm total length) alligators by noose or hand from an airboat between 2000 and 0530 hours in June of 2013 and 2014. We began captures immediately after bird nesting had largely been completed, to minimize our disturbance to active nests. Because female alligators have smaller home ranges and move less than males in the Everglades [51], we assumed that female body condition should be more reflective of food opportunities from an individual tree island than male body condition. Because of this and the fact that adult females have the greatest influence upon alligator population dynamics [53], we only used females for analyses. We determined sex by cloacal examination and recorded geographical coordinates of capture location. We measured snout-vent length dorsally (± 0.1 cm) and body mass (± 0.5 kg) using a spring scale.
For hematological indices, we extracted 5 mL of blood from the cranial sinus of each captured alligator using a 20-G needle, which was immediately transferred to either sodium (2013) or lithium (2014) heparin Vacutainer tubes and centrifuged (3,400 rpm for 10 minutes). Because this location of blood draw is subject to dilution from lymph or cerebrospinal fluid, we cross-referenced plasma total protein concentrations from other alligator studies [54]. Blood samples were all taken within fifteen minutes of initial noosing or hand-capture. We pipetted the plasma into vials and stored them in a cooler of ice until we returned from the field. The plasma was stored in a freezer at −20°C (2013) [55] or on dry ice or in a −80°C freezer (2014) until the tests were run. The frozen plasma was transported to the Avian & Wildlife Laboratory, University of Miami (Miami, FL) for analyses.
Condition Indices
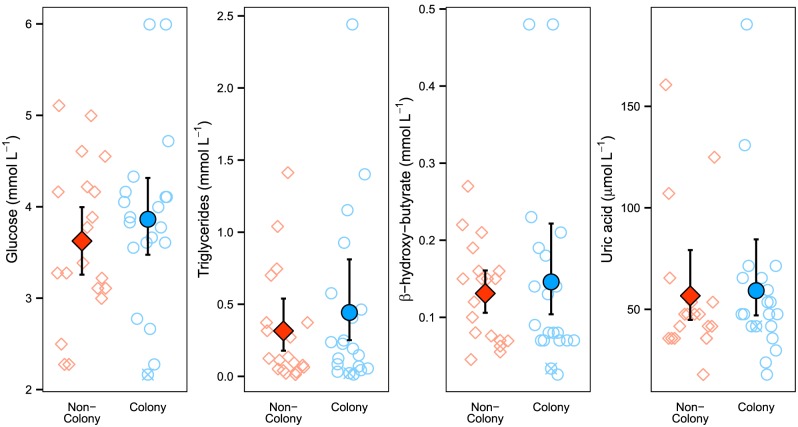
Four hematological markers were used as indicators of nutritional status, which are collectively called intermediary plasma metabolites (IPMs). The IPMs glucose, triglycerides, β-hydroxy-butyrate (BHB), and uric acid have been used in birds and crocodilians to detect nutritional deficiencies [55–58]. In these studies, elevated plasma concentrations of glucose and triglycerides indicated little to no starvation, while elevated BHB and drops in glucose and triglycerides signified intermediate starvation; increased uric acid was associated with severe starvation. We predicted alligators in nesting colonies would have higher plasma glucose and triglycerides, and lower BHB and uric acid than those not found in colonies.
Morphometric body condition indices can be used to indicate an animal’s energy reserves relative to size [59]. These indices have been shown to be positively correlated with reproductive success in birds [60], turtles [61], and snakes [62]. They have also been used as reliable, efficient indictors of alligator population health [63–66]. Fulton’s condition factor (K) was used because it has been used previously in alligators; K was calculated as such: K = M * SVL−3 * 105, where M is mass and SVL is snout-vent length [67–69].
Because K may bias condition scores due to allometry [70,71], the scaled mass index () was also used. was calculated using the following equation: = Mi * [SVL0 / SVLi] ^bSMA, where Mi and SVLi are mass and snout-vent length of individual i respectively, SVL0 is an arbitrary length (we used 100 cm), and bSMA is the scaling exponent as determined by a standardized major axis (SMA) regression of ln(M) on ln(SVL) [71,72]. For the SMA regression we used a reference population of female alligators caught in our study area from 1999–2014 (n = 565, mean ± SD [range], M: 16.38 kg ± 11.10 [0.82–56.00], SVL: 85.55 cm ± 21.39 [35.0–135.9]). We could not determine whether individuals from this reference population were associated with nesting colonies. However, since Everglades alligators are particularly thin [64,73], a study-area-specific reference population better-informed our prediction of how mass scales with length in this population. We also checked that growth was approximately isometric (i.e., mass ∝ length3) within our capture sample by conducting an ordinary least squares (OLS) regression of ln(M) on ln(SVL) and testing the hypothesis that the regression coefficient for length was significantly different from three [74].
Environmental Covariates
We included four environmental covariates in our models: (1) yearly minimum water depth, (2) range in water depth, (3) tree island area, and (4) counts of nearby alligator-maintained ponds (alligator holes). (1) We predicted a unimodal relationship between alligator body condition and yearly minimum water depth [49,50,69]. In the Everglades, low water levels confine prey and make them more available for capture by alligators, yet in particularly dry years alligator populations decline [75]. (2) Greater range in water depth has been shown to increase wetland productivity [76–79], (3) tree islands are nutrient hotspots in the Everglades that increase local productivity [80–83], and (4) during low water conditions in the Everglades, fish and other aquatic prey congregate into alligator holes [84,85]; we predict each of factors 2–4 to have positive effects on alligator body condition through higher local prey abundance. From this information, we created a suite of a priori hypotheses upon which we based our statistical models (S1 Table).
