Abstract
Purpose of review
This review summarizes clinical and basic science evidence linking trauma and nonsteroidal anti-inflammatory drug (NSAID) use to initiation and progression of severe group A streptococcal (GAS) soft tissue infection.
Recent findings
New evidence includes recent clinical series and controlled studies that lend support to an NSAID/GAS association, basic science studies that demonstrate unique roles for nonpenetrating injury and NSAID administration in initiation of cryptogenic GAS infection and experimental studies showing that nonselective NSAIDs accelerate disease progression and limit antibiotic efficacy in established GAS soft tissue infections. Potential mechanisms for these processes are discussed.
Summary
NSAIDs are important anti-inflammatory and analgesic drugs; however, new experimental data suggest that nonselective NSAIDs do more than simply mask the signs and symptoms of developing GAS infection. A more thorough understanding of the triadic interplay of injury-triggered immune signaling, GAS soft tissue infection and NSAIDs is of significant clinical importance and could shift the current paradigm of pain management to avert the consequences of such devastating infections.
Keywords: Group A streptococcus, myonecrosis, necrotizing fasciitis, NSAIDs, trauma
INTRODUCTION
Group A streptococcal (GAS) necrotizing soft tissue infections remain important causes of morbidity and mortality worldwide (reviewed in [1]). Mortality ranges from 30 to 85%; survivors require aggressive surgical intervention and intensive care management. Although epidemiologic factors that increase the risk of death have been defined, risk factors for initiation of infection are less clear. Nonpenetrating trauma and use of nonsteroidal anti-inflammatory drugs (NSAIDs) have been associated with infection onset and/or worse outcomes. This review discusses the clinical and experimental evidence that supports/refutes these associations and the potential molecular mechanisms involved.
NONPENETRATING INJURY AND CRYPTOGENIC GROUP A STREPTOCOCCAL SOFT TISSUE INFECTION
A critical role for antecedent soft tissue injury has been well established for some bacterial infections such as clostridial myonecrosis wherein deep, penetrating trauma introduces organisms (or spores) into devitalized tissues. Though the rates of tissue destruction are comparable in streptococcal and clostridial myonecrosis, the types of injuries predisposing to GAS infection are distinctly different. Here, a minor muscle strain, sprain or bruise is often the rule. For instance, in the 20 cases of invasive GAS infection described by Stevens et al. [2], eight patients (40%) had no known portal of entry and mortality was 30%. Similarly, Adams et al. [3] documented 21 cases of life-threatening GAS infection in which 19 lacked an obvious portal of entry; 18 (85.7%) died. Finally, a 2007 case-controlled study found that nonpenetrating trauma was significantly associated with GAS necrotizing fasciitis [4]. Without an obvious portal of bacterial entry, the correct diagnosis is often delayed until after shock and organ failure are manifest, causing the mortality to exceed 70%. Survivors undergo emergent amputation or extensive surgical debridement and prolonged hospitalization [1]. Several authors have concluded that nonpenetrating muscle injury may be a prerequisite for GAS necrotizing fasciitis or myonecrosis [3,4]. This implies that a specific GAS/skeletal muscle interaction exists that initiates these cryptogenic GAS infections.
KEY POINTS.
This review summarizes clinical and basic science evidence linking trauma and NSAID use to initiation and progression of severe GAS soft tissue infection.
New experimental evidence suggests NSAIDs actively contribute to initiation of secondary infection after injury, increase severity of established infection and reduce antibiotic efficacy.
Understanding the relationship between injury, inflammation and infection may alter the current paradigm of clinical pain management.
Our studies demonstrated that injury of cultured human skeletal muscle cells increased the binding of GAS [5] and that the intermediate filament protein, vimentin, was the principal adhesin responsible [5]. This was curious at first, as vimentin was a well known cytoskeletal protein found within many cell types including immature, undifferentiated skeletal muscle cell precursors (satellite cells) [6]. Our studies clearly demonstrated that injured muscle cells also display vimentin on their surface [5]. This finding extended other reports describing a cell-surface form of vimentin in platelets, endothelial cells and lymphocytes [7–9]. We further demonstrated that GAS, but not Staphylococcus aureus, bound soluble vimentin in vitro (authors’ unpublished observations) and were associated with vimentin-positive necrotic muscle in a human case of GAS necrotizing fasciitis [5].
To examine the relationship between nonpenetrating muscle injury, vimentin expression and GAS infection, we developed a murine model of injury-associated cryptogenic GAS infection [10]. In this model, repeated eccentric contraction exercise creates a moderate muscle injury by forcing an electrically stimulated, fully contracted muscle to lengthen. This regimen causes a loss of function and promotes influx of inflammatory cells (Fig. 1) – two criteria that define postexercise muscle injury [11]. It also stimulates the physiological, biochemical and transcriptomic responses characteristic of muscle regeneration and strengthening [12–14]. As it relates to cryptogenic GAS infection, the model mimics a simple muscle strain.
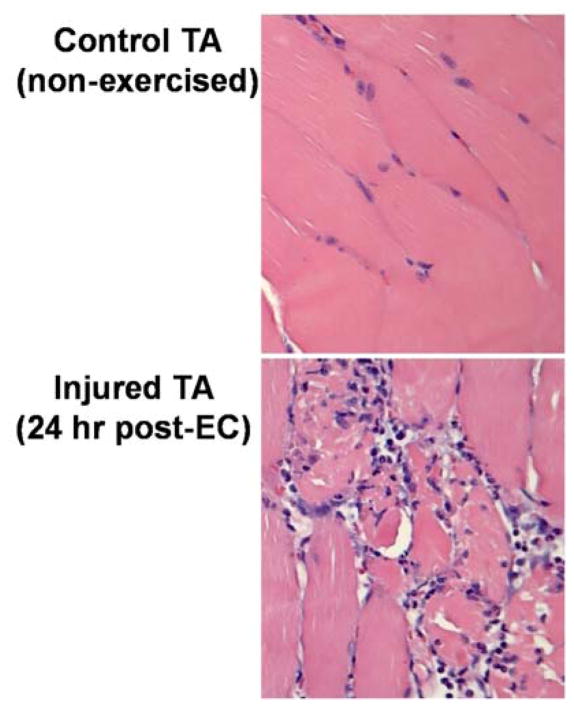
FIGURE 1.
Eccentric contraction-induced muscle injury results in disruption of myofiber architecture and marked influx of inflammatory cells. Mice underwent our published eccentric contraction exercise regimen (described in the text). At 24 hr post-eccentric contraction, the exercised and nonexercised tibialis anterior muscles were harvested for routine histopathology. One representative animal of 3 is depicted. The exercised muscle, but not the control tibialis anterior, displayed marked changes in muscle architecture and a marked inflammatory cell influx. These pathologies, and the corresponding reduction in muscle force (isometric torque), are the two accepted criteria that define muscle injury in this model.
After contraction injury, vimentin expression was significantly increased by 6 h, peaked at 48 h and remained elevated over 72 h [10]. Intravenous infusion of M-type 3 GAS at the peak of vimentin expression resulted in the homing of the organism to the injured site [10]. As only immature or regenerating muscles express vimentin [15], our findings provided the first molecular mechanism to explain the development of severe GAS soft tissue infections precisely at sites of prior minor muscle trauma.
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS AND SEVERE GROUP A STREPTOCOCCAL INFECTION
In 1985, Brun-Buisson et al. suggested a possible association between NSAID use and development of GAS necrotizing fasciitis [16]. They identified six previously healthy individuals with no underlying conditions in whom necrotizing fasciitis developed spontaneously (two of six) or following minor nonpenetrating trauma (four of six). All had received at least one NSAID in 4–10 days prior to hospitalization. One patient died; survivors underwent multiple surgeries. Based on these findings and the known effects of NSAIDs on immune cell function, the authors concluded that NSAIDs contributed to development or extension of the disease process.
In 1995, Stevens [17] proposed that NSAIDs may contribute to more severe GAS infection by interrupting the negative feedback loop that limits production of tumor necrosis factor (TNF)α – a key mediator of septic shock. Others argued that NSAIDs merely mask the signs and symptoms of developing infection, such that diagnosis and antibiotic treatment are delayed. Since then, numerous clinical and epidemiological reports have investigated this issue.
In 2003, Aronoff and Bloch [18] published a careful review of these reports. From 1966 to 2002, they identified eight case reports, 12 retrospective and five prospective studies. Cases were analyzed for the presence of risk factors, portals of bacterial entry, timing of NSAID use relative to the onset of necrotizing fasciitis and other variables. Because most studies, including that by Brun-Buisson et al., lacked matched, NSAID-unexposed control groups or had other significant limitations, they concluded that, in total, these data do not support a causal role for NSAIDs in the development of GAS necrotizing fasciitis or to a worsening of the infection once established, but suggested further investigations were warranted.