From the Everglades Depth Estimation Network project website (http://sofia.usgs.gov/eden/) we extracted predicted water depths for the 400×400 m grid cells in which each capture occurred; this water depth model has been validated to RMSE = 3.3 cm [86]. We used water depths from within each capture’s calendar year for minimum yearly water depth, and from ≤ 365 days prior to capture for range in water depth. From the Ecological Modeling Team at Everglades National Park (http://simglades.org/) we downloaded habitat type by 50×50 m grid cells (for tree island area calculations) and locations of alligator holes in our study area (see [49] for how these data were derived). For tree island area, we calculated the proportion of nearby grid cells that we categorized as tree island, while for alligator holes we took counts of nearby holes. We only considered habitat-type cells or alligator holes within sqrt{4002 / π} m of each capture location, so that the area concerned was the same as for the water-depth data.
Statistical Analyses
All analyses were conducted in R 3.1.2 (R Core Team 2014). Due to non-normality of response variables, we used one-way percentile bootstrap hypothesis tests (106 simulations) [87,88] of H0: μnc ≥ μc, where c is colony and nc is non-colony, for K, , and glucose, and H0: μnc ≤ μc for uric acid. Influential points were determined using plots of jackknife influence values. Triglycerides and BHB had samples with undetected levels, so we used the Gρ family [89] equivalent to the Peto and Peto [90] modification of the Gehan-Wilcoxon test, to test for differences in the empirical cumulative distribution functions for colony and non-colony values [91]. To conduct these tests, we used the R package ‘NADA’ [92], which adjusts routines from the package ‘survival’ [93] to handle left-censored data.
If a variable was different between colony and non-colony females, we assessed potential covariate effects using linear models. Diagnostic plots indicated heteroscedasticity and non-normality in some models, so we used Box-Cox [94] data transformations. Plots also indicated influential points, so we employed robust regression techniques: Huber’s [95] M-estimator and iterated median absolute deviations. These techniques were carried out by the functions ‘boxcox’ and ‘rlm’ in the ‘MASS’ package [96]. We evaluated support for robust linear models (RLMs) using the second-order variant of Akaike’s Information Criterion (AICc), the difference in AICc between model i and the top model (Δi), and Akaike weights (wi); the latter represents Pr(modeli is the best model | data) [97].
Results
We captured thirty-nine female alligators (20 colony, 19 non-colony), ranging from 7.5 to 46.0 kg (mean ± SD, 21.3 kg ± 9.7) total mass and 146.6 to 239.1 cm (194.1 cm ± 27.1) total length. Because female alligators in the Everglades can reproduce at 1.5 m total length [64], we considered all individuals potentially reproductively active and hereafter refer to them as adults.
The bird nesting colonies at which we captured alligators were primarily comprised of great egrets (Ardea alba), with smaller numbers of little blue herons (Egretta caerulea), tricolored herons (E. tricolor), snowy egrets (E. thula), and anhingas (Anhinga anhinga). Yearly minimum water depth was much deeper for colony (25.5 cm ± 15.8) than non-colony (14.9 cm ± 12.5) sites in WCA 3A (one-way Welch’s t-test: t26.4 = 2.02, P = 0.027), while it was similar in LOX (colony: 13.2 cm ± 8.26, non-colony: 11.4 cm ± 7.40, t7.91 = 0.363, P = 0.363).
Glucose (P = 0.207) and uric acid (P = 0.585) were not significantly different between colony and non-colony females, and hypothesis tests for left-censored data showed no differences in triglycerides (χ21 = 0.647, P = 0.421) or BHB (χ21 = 7.95×10−4, P = 0.978) (Fig 2). Total protein values (43.9 ± 12.7 g L−1) were similar to those from other alligator studies [54,98], which suggests that our samples were not markedly diluted with lymph or cerebrospinal fluid [54].
Fig 2. Intermediary plasma metabolites (IPMs) for colony and non-colony alligators.
Comparison of the IPMs glucose, triglycerides, β-hydroxy-butyrate (BHB), and uric acid for adult female alligators caught near Everglades tree islands with and without wading bird nesting colonies present. Error bars are CI95% via bias-corrected and accelerated bootstrapping; crossed points indicate the individual caught with a broken tail. For triglycerides and BHB, censored values were replaced with model estimates from separate regressions on order statistics for colony and non-colony subpopulations.
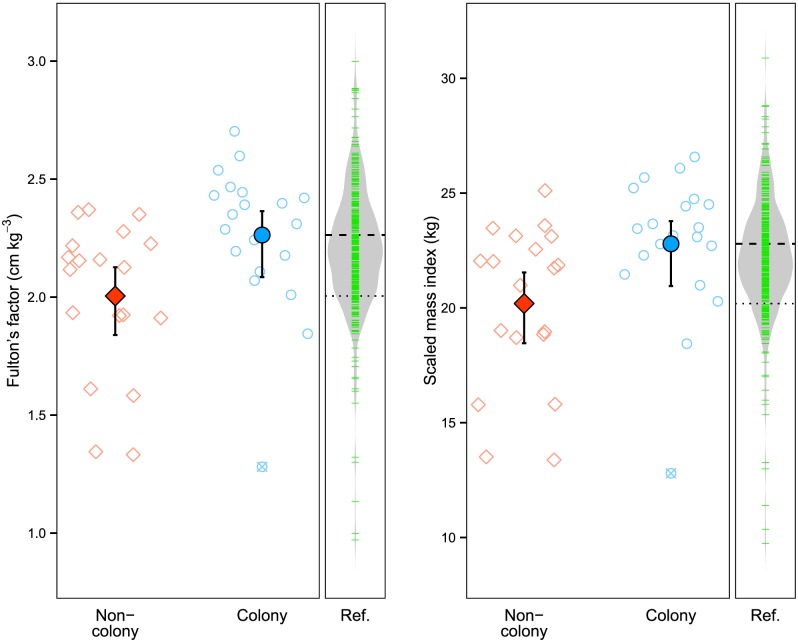
Both morphometric indices were significantly higher (K: P = 0.008, : P = 0.010) in colony (K = 2.26 cm kg−3 ± 0.310, = 22.79 kg ± 3.09) than non-colony (K = 2.00 cm kg−3 ± 0.325, = 20.19 kg ± 3.48) female alligators (Fig 3). Jackknife-after-bootstrap plots indicated one colony female as a potential outlier for these bootstrap hypothesis tests, an individual with a broken tail that likely influenced its ability to forage. As we had biological and statistical evidence that this point was a likely outlier, we identified it in Figs 2 and 3.