Since then, additional reports have emerged. In 2001, Lesko et al. [19] conducted a prospective case-controlled study of NSAID use and development of invasive GAS infection in children with varicella. They found that the risk of necrotizing GAS soft tissue infection was not increased with ibuprofen use [19]. However, they did observe an increased risk of non-necrotizing invasive GAS infections in patients receiving this NSAID. Their follow-up study in 2003 found that the combined use of ibuprofen and paracetamol during varicella infection was significantly associated with development of GAS necrotizing fasciitis [20]. In 2008, a prospective study by Lamagni et al. [21] demonstrated that NSAID use was independently associated with a three-fold increased risk for development of streptococcal toxic shock syndrome. Also in 2008, a French multicentre prospective study by Dubos et al. [22] and a nested case-controlled study in the UK by Mikaeloff et al. [23] each found that NSAID use was independently associated with severe secondary bacterial skin complications in children with varicella infection. In an active surveillance study, Leroy et al. [24] found that severe bacterial soft tissue infections – largely because of GAS or S. aureus – were increased in children who had received NSAIDs, principally ibuprofen, in the 15 days prior to infection onset, leading them to conclude that the increased frequency of severe bacterial infections may have been an adverse effect of NSAIDs. Lastly, in 2012 chart review by Das et al. [25] of GAS necrotizing fasciitis in eight New Zealand hospitals from 2000–2006, it was noted that nearly one-fourth of the patients were taking NSAIDs, though these authors concluded that the role of NSAIDs needed further study.
Experimental evidence for or against an NSAID/GAS association is limited. An early study by Guibal et al. used a rabbit model of GAS necrotizing fasciitis in which animals were challenged with a combination of viable S. pyogenes plus S. aureus alpha toxin and treated immediately, and again 12 h later, with diclofenac [26]. This relatively COX-2-selective NSAID [27] limited the extent of necrotizing fasciitis and lowered the bacterial numbers in the tissues [26]. These authors concluded that the any NSAID-induced increase in severity of human necrotizing fasciitis is likely because of the therapeutic delay induced by the clinical effects of the NSAID, and not to inhibition of antibacterial defense [26]. Using a GAS bacteremia model, Goldmann et al. showed that a highly COX-2-selective NSAID (NS-398), delivered 2 hr before and again 2 hr after challenge, significantly but transiently delayed mortality of mice [28]. Our studies of experimental GAS myonecrosis demonstrated that a highly COX-2-selective NSAID (SC-236), beginning 1 h after intramuscular challenge and continuing for 3 days, did not significantly alter the disease course or outcome [29▪▪]. Together these limited data suggest that COX-2-selective NSAIDs do not worsen severe GAS infection, nor do they provide significant therapeutic benefit.
In striking contrast to the COX-2-selective NSAIDs, our studies [29▪▪] and those of Weng et al. [30] clearly demonstrated that nonselective NSAIDs accelerated the disease course and worsened outcomes of experimental GAS myonecrosis. A nonselective NSAID also significantly augmented initial GAS infection of experimentally injured muscles [10].
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS AND OTHER BACTERIAL INFECTIONS
Several studies have suggested that NSAIDs contribute to severe necrotizing soft tissue infections (NSTI) caused by pathogens other than GAS [31–34]. For example, a 2008 retrospective, case-controlled study by Souyri et al. [31] found a strong association between NSAID use and severe NSTI in which 23% of cases were caused by staphylococci and others were caused by various Gram-negative species. Similarly, S. aureus and Streptococcus pneumoniae were identified in the study by Leroy et al. [24] of NSAID-associated severe bacterial infections. Dial et al. [32] saw an unexpected association between severe C. difficile infection and NSAID use. In a multicenter, placebo-controlled trial by Ott et al.[33], patients undergoing coronary artery bypass surgery and receiving a combination or parecoxib/valdecoxib (both COX-2 selective NSAIDs) for postoperative analgesia had a significantly increased incidence of severe sternal wound infections compared with patients treated with morphine, opioids or acetaminophen. Lastly, a 2014 report by Messika et al. [34] found that patients with community-acquired pneumococcal pneumonia exposed to NSAIDs had more pulmonary complications and required more ventilator support compared with matched non-NSAID-exposed controls.
Thus, a preponderance of clinical evidence and some experimental data suggest that certain NSAIDs may predispose to development or progression of severe bacterial infections, including necrotizing infections caused by GAS, though the strength of the evidence varies by study design and model system used.
MECHANISMS OF NONSTEROIDAL ANTI-INFLAMMATORY DRUG ACTION
NSAIDs are competitive inhibitors of cyclooxygenase (COX), of which two principal isoforms have been recognized. The first, COX-1, is constitutively expressed by virtually all tissues and COX-1-derived PGs have general housekeeping functions. COX-1 drives the immediate (i.e. minutes) PG biosynthesis response and is the preferred enzyme for rapid generation of PGD2 and 15d-PGJ2. NSAID-induced suppression of 15d-PGJ2 production enhances pro-inflammatory cytokine production and inhibits opsonophagocytosis. COX-2 mediates the delayed and sustained PG biosynthetic response. It largely generates PGs that mediate pain and inflammation (principally PGE2 and PGF2α). This strict division of labor between the two COX isoforms has been recently questioned (reviewed in [27]).
NSAIDs are classified based on their COX-1/COX-2 inhibitory concentration50 (IC50) ratios [27,35,36] (Table 1). Nonselective NSAIDs inhibit both COX isoforms (ratio = ~1–10). This group includes ibuprofen, indomethacin and ketorolac tromethamine. Selective NSAIDs preferentially inhibit one of the two COX isoforms. For example, acetylsalicylic acid and SC-560 are COX-1-specific inhibitors (ratios ≪1), whereas celecoxib and SC-236 inhibit COX-2 (ratios >10). Newer third-generation “dual-acting” NSAIDs inhibit both COX isoforms as well as the 5-lipoxygenase (LOX) [37]. Although some products of the LOX pathway are proinflammatory (e.g. leukotrienes), others such as lipoxins are proresolution molecules.
Table 1.
NSAID characteristics
| Class | NSAID (trade name) | COX-1/COX-2 IC50 ratio | Subclass |
|---|---|---|---|
| Selective COX-1 inhibitors | SC-560 | 0.001 | Coxib |
| Aspirin | 0.01 | Salicylates | |
|
| |||
| Non-selective COX inhibitors | Naproxen (Aleve) | 0.33 | Acetic acid |
| Ketorolac tromethamine (Toradol) | 0.35 | Propionic acid | |
| Ibuprofen (Advil, Motrin) | 0.5 | Propionic acid | |
| Ketoprofen | 0.6 | Propionic acid | |
| Indomethacin (Indocin) | 1.9 | Acetic acid | |
|
| |||
| Relatively selective COX-2 inhibitors | Melioxicam | 11.6 | Enolic acid |
| Diclofenac | 18.9 | Acetic acid | |
|
| |||
| Selective COX-2 inhibitors | NS-398 | 168.0 | |
| Celecoxib (Celebrex) | 375.0 | Coxib | |
| Rofecoxib (Vioxx) | 410.0 | Coxib | |
| SC-236 | 1780 | Benezene sulfonamide | |
NSAIDs also have non-COX-dependent activities including inhibition of nuclear factor kappa B (NFkB) and activation of nuclear peroxisome proliferator-activated receptors (PPARs). When activated on macrophages, PPARγ downregulates proinflammatory cytokine production and increases nonphlogistic (proresolution) phagocytosis. Paradoxically, NSAID-induced activation of PPARγ also increases COX-2 gene expression (reviewed in [38]), though this effect varies by cell type.
INJURY, INFLAMMATION AND NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
Inflammation and myogenesis after injury are tightly linked (reviewed in [39]). In response to injury, a rapid (within hours) but transient neutrophilic influx is initiated, largely driven by production of CC chemokines (CCL-2, 3, 4) by multiple cell types. Invading neutrophils release proteolytic enzymes (e.g. ADAM-8 [40]) to degrade damaged muscle cells. The waning neutrophil influx is followed 24–48 h later by arrival of proinflammatory M1 macrophages and eosinophils recruited via cell-specific chemoattractants (e.g. MCP-1, μPAR for macrophages; eotaxin, 5-oxo-ETE for eosinophils). Production of growth factors (e.g. hepatocyte growth factor) and proinflammatory cytokines (e.g. TNFα, interleukin-6) stimulates proliferation of muscle satellite cells [41], but prevents their differentiation [41] – a process that is enhanced by canonical NFkB activation. Peak vimentin expression is directly related to maximal proliferation of satellite cells [42]. Phagocytosis of necrotic muscles and end-stage neutrophils prompts proinflammatory M1 macrophages to switch to a proresolution M2 phenotype [41]. Production of interleukin-4 by M2 macrophages, infiltrating eosinophils and resident mast cells is required for muscle regeneration after injury [43▪▪]. Interleukin-4 activates resident fibro-adipocytes (FAPs), which in turn support differentiation of proliferating myogenic precursors [44] by an unknown mechanism. Interleukin-4 signaling also promotes FAP-mediated phagocytic clearance of necrotic debris [43▪▪] – a process that has been previously attributed solely to macrophages. The absence of eosinophils, IL4 or FAPs, or disruption of FAPs response to Interleukin-4, significantly delays muscle regeneration [43▪▪]. Healing phagocytosis is further facilitated by increased phagocyte expression of the scavenger receptor CD36, and by prod0uction of lipoxins (reviewed in [45]) and PPARγ activators such as prostaglandin 15d-PGJ2. At this stage, inflammation is curtailed, mature myofibril regeneration is ensured and vimentin expression declines [15]. This cycle is illustrated in Fig. 2.