Fig 3. Morphometric body condition for colony and non-colony alligators.
Comparison of morphometric body condition (Fulton’s factor, K, and scaled mass index, ) for adult female alligators caught near Everglades tree islands with and without wading bird nesting colonies present. Error bars are CI95% via bias-corrected and accelerated bootstrapping, and crossed points indicate the individual caught with a broken tail. Beanplots represent values from the reference population of alligators caught in our study area between 1999–2014, filtered for those within the range of snout-vent lengths from our captured females (n = 387); dashed and dotted lines are located at the mean values for colony and non-colony alligators, respectively.
The 5 RLMs for K that included colony presence as a predictor represented the 5 most highly supported models (Table 2). The highest-supported model also included range in water depth and alligator hole counts as additive terms (wi = 0.73), while the second-best model included colony presence as the lone predictor (wi = 0.13).
Table 2. Model selection on RLMs predicting alligator body condition (Fulton’s factor, K).
| Model | AICc | Δi | wi | k |
|---|---|---|---|---|
| Colony presence + Water depth range + Alligator holes | 215.80 | 0.00 | 0.73 | 5 |
| Colony presence | 219.23 | 3.43 | 0.13 | 3 |
| Colony presence + Water depth range | 220.38 | 4.58 | 0.07 | 4 |
| Colony presence + Tree island area | 221.83 | 6.02 | 0.04 | 4 |
| Colony presence × Minimum water depth† | 224.49 | 8.69 | 0.01 | 7 |
| Water depth range | 225.13 | 9.33 | 0.01 | 3 |
| Tree island area | 226.91 | 11.10 | 0.00 | 3 |
| Minimum water depth† × Water depth range | 227.05 | 11.25 | 0.00 | 7 |
| Minimum water depth† | 229.19 | 13.38 | 0.00 | 4 |
| Minimum water depth† × Tree island area | 231.73 | 15.93 | 0.00 | 7 |
| Minimum water depth† × Alligator holes | 236.19 | 20.39 | 0.00 | 7 |
AICc, second-order variant of Akaike’s Information Criterion; Δi, difference in AICc between model i and the top model; wi, relative likelihood of model i [i.e., Pr(modeli is the best model | data)]; k, number of model parameters
† Quadratic term included
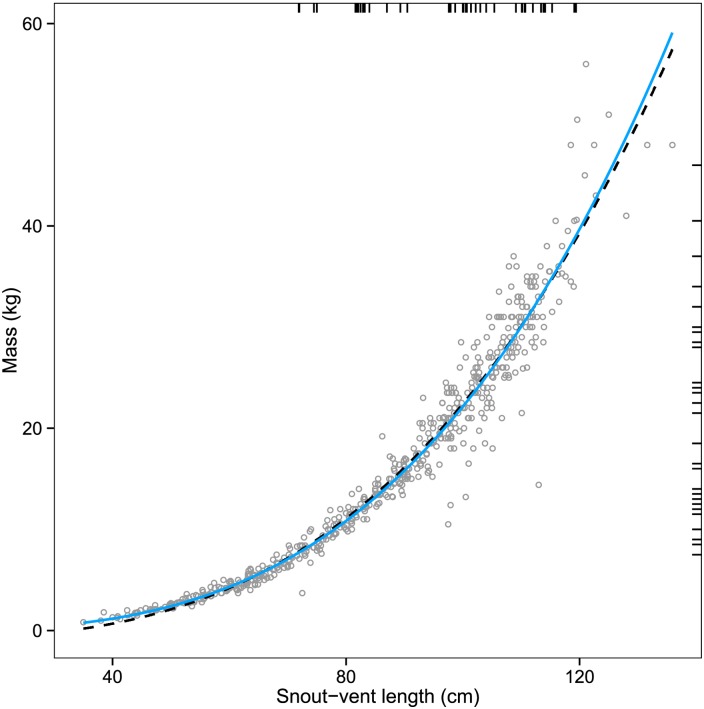
showed similar results as K with respect to model selection (S2 Table). The SMA regression of ln(M) on ln(SVL) using the reference population (n = 565, r2 = 0.981, P << 0.001) indicated a scaling exponent of 3.20 (CI95% = 3.17–3.24) for the calculation of . However, the M–SVL relationship within the population of animals captured was not significantly different from that predicted for isometric growth (OLS regression: t37 = −0.471, P = 0.354, coefficient CI95% = 2.51–3.30), nor did the curves for M ∝ SVL3 differ markedly from M ∝ SVL3.20 within the size-range of alligators captured herein (Fig 4).
Fig 4. Mass versus length for the reference population.
Plot of mass against snout-vent length for a reference population of alligators caught in the study area from 1999–2014 (n = 565). Regression lines are for standardized major axis (dashed) and ordinary least squares (solid) regressions, and rug plots along vertical and horizontal axes represent masses and snout-vent lengths respectively for alligators in this study.
Discussion
Our results using morphometric indices are consistent with the hypothesis that wading birds are facilitators of alligators by providing localized nutritional subsidies. Both Fulton’s factor (K) and the scaled mass index () indicated that alligators near wading bird nesting colonies were in better body condition than those in similar habitat without active colonies (Fig 3), an effect that was statistically independent of environmental factors (Table 2 and S2 Table). Because (i) the results were similar for K and , (ii) the mass—length relationship within our capture population did not differ significantly from 3 (CI95% = 2.51–3.30; Fig 4), and (iii) K afforded us opportunities to compare to past studies, we hereafter only use K as our morphometric condition index and refer to it as simply “body condition.” However, we recommend future studies to utilize the scaled mass index if analyzing a wide size-range of alligators.
Results from IPMs (glucose, triglycerides, BHB, and uric acid) did not support our hypothesis (Fig 2), which contrasts previous work using IPMs in Yacare caiman (Caiman crocodilus yacare) [55]. However, their lower-condition population was caiman on dry land, where they likely have few or no feeding opportunities. All alligators in our study were captured in aquatic habitat and were not likely to have been completely deprived of food. The effects of intermittent feeding on IPMs is unclear in crocodilians, and even our most emaciated alligator via morphometric indices had inconclusive IPM results (crossed points in Figs 2 and 3). Additional studies in birds and squamates with consistent IPM results are typically either on laboratory-starved animals (e.g., [57]) or wild populations suffering extreme resource limitations and/or environmental contamination (e.g., [56,58]). Combined with the differences we found using morphometric indices, we infer that these blood parameters are only sensitive enough to discern severe nutritional differences.
Indeed, morphometric body condition results suggest biologically relevant effects of nesting colonies. Using those female alligators from our reference population that were within the range of snout-vent lengths reported herein (n = 387), mean body condition for colony-associated females we captured ranked as the 63rd percentile, while that for non-colony females ranked as the 17th (Fig 3). Moreover, the observed disparity between colony and non-colony alligator body condition (13%) is greater than the differences in pre-breeding-season condition for blue petrel (Halobaena caerulea) females that did and did not “decide” to breed (11%) [60], and in green turtle (Chelonia mydas) condition for those caught in years least and most associated with density-dependent reductions in growth rates (~8%) [99]. Thus the body condition difference we report is likely large enough to be associated with breeding potential for these subpopulations of alligators.
In seasonal wetlands in the Everglades, crocodilian food availability is thought to increase during dry months [69,73,100]. The magnitude of the increase in alligator body condition through colony association was similar to body condition changes effected through dry versus wet seasons (13%) [69] and two years of elevated water levels (−15%) [101]. Yet the differences we reported occurred despite colony sites having equal or greater water depths than non-colony sites, which might suggest that, on the scale of an individual tree island, colony association can buffer the effects of hydrology on nearby alligators. Alternatively, size and dry-season fish abundance are positively correlated for aquatic refuge (i.e., drought-resistant) sites in the Everglades [50], so the deeper modeled water depths near colonies in WCA 3A might indicate larger, deeper-water refuge sites with greater prey abundance. However, the colony-associated difference in body condition was greater for captures in LOX (16.7%), where water depths were similar between colony and non-colony sites, than in WCA 3A (11.6%). This sheds doubt upon deeper refuge sites near colonies causing the observed differences.
The apparent mutually facilitative association between nesting wading birds and alligators is a novel nest protection association, as the protector receives potentially substantial nutritional benefits from the protectee. The magnitude of benefits demonstrated here indicates that for alligators there should be selective pressure toward behaviors that enhance the benefits they derive from this association. We hypothesize that alligators are attracted to and, given their territorial behavior, may even compete for territories that include wading bird colonies. We predict from this that alligators should display movements towards bird colonies upon their formation, and alligators occupying colonies should be larger and/or occur more densely than in non-colony sites. Testing the above predictions will be key to understanding how the close spatial association between these two species arises, specifically whether alligator behavior has interactive effects with wading birds’ previously demonstrated attraction to alligator-present sites [40].
In comparison to other nest protectors described (Table 1; Appendix 1 in [26]) alligators are much larger-bodied, more indiscriminate in food choice, and less capable of reaching nests in trees. This has two important effects which could serve to reinforce this relationship: First, a comparatively large portion of nutrition from breeding birds should directly or indirectly reach alligators, as the latter could take advantage of an increase in small aquatic prey that may be fuelled by bird-guano deposition yet are large enough to consume chicks of all sizes. Brood reduction is common in all wading birds [36,102,103], and the resulting chick carcasses from wading bird nesting colonies represent the most substantive food source for associate alligators [37]. Because alligators can utilize this food source, brood reduction by wading birds is likely another vital component of this relationship, providing a steady flow of nutrients from protectee to protector.
Second, the risk of alligator predation on wading birds should drop quickly to zero with distance of nests above alligators. Although crocodilians are capable of jumping vertically, even large adult alligators are unlikely to reach heights of over ~1.5–2 m [104,105], particularly in the relatively shallow water and thick vegetation within tree islands. Nesting at such a height is a relatively small price to pay if the remuneration for birds is protection from nest predation by mammalian predators, and the ability of birds to nest directly over alligators with relatively little threat of predation should allow for a close connection between protector and protectee. This would increase the likelihood of both partners receiving benefits, as alligators in close proximity to bird nests should be more likely to (1) detect and consume fallen chick carcasses and (2) deter mammalian predators.
The oligotrophic Everglades is a particularly harsh environment for crocodilians, as it induces high energetic demands for resident ectotherms but offers a relatively poor food base [64,73,106]. We suggest that further research should seek to replicate these findings in other wetlands less energetically demanding for crocodilians. We also suggest that the basic mechanisms of this apparent two-way ecological facilitation could apply broadly to analogous species-groups of colonially nesting wetland birds and crocodilians in many other tropical and subtropical regions (e.g., floodplains and wetlands of southeastern USA, Western Australia, India, Africa, the Amazon, the Pantanal, and the Llanos).
Supporting Information
Alligators (red) are observed under white ibis chicks (blue) in wading bird nesting colonies (A) “Alley North” (26.201°, −80.529°) and (B) “163” (25.773°, −80.833°). (C) In the image from colony “Tamiami West” (25.758°, −80.545°), the camera is facing down from an anhinga nest. Reprinted under a CC BY license, with permission from (A) Nicholas E. Vitale and (B, C) Lucas A. Nell, original copyrights 2014.
(PDF)
(PDF)
AICc, second-order variant of Akaike’s Information Criterion; Δi, difference in AICc between model i and the top model; wi, relative likelihood of model i [i.e., Pr(modeli is the best model | data)]; k, number of model parameters.