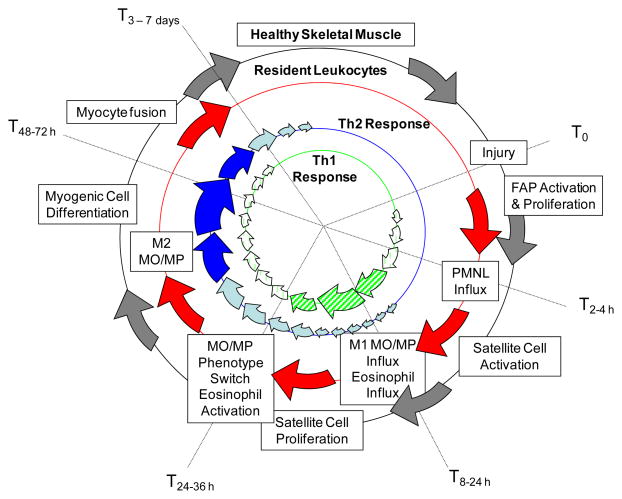
FIGURE 2.
Muscle injury, inflammation and regeneration. Injury stimulates polymorphonuclear leukocyte (PMNL) influx as well as satellite cell and fibro-adipocyte (FAP) activation. Satellite cells and others produce chemoattractants that stimulate influx of eosinophils and M1 monocyte/macrophages (MO/MP). Proinflammatory (Th1-type) cytokines produced by M1 cells promote satellite cell proliferation, but prevent their differentiation. Phagocytosis of muscle debris and dying PMNL and interleukin-4 production by eosinophils prompts the M1 to M2 MO/MP phenotype switch, resulting in a Th2-type cytokine response. The Th2 cytokines, including interleukin-4, activate resident fibroadipocytes (FAPs) to promote myogenic precursor cell differentiation/maturation to resolve injury.
Several steps in this process are confirmed to be, or are likely, NSAID-sensitive. The different COX isoforms, and the prostaglandins they generate, contribute in unique and in overlapping ways to the acute and resolution phases of myogenesis including proliferation, differentiation and fusion (reviewed in [46]). Experimental studies in mice demonstrate that COX-2 is increased early in injured muscles and declines by day 7, whereas COX-1 expression is only modestly affected [47]. COX-2-derived PGs stimulate myoblast proliferation in vitro and NSAIDs inhibit this proliferation [47,48]. Administration of NSAIDs during early stages of muscle repair in vivo also reduces the number of myoblasts [47]. Until a critical threshold number of myoblasts is generated, myofiber repair is delayed [47]. Similarly, blocking infiltration of proinflammatory M1 macrophages into injured muscle, either by systemic depletion of these cells [41] or by early administration of NSAIDs [47], significantly prolongs or prevents muscle regeneration. Although COX-2 deficiency is also associated with decreased numbers of infiltrating macrophages [47], it has been shown that myogenesis does occur, though much more slowly, in the absence of these inflammatory cells [49]. Administration of NSAIDs at later times after injury has no effect of muscle regeneration, suggesting that COX-2-derived PGs are critical only during the early stages of muscle regeneration [47]. Though the mechanism is not known, prostaglandin-mediated activation of PPARγ suggests a role for COX in this process and thus is potentially NSAID-sensitive.
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS AND SUSCEPTIBILITY TO POST-INJURY INFECTION
Nonselective NSAIDs are commonly used to alleviate pain associated with muscle injuries. To investigate their role in predisposing injured muscles to GAS infection, we treated EC-exercised animals with ketorolac tromethamine or saline at 47 h postinjury, followed 1 h later with intravenous GAS. Ketorolac significantly increased in the number of GAS in the injured muscles compared with saline-treated controls [10]. Thus, NSAIDs, given at a time postinjury that correlates to the peak of perceived muscle soreness in humans, and to maximal vimentin expression during muscle regeneration, increases the susceptibility of injured muscles to secondary GAS infection. These data demonstrated a direct link between muscle injury, NSAID use and cryptogenic GAS infection.
How NSAIDs augment susceptibility to postinjury GAS infection is wholly unknown. Our preliminary studies suggest that an NSAID-induced delay in muscle cell maturation prolongs the availability of the GAS-binding target. Although macrophage switching from a pro- to anti-inflammatory phenotype facilitates timely muscle regeneration after injury, our work suggests that NSAIDs do not affect this transition in vivo (author’s unpublished observations). Other possible mechanisms are currently under investigation.
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS AND THE PROGRESSION OF ESTABLISHED GROUP A STREPTOCOCCAL INFECTION
Most authors will concede that NSAIDs confound early recognition of GAS infection and thereby delay treatment. However, controversy remains regarding whether NSAIDs directly contribute to more severe GAS soft tissue infection. To investigate this issue, we recently studied the effects of selective and nonselective NSAIDs, alone and in combination with antibiotic therapy, on the outcome of experimental GAS myonecrosis [29▪▪]. NSAIDs were initiated 1 h after bacterial challenge and 7 h prior to antibiotic treatment. This design mimics the human scenario in which NSAID administration (usually nonselective NSAIDs) often precedes antibiotic therapy, as patients and physicians frequently do not suspect an existing deep necrotizing soft tissue infection, especially in the ~50% of cases that lack an obvious portal of bacterial entry [2].