(PDF)
(XLSX)
(XLSX)
Acknowledgments
We thank Carolyn Cray, Valentine Lance, Darryl Heard, Richard Hill, Frank Ridgley, and Marshall McCue for their valuable advice on our sample design. We also thank Esther Greenbarg, Jeff Beauchamp, Brian Jeffery, Noah Burrell, Lindsey Garner, Nicholas Vitale, Seth Farris, Matt Denton, and Michiko Squires for help with fieldwork. We acknowledge the Everglades Depth Estimation Network (EDEN) project and the US Geological Survey for providing the water depth data for the purpose of this research. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the US Fish and Wildlife Service.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant to PCF from the U.S. Army Corps of Engineers (http://www.usace.army.mil/), grant number W912HZ-10-2-0013. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bronstein JL. The evolution of facilitation and mutualism. J Ecol. 2009;97: 1160–1170. [Google Scholar]
- 2.Valiente-Banuet A, Rumebe AV, Verdu M, Callaway RM. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proc Natl Acad Sci USA. 2006;103: 16812–16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kéfi S, van Baalen M, Rietkerk M, Loreau M. Evolution of local facilitation in arid ecosystems. Am Nat. 2008;172: E1–E17. 10.1086/588066 [DOI] [PubMed] [Google Scholar]
- 4.Green PT, O'Dowd DJ, Abbott KL, Jeffery MM, Retallick KK, MacNally RR. Invasional meltdown: invader-invader mutualism facilitates a secondary invasion. Ecology. 2011;92: 1758–1768. [DOI] [PubMed] [Google Scholar]
- 5.Palmer TM, Stanton ML, Young TP, Lemboi JS, Goheen JR, Pringle RM. A role for indirect facilitation in maintaining diversity in a guild of African acacia ants. Ecology. 2013;94: 1531–1539. [DOI] [PubMed] [Google Scholar]
- 6.Shchekinova EY, Löder MGJ, Boersma M, Wiltshire KH. Facilitation of intraguild prey by its intraguild predator in a three-species Lotka-Volterra model. Theor Popul Biol. 2014;92: 55–61. [DOI] [PubMed] [Google Scholar]
- 7.Crocker RL, Major J. Soil development in relation to vegetation and surface age at Glacier Bay, Alaska. J Ecol. 1955;43: 427–448. [Google Scholar]
- 8.Bruno JF. Facilitation of cobble beach plant communities through habitat modification by Spartina alterniflora. Ecology. 2000;81: 1179–1192. [Google Scholar]
- 9.Cavieres LA, Badano EI. Do facilitative interactions increase species richness at the entire community level? J Ecol. 2009;97: 1181–1191. [Google Scholar]
- 10.Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends Ecol Evol. 2003;18: 119–125. [Google Scholar]
- 11.Crotty SM, Bertness MD. Positive interactions expand habitat use and the realized niches of sympatric species. Ecology. 2015;96: 2575–2582. [DOI] [PubMed] [Google Scholar]
- 12.Bulleri F, Bruno JF, Silliman BR, Stachowicz JJ. Facilitation and the niche: implications for coexistence, range shifts and ecosystem functioning. Funct Ecol. 2015. [Google Scholar]
- 13.Bertness MD, Callaway RM. Positive interactions in communities. Trends Ecol Evol. 1994;9: 191–193. 10.1016/0169-5347(94)90088-4 [DOI] [PubMed] [Google Scholar]
- 14.Callaway RM. Positive interactions and interdependence in plant communities. Dordrecht, The Netherlands: Springer; 2007. [Google Scholar]
- 15.Ricklefs RE. An analysis of nesting mortality in birds. Smithson C Zool. 1969;9: 1–48. [Google Scholar]
- 16.Wilcove DS. Nest predation in forest tracts and the decline of migratory songbirds. Ecology. 1985;66: 1211–1214. [Google Scholar]
- 17.Robinson SK, Thompson FR, Donovan TM, Whitehead DR, Faaborg J. Regional forest fragmentation and the nesting success of migratory birds. Science. 1995;267: 1987–1990. [DOI] [PubMed] [Google Scholar]
- 18.Crooks KR, Soulé ME. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999;400: 563–566. [Google Scholar]
- 19.Ghalambor CK, Peluc SI, Martin TE. Plasticity of parental care under the risk of predation: how much should parents reduce care? Biol Letters. 2013;9: 20130154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tremblay J-P, Gauthier G, Lepage D, Desrochers A. Factors affecting nesting success in greater snow geese: effects of habitat and association with snowy owls. Wilson Bull. 1997;109: 449–461. [Google Scholar]
- 21.Ueta M. Azure-winged magpies avoid nest predation by nesting near a Japanese lesser sparrowhawk's nest. Condor. 1998;100: 400–402. [Google Scholar]
- 22.Beier P, Tungbani AI. Nesting with the wasp Ropalidia cincta increases nest success of red-cheeked cordonbleu (Uraeginthus bengalus) in Ghana. Auk. 2006;123: 1022–1037. [Google Scholar]
- 23.Hipfner JM, Morrison KW, Darvill R. Peregrine falcons enable two species of colonial seabirds to breed successfully by excluding other aerial predators. Waterbirds. 2011;34: 82–88. [Google Scholar]
- 24.Haemig PD. Symbiotic nesting of birds with formidable animals: a review with applications to biodiversity conservation. Biodivers Conserv. 2001;10: 527–540. [Google Scholar]
- 25.Caro TM. Antipredator defenses in birds and mammals. Chicago, IL, USA: University of Chicago Press; 2005. [Google Scholar]
- 26.Quinn JL, Ueta M. Protective nesting associations in birds. Ibis. 2008;150: 146–167. [Google Scholar]
- 27.Pugnaire FI, Haase P, Puigdefábregas J. Facilitation between higher plant species in a semiarid environment. Ecology. 1996;77: 1420–1426. [Google Scholar]
- 28.Larsen T, Moldsvor J. Antipredator behavior and breeding associations of bar-tailed godwits and whimbrels. Auk. 1992;109: 601–608. [Google Scholar]
- 29.Groom MJ. Sand-colored nighthawks parasitize the antipredator behavior of three nesting bird species. Ecology. 1992;73: 785–793. [Google Scholar]
- 30.Lindell C. Benefits and costs to plain-fronted thornbirds (Phacellodomus rufifrons) of interactions with avian nest associates. Auk. 1996;113: 565–577. [Google Scholar]
- 31.Wiklund CG. Fieldfare (Turdus pilaris) breeding success in relation to colony size, nest position and association with merlins (Falco columbarius). Behav Ecol Sociobiol. 1982;11: 165–172. [Google Scholar]
- 32.Campobello D, Sarà M, Hare JF. Under my wing: lesser kestrels and jackdaws derive reciprocal benefits in mixed-species colonies. Behav Ecol. 2012;23: 425–433. [Google Scholar]
- 33.Zelickman EA, Golovkin AN. Composition, structure and productivity of neritic plankton communities near the bird colonies of the northern shores of Novaya Zemlya. Mar Biol. 1972;17: 265–274. [Google Scholar]
- 34.McColl JG, Burger J. Chemical inputs by a colony of Franklin's gulls nesting in cattails. Am Midl Nat. 1976;96: 270–280. [Google Scholar]
- 35.Giroux M-A, Berteaux D, Lecomte N, Gauthier G, Szor G, Bêty J. Benefiting from a migratory prey: spatio-temporal patterns in allochthonous subsidization of an arctic predator. J Anim Ecol. 2012;81: 533–542. 10.1111/j.1365-2656.2011.01944.x [DOI] [PubMed] [Google Scholar]
- 36.Mock DW, Parker GA. The evolution of sibling rivalry. New York, NY, USA: Oxford University Press; 1997. [Google Scholar]
- 37.Nell LA, Frederick PC. Fallen nestlings and regurgitant as mechanisms of nutrient transfer from nesting wading birds to crocodilians. Wetlands. 2015;35: 723–732. [Google Scholar]
- 38.Rodgers JA Jr. On the antipredator advantages of coloniality: a word of caution. Wilson Bull. 1987;99: 269–271. [Google Scholar]
- 39.Frederick PC, Collopy MW. The role of predation in determining reproductive success of colonially nesting wading birds in the Florida Everglades. Condor. 1989;91: 860–867. [Google Scholar]
- 40.Burtner BF. Symbiosis between long legged wading birds (Ciconiiformes) and alligators (Alligator mississippiensis)?: testing the “nest protector” hypothesis. M.Sc. Thesis, University of Florida. 2011. Available: http://ufdc.ufl.edu/UFE0043815/00001.
- 41.Wolfe JL, Bradshaw DK, Chabreck RH. Alligator feeding habits: new data and a review. Northeast Gulf Science. 1987;9: 1–8. [Google Scholar]
- 42.Barr BR. Food habits of the American alligator, Alligator mississippiensis, in the southern Everglades. Ph.D. Thesis, University of Miami. 1997.
- 43.Rice AN. Diet and condition of American alligators (Alligator mississippiensis) in three central Florida lakes. M.Sc. Thesis, University of Florida. 2004. Available: http://www.myfwc.com/media/310266/Alligator_Rice_A.pdf.
- 44.Rosenblatt AE, Nifong JC, Heithaus MR, Mazzotti FJ, Cherkiss MS, Jeffery BM, et al. Factors affecting individual foraging specialization and temporal diet stability across the range of a large “generalist” apex predator. Oecologia. 2015;178: 5–16. 10.1007/s00442-014-3201-6 [DOI] [PubMed] [Google Scholar]
- 45.Oliver JD, Schoenberg SA. Residual influence of macronutrient enrichment on the aquatic food web of an Okefenokee Swamp abandoned bird rookery. Oikos. 1989;55: 175–182. [Google Scholar]
- 46.Frederick PC, Powell GVN III. Nutrient transfer by wading birds in the Everglades In: Davis SM, Ogden JC, editors. Everglades: the ecosystem and its restoration. Delray Beach, FL, USA: St. Lucie Press; 1994. pp. 571–584. [Google Scholar]
- 47.Irick DL, Gu B, Li YC, Inglett PW, Frederick PC, Ross MS, et al. Wading bird guano enrichment of soil nutrients in tree islands of the Florida Everglades. Science of The Total Environment. 2015;532: 40–47. 10.1016/j.scitotenv.2015.05.097 [DOI] [PubMed] [Google Scholar]
- 48.Frederick PC, Ogden JC. Monitoring wetland ecosystems using avian populations: seventy years of surveys in the Everglades In: Busch DE, Trexler JC, editors. Monitoring ecosystems: interdisciplinary approaches for evaluating ecoregional initiatives. Washington, DC, USA: Island Press; 2002. pp. 321–350. [Google Scholar]
- 49.Shinde D, Pearlstine LG, Brandt LA, Mazzotti FJ, Parry MW, Jeffery BM, et al. Alligator production suitability index model (GATOR—PSIM v. 2.0): ecological and design documentation. Homestead, FL, USA: South Florida Natural Resources Center, Everglades National Park; 2014. p. 18. [Google Scholar]
- 50.Parkos JJ III, Ruetz CR III, Trexler JC. Disturbance regime and limits on benefits of refuge use for fishes in a fluctuating hydroscape. Oikos. 2011;120: 1519–1530. [Google Scholar]
- 51.Morea CR. Home range, movement, and habitat use of the American alligator in the Everglades. M.Sc. Thesis, University of Florida. 1999.