Initial experiments examined the effects of ketorolac tromethamine on survival in animals challenged with LD100-high GAS. As expected, this dose of GAS resulted in fulminant infection in which untreated animals died by day 6 [29▪▪]. Ketorolac-treated animals developed a significantly more severe and rapidly progressive disease course resulting in 100% mortality by day 2 [29▪▪]. To ensure that these results were independent of both the inoculum size used and the nonselective NSAID chosen, the experiment was repeated using a four-fold lower LD100 dose of GAS (LD100-low) and two additional nonselective NSAIDs, ibuprofen and indomethacin, the latter being in a different NSAID chemical subclass and considered ‘dual-acting’ (i.e. also inhibits 5-lipoxygenase) [35]. All three nonselective COX inhibitors greatly accelerated the disease progression and significantly shortened the times to 50 and 100% mortality by as much as 4 days [29▪▪]. These findings extend the observations of Weng et al. [30] in which the nonselective NSAID, ibuprofen, significantly increased mortalityin animals challenged intramuscularly with a sublethal dose of GAS.
The effects of NSAIDs on antibiotic efficacy were similarly studied. To ensure that any beneficial or adverse impacts of NSAIDs would be observed, antibiotics (penicillin, clindamycin, or a combination of penicillin plus clindamycin) were used at 50% effective doses. Antibiotics were begun 8 h after infection and continued for 72 h either alone or in combination with selective or nonselective NSAIDs. As expected, antibiotics alone demonstrated significant, but transient, therapeutic benefits [29▪▪]. Addition of nonselective NSAIDs to each of the antibiotic regimens significantly accelerated disease progression, shortening the time to 50% death and resulting in significantly greater mortality at day 14 compared with antibiotic treatment alone [29▪▪].
Early administration of a COX-2-selective NSAID alone or in combination with antibiotics had little effect on the disease course or outcome of infection in our model [29▪▪]. These results contrast with other experimental murine infection studies in which strategies targeting COX-2 provided protection against group B streptococcus peritonitis [50] or Pseudomonas aeruginosa pneumonia [51]. Such results have prompted some author to conclude that COX-2-selective NSAIDs hold potential as an adjunct to antibiotic therapy for treatment of severe infections. The conclusion should be considered with caution in that the inbred mouse species utilized had preexisting immune biases that could have influenced outcome. In addition, the different routes of bacterial administration and the type and timing of NSAID administration could also be important factors.
Our studies demonstrated a nonsignificant trend toward improved outcome in animals receiving antibiotics plus the COX-1-selective NSAID [29▪▪]. This supports studies demonstrating that pharmacologic blockade of COX-1 improves neutrophil-mediated clearance of pathogens, especially when used in combination with antibiotics [50]. However, the precise role of COX-1 in severe GAS soft tissue infection requires further study.
In total, these findings support the notion that in GAS soft tissue infections, nonselective NSAIDs shift the host/pathogen interaction in favor of the pathogen, and suggest that once infection is established, even a brief exposure to such NSAIDs limits antibiotic efficacy and contributes to worse outcome.
CONCLUSION
NSAIDs are important anti-inflammatory and analgesic drugs; however, new experimental data suggest that nonselective NSAIDs do more than simply mask the signs and symptoms of developing infection. Instead, these agents delay muscle regeneration, increase susceptibility to postinjury GAS infection and dramatically accelerate GAS disease progression in established soft tissue infection. Even short-term exposure to such NSAIDs may reduce antibiotic efficacy.
We propose the following model relating injury and NSAID use to severe GAS soft tissue infection. At 24–48 h after acute injury, regenerating myoblasts maximally express the vimentin on their surface. In the absence of a penetrating wound, GAS traffic to the injured site likely via a transient bacteremia from the oropharynx, and attach specifically to vimentin expressed on injured muscles in sufficient numbers to initiate infection. NSAID-induced delays in muscle regeneration and cellular immunosuppression further enhance GAS-binding and facilitate unrestrained bacterial proliferation. Once established, proliferating organisms release potent cytolysins, causing further muscle cell injury and rendering intracellular vimentin accessible to amplify the GAS/muscle interaction.
The innate and acquired immune status of the host, the class of NSAID used and the timing of NSAID administration relative to the stage of muscle repair are clearly of fundamental importance in this scenario and each would likely influence the outcome of the host/pathogen interaction. A more thorough understanding of the molecular mechanisms that control cellular responses to tissue injury and infection, and the role of NSAIDs in this process, is of significant clinical importance and could shift the current paradigm of pain management in a myriad of settings and avert the devastating consequences of severe GAS infection.
Footnotes
Financial support and sponsorship
None.