- 52.Google Inc. Google Earth (version 7.1.2.2041) [Internet]. Mountain View, CA, USA: Google Inc; 2013. Available: www.google.com/earth/. [Google Scholar]
- 53.Taylor D, Kinler N, Linscombe G. Female alligator reproduction and associated population estimates. J Wildlife Manage. 1991;55: 682–688. [Google Scholar]
- 54.Lance VA, Elsey RM, Butterstein G, Trosclair PL III. Rapid suppression of testosterone secretion after capture in male American alligators (Alligator mississippiensis). Gen Comp Endocr. 2004;135: 217–222. [DOI] [PubMed] [Google Scholar]
- 55.Campbell HA, Micheli MA, Abe A. A seasonally dependent change in the distribution and physiological condition of Caiman crocodilus yacare in the Paraguay River Basin. Wildlife Res. 2008;35: 150. [Google Scholar]
- 56.Hollmén T, Franson JC, Hario M, Sankari S, Kilpi M, Lindström K. Use of serum biochemistry to evaluate nutritional status and health of incubating common eiders (Somateria mollissima) in Finland. Physiol Biochem Zool. 2001;74: 333–342. [DOI] [PubMed] [Google Scholar]
- 57.Rodríguez P, Tortosa FS, Villafuerte R. The effects of fasting and refeeding on biochemical parameters in the red-legged partridge (Alectoris rufa). Comp Biochem Phys A. 2005;140: 157–164. [DOI] [PubMed] [Google Scholar]
- 58.Artacho P, Soto-Gamboa M, Verdugo C, Nespolo RF. Blood biochemistry reveals malnutrition in black-necked swans (Cygnus melanocoryphus) living in a conservation priority area. Comp Biochem Phys A. 2007;146: 283–290. [DOI] [PubMed] [Google Scholar]
- 59.Green AJ. Mass/length residuals: measures of body condition or generators of spurious results? Ecology. 2001;82: 1473–1483. [Google Scholar]
- 60.Chastel O, Weimerskirch H, Jouventin P. Influence of body condition on reproductive decision and reproductive success in the blue petrel. Auk. 1995;112: 964–972. [Google Scholar]
- 61.Litzgus JD, Bolton F, Schulte-Hostedde AI. Reproductive output depends on body condition in spotted turtles (Clemmys guttata). Copeia. 2008;2008: 86–92. [Google Scholar]
- 62.Gibbs HL, Chiucchi JE. Inbreeding, body condition, and heterozygosity-fitness correlations in isolated populations of the endangered eastern massasauga rattlesnake (Sistrurus c. catenatus). Conserv Genet. 2012;13: 1133–1143. [Google Scholar]
- 63.Elsey RM, Joanen T, McNease LL, Kinler N. Growth rates and body condition factors of Alligator mississippiensis in coastal Louisiana wetlands: a comparison of wild and farm-released juveniles. Comp Biochem Phys A. 1992;103: 667–672. [Google Scholar]
- 64.Dalrymple GH. Growth of American alligators in the Shark Valley region of Everglades National Park. Copeia. 1996;1996: 212. [Google Scholar]
- 65.Saalfeld DT, Webb KK, Conway WC, Calkins GE, Duguay JP. Growth and condition of American alligators (Alligator mississippiensis) in an inland wetland of East Texas. Southeast Nat. 2008;7: 541–550. [Google Scholar]
- 66.Mazzotti FJ, Best GR, Brandt LA, Cherkiss MS, Jeffery BM, Rice KG. Alligators and crocodiles as indicators for restoration of Everglades ecosystems. Ecol Indic. 2009;9: S137–S149. [Google Scholar]
- 67.Zweig CL. Body condition index analysis for the American alligator (Alligator mississippiensis). M.Sc. Thesis, University of Florida. 2003. Available: http://etd.fcla.edu/UF/UFE0000836/zweig_c.pdf.
- 68.Rice AN, Ross JP, Woodward AR, Carbonneau DA, Percival HF. Alligator diet in relation to alligator mortality on Lake Griffin, FL. Southeast Nat. 2007;6: 97–110. [Google Scholar]
- 69.Fujisaki I, Rice KG, Pearlstine LG, Mazzotti FJ. Relationship between body condition of American alligators and water depth in the Everglades, Florida. Hydrobiologia. 2009;635: 329–338. [Google Scholar]
- 70.Stevenson RD, Woods WA Jr. Condition indices for conservation: new uses for evolving tools. Integr Comp Biol. 2006;46: 1169–1190. 10.1093/icb/icl052 [DOI] [PubMed] [Google Scholar]
- 71.Peig J, Green AJ. The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct Ecol. 2010;24: 1323–1332. [Google Scholar]
- 72.Peig J, Green AJ. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos. 2009;118: 1883–1891. [Google Scholar]
- 73.Mazzotti FJ, Brandt LA. Ecology of the American alligator in a seasonally fluctuating environment In: Davis SM, Ogden JC, editors. Everglades: the ecosystem and its restoration. Delray Beach, FL, USA: St. Lucie Press; 1994. pp. 485–505. [Google Scholar]
- 74.Cone RS. The need to reconsider the use of condition indices in fishery science. T Am Fish Soc. 1989;118: 510–514. [Google Scholar]
- 75.Waddle JH, Brandt LA, Jeffery BM, Mazzotti FJ. Dry years decrease abundance of American alligators in the Florida Everglades. Wetlands. 2015;35: 865–875. [Google Scholar]
- 76.Kushlan JA. Avian use of fluctuating wetlands In: Sharitz RR, Gibbons JW, editors. Freshwater wetlands and wildlife. Oak Ridge, TN: US Department of Energy; 1989. pp. 593–604. [Google Scholar]
- 77.Nuttle WK. Measurement of wetland hydroperiod using harmonic analysis. Wetlands. 1997;17: 82–89. [Google Scholar]
- 78.Leira M, Cantonati M. Effects of water-level fluctuations on lakes: an annotated bibliography. Hydrobiologia. 2008;613: 171–184. [Google Scholar]
- 79.Bedford B, Labisky R, van der Valk A, Volin J. Ecological effects of extreme hydrological events on the Greater Everglades. Independent Scientific Review Panel Report to RECOVER; 2012.