Conflicts of interest
This work was supported in part by a Merit Review Award (#I01BX007080, AEB) from the United States Department of Veterans Affairs, Biomedical Laboratory Research and Development Program and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant #P20GM103408. None of the authors have any conflicts of interest to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Bryant AE, Stevens DL. Streptococcus pyogenes. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8. Philadelphia, PA: Elsevier Saunders; 2015. pp. 2285–2299. [Google Scholar]
- 2.Stevens DL, Tanner MH, Winship J, et al. Reappearance of scarlet fever toxin A among streptococci in the Rocky Mountain West: Severe group A streptococcal infections associated with a toxic shock-like syndrome. N Eng J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 3.Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med. 1985;145:1020–1023. [PubMed] [Google Scholar]
- 4.Nuwayhid ZB, Aronoff DM, Mulla ZD. Blunt trauma as a risk factor for group A streptococcal necrotizing fasciitis. Ann Epidemiol. 2007;17:878–881. doi: 10.1016/j.annepidem.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193:1685–1692. doi: 10.1086/504261. [DOI] [PubMed] [Google Scholar]
- 6.Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106(Pt 4):1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- 7.Xu B, deWaal RM, Mor-Vaknin N, et al. The endothelial cell-specific antibody PAL-E identifies a secreted form of vimentin in the blood vasculature. Mol Cell Biol. 2004;24:9198–9206. doi: 10.1128/MCB.24.20.9198-9206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podor TJ, Singh D, Chindemi P, et al. Vimentin exposed on activated platelets and platelet microparticles localizes vitronectin and plasminogen activator inhibitor complexes on their surface. J Biol Chem. 2002;277:7529–7539. doi: 10.1074/jbc.M109675200. [DOI] [PubMed] [Google Scholar]
- 9.Boilard E, Bourgoin SG, Bernatchez C, Surette ME. Identification of an autoantigen on the surface of apoptotic human T cells as a new protein interacting with inflammatory group IIA phospholipase A2. Blood. 2003;102:2901–2909. doi: 10.1182/blood-2002-12-3702. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton SM, Bayer CR, Stevens DL, et al. Muscle injury, vimentin expression, and nonsteroidal anti-inflammatory drugs predispose to cryptic group A streptococcal necrotizing infection. J Infect Dis. 2008;198:1692–1698. doi: 10.1086/593016. This basic science study utilizes a unique model of cryptogenic group A streptococcal infection and links nonpenetrating muscle injiury, vimentin expression and NSAID use to initiation of infection at the injured site. [DOI] [PubMed] [Google Scholar]
- 11.Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev. 2012;18:42–97. [PubMed] [Google Scholar]
- 12.Martineau LC, Gardiner PF. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J Appl Physiol. 2001;91:693–702. doi: 10.1152/jappl.2001.91.2.693. [DOI] [PubMed] [Google Scholar]
- 13.Barash IA, Mathew L, Ryan AF, et al. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol. 2004;286:C355–C364. doi: 10.1152/ajpcell.00211.2003. [DOI] [PubMed] [Google Scholar]
- 14.Warren GL, Summan M, Gao X, et al. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol. 2007;582:825–841. doi: 10.1113/jphysiol.2007.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaittinen S, Lukka R, Sahlgren C, et al. The expression of intermediate filament protein nestin as related to vimentin and desmin in regenerating skeletal muscle. J Neuropathol Exp Neurol. 2001;60:588–597. doi: 10.1093/jnen/60.6.588. [DOI] [PubMed] [Google Scholar]
- 16.Brun-Buisson CJL, Saada M, Trunet P, et al. Haemolytic streptococcal gangrene and nonsteroidal anti-inflammatory drugs. British Med J. 1985;290:1786. doi: 10.1136/bmj.290.6484.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens DL. Could nonsteroidal anti-inflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome? Clin Infect Dis. 1995;21:977–980. doi: 10.1093/clinids/21.4.977. [DOI] [PubMed] [Google Scholar]
- 18.Aronoff DM, Bloch KC. Assessing the relationship between the use of nonsteroidal antiinflammatory drugs and necrotizing fasciitis caused by group A streptococcus. Medicine (Baltimore) 2003;82:225–235. doi: 10.1097/01.md.0000085060.63483.bb. [DOI] [PubMed] [Google Scholar]
- 19.Lesko SM, O’Brien KL, Schwartz B, et al. Invasive group A streptococcal infection and nonsteroidal antiinflammatory drug use among children with primary varicella. Pediatrics. 2001;107:1108–1115. doi: 10.1542/peds.107.5.1108. [DOI] [PubMed] [Google Scholar]
- 20.Lesko SM. The safety of ibuprofen suspension in children. Int J Clin Pract Suppl. 2003:50–53. [PubMed] [Google Scholar]
- 21.Lamagni TL, Neal S, Keshishian C, et al. Severe Streptococcus pyogenes Infections, United Kingdom, 2003–2004. Emerg Infect Dis. 2008;14:201–209. doi: 10.3201/eid1402.070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubos F, Hue V, Grandbastien B, et al. Bacterial skin infections in children hospitalized with varicella: a possible negative impact of nonsteroidal anti-inflammatory drugs? Acta Derm Venereol. 2008;88:26–30. doi: 10.2340/00015555-0333. [DOI] [PubMed] [Google Scholar]
- 23.Mikaeloff Y, Kezouh A, Suissa S. Nonsteroidal anti-inflammatory drug use and the risk of severe skin and soft tissue complications in patients with varicella or zoster disease. Br J Clin Pharmacol. 2008;65:203–209. doi: 10.1111/j.1365-2125.2007.02997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leroy S, Marc E, Bavoux F, et al. Hospitalization for severe bacterial infections in children after exposure to NSAIDs: a prospective adverse drug reaction reporting study. Clin Drug Investig. 2010;30:179–185. doi: 10.2165/11532890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Das DK, Baker MG, Venugopal K. Risk factors, microbiological findings and outcomes of necrotizing fasciitis in New Zealand: a retrospective chart review. BMC Infect Dis. 2012;12:348. doi: 10.1186/1471-2334-12-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guibal F, Muffat-Joly M, Terris B, et al. Effects of diclofenac on experimental streptococcal necrotizing fasciitis (NF) in rabbit. Arch Dermatol Res. 1998;290:628–633. doi: 10.1007/s004030050363. [DOI] [PubMed] [Google Scholar]
- 27.Suleyman H, Demircan B, Karagoz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. 2007;59:247–258. [PubMed] [Google Scholar]
- 28.Goldmann O, Hertzen E, Hecht A, et al. Inducible cyclooxygenase released prostaglandin E2 modulates the severity of infection caused by Streptococcus pyogenes. J Immunol. 2010;185:2372–2381. doi: 10.4049/jimmunol.1000838. [DOI] [PubMed] [Google Scholar]
- 29▪▪.Hamilton SM, Bayer CR, Stevens DL, Bryant AE. Effects of selective and nonselective nonsteroidal anti-inflammatory drugs on antibiotic efficacy of experimental group A streptococcal myonecrosis. J Infect Dis. 2014;209:1429–1435. doi: 10.1093/infdis/jit594. This report demonstrates that three different nonselective NSAIDs each accelerated the course of disease, increased mortality and limited antibiotic efficacy in experimental streptococcal myonecrosis. COX-selective NSAIDs were largely without effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng TC, Chen CC, Toh HS, Tang HJ. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J Microbiol Immunol Infect. 2011;44:418–423. doi: 10.1016/j.jmii.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Souyri C, Olivier P, Grolleau S, Lapeyre-Mestre M. Severe necrotizing soft-tissue infections and nonsteroidal anti-inflammatory drugs. Clin Exp Dermatol. 2008;33:249–255. doi: 10.1111/j.1365-2230.2007.02652.x. [DOI] [PubMed] [Google Scholar]
- 32.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 33.Ott E, Nussmeier NA, Duke PC, et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125:1481–1492. doi: 10.1016/s0022-5223(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 34.Messika J, Sztrymf B, Bertrand F, et al. Risks of nonsteroidal antiinflammatory drugs in undiagnosed intensive care unit pneumococcal pneumonia: younger and more severely affected patients. J Crit Care. 2014;29:733–738. doi: 10.1016/j.jcrc.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Brooks PM, Day RO. Nonsteroidal antiinflammatory drugs–differences and similarities. N Engl J Med. 1991;324:1716–1725. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- 36.Warner TD, Giuliano F, Vojnovic I, et al. Nonsteroid drug selectivities for cyclooxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leone S, Ottani A, Bertolini A. Dual acting anti-inflammatory drugs. Curr Top Med Chem. 2007;7:265–275. doi: 10.2174/156802607779941341. [DOI] [PubMed] [Google Scholar]
- 38.Pang L, Nie M, Corbett L, Knox AJ. Cyclooxygenase-2 expression by nonsteroidal anti-inflammatory drugs in human airway smooth muscle cells: role of peroxisome proliferator-activated receptors. J Immunol. 2003;170:1043–1051. doi: 10.4049/jimmunol.170.2.1043. [DOI] [PubMed] [Google Scholar]
- 39.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura D, Sakai H, Sato T, et al. Roles of ADAM8 in elimination of injured muscle fibers prior to skeletal muscle regeneration. Mech Dev. 2015;135:58–67. doi: 10.1016/j.mod.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vater R, Cullen MJ, Harris JB. The expression of vimentin in satellite cells of regenerating skeletal muscle in vivo. Histochem J. 1994;26:916–928. [PubMed] [Google Scholar]
- 43▪▪.Heredia JE, Mukundan L, Chen FM, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. This elegant study documents the roles of eosinophil-derived interleukin-4 and fibroadipocytes in driving muscle