- 80.Wetzel PR, van der Valk AG, Newman S, Gawlik DE, Troxler TG, Coronado-Molina CA, et al. Maintaining tree islands in the Florida Everglades: nutrient redistribution is the key. Front Ecol Environ. 2005;3: 370–376. [Google Scholar]
- 81.Givnish TJ, Volin JC, Owen VD, Volin VC, Muss JD, Glaser PH. Vegetation differentiation in the patterned landscape of the central Everglades: importance of local and landscape drivers. Global Ecol Biogeogr. 2008;17: 384–402. [Google Scholar]
- 82.Ross MS, Sah JP. Forest resource islands in a sub-tropical marsh: soil—site relationships in Everglades hardwood hammocks. Ecosystems. 2011;14: 632–645. [Google Scholar]
- 83.Gaiser EE, Trexler JC, Wetzel PR. The Florida Everglades In: Batzer DP, Baldwin AH, editors. Wetland habitats of North America: ecology and conservation concerns. Berkeley, CA: University of California Press; 2012. pp. 231–252. [Google Scholar]
- 84.Kushlan JA. Observations on the role of the American alligator (Alligator mississippiensis) in the southern Florida wetlands. Copeia. 1974;1974: 993–996. [Google Scholar]
- 85.Palmer ML, Mazzotti FJ. Structure of Everglades alligator holes. Wetlands. 2004;24: 115–122. [Google Scholar]
- 86.Liu Z, Volin JC, Owen VD, Pearlstine LG, Allen JR, Mazzotti FJ, et al. Validation and ecosystem applications of the EDEN water-surface model for the Florida Everglades. Ecohydrology. 2009;2: 182–194. [Google Scholar]
- 87.Hall P, Wilson SR. Two guidelines for bootstrap hypothesis testing. Biometrics. 1991;47: 757–762. [Google Scholar]
- 88.MacKinnon JG. Bootstrap hypothesis testing In: Belsley DA, Kontoghiorghes EJ, editors. Handbook of computational econometrics. New York, NY, USA: John Wiley and Sons; 2009. pp. 183–214. [Google Scholar]
- 89.Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika. 1982;69: 553–566. [Google Scholar]
- 90.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A-G. 1972;135: 185–207. [Google Scholar]
- 91.Helsel DR. Statistics for censored environmental data using Minitab and R. 2nd ed Hoboken, NJ, USA: John Wiley and Sons; 2012. [Google Scholar]
- 92.Lee L. NADA: nondetects and data analysis for environmental data. 2013. Available: http://CRAN.R-project.org/package=NADA.
- 93.Therneau TM. A package for survival analysis in S. 2014. Available: http://CRAN.R-project.org/package=survival.
- 94.Box GEP, Cox DR. An analysis of transformations. J Roy Stat Soc B Met. 1964;26: 211–252. [Google Scholar]
- 95.Huber PJ. Robust estimation of a location parameter. Ann Math Stat. 1964;35: 73–101. [Google Scholar]
- 96.Venables WN, Ripley BD. Modern applied statistics with S. New York, NY, USA: Springer; 2002. [Google Scholar]
- 97.Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed New York, NY, USA: Springer; 2002. [Google Scholar]
- 98.Guillette LJ Jr, Woodward AR, Crain DA, Masson GR, Palmer BD, Cox MC, et al. The reproductive cycle of the female American alligator (Alligator mississippiensis). Gen Comp Endocr. 1997;108: 87–101. [DOI] [PubMed] [Google Scholar]
- 99.Bjorndal KA, Bolten AB, Chaloupka MY. Green turtle somatic growth model: evidence for density dependence. Ecology. 2008;10: 269–282. [Google Scholar]
- 100.Percival HF, Rice KG, Howarter SR. American alligator distribution, thermoregulation, and biotic potential relative to hydroperiod in the Everglades. Gainesville, FL, USA: Florida Cooperative Fish and Wildlife Unit; 2000. p. 155. [Google Scholar]
- 101.Dalrymple GH. The effect of prolonged high water levels in 1995 on the American alligator in the Shark Valley area of Everglades National Park In: Armentano TV, editor. Proceedings of the Conference on Ecological Assessment of the 1994–1995 High Water Conditions in the Southern Everglades. Homestead, FL, USA: National Park Service, South Florida Natural Research Center; 1996. pp. 125–136. [Google Scholar]
- 102.Kahl MP Jr. Food ecology of the wood stork (Mycteria americana) in Florida. Ecol Monogr. 1964;34: 97–117. [Google Scholar]
- 103.Kushlan JA. Population energetics of the American white ibis. Auk. 1977;94: 114–122. [Google Scholar]
- 104.Davenport J, Sayer MDJ. Observations on the aquatic locomotion of young salt-water crocodiles (Crocodylus porosus Schneider). Herpetol J. 1989;1: 356–361. [Google Scholar]
- 105.Britton ARC. Crocodilian biology database [Internet]. 2012 [cited 10 May 2015]. Available: http://crocodilian.com/cnhc/cbd.html.
- 106.Jacobsen T, Kushlan JA. Growth dynamics in the American alligator (Alligator mississippiensis). J Zool. 1989;219: 309–328. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alligators (red) are observed under white ibis chicks (blue) in wading bird nesting colonies (A) “Alley North” (26.201°, −80.529°) and (B) “163” (25.773°, −80.833°). (C) In the image from colony “Tamiami West” (25.758°, −80.545°), the camera is facing down from an anhinga nest. Reprinted under a CC BY license, with permission from (A) Nicholas E. Vitale and (B, C) Lucas A. Nell, original copyrights 2014.
(PDF)
(PDF)
AICc, second-order variant of Akaike’s Information Criterion; Δi, difference in AICc between model i and the top model; wi, relative likelihood of model i [i.e., Pr(modeli is the best model | data)]; k, number of model parameters.
(PDF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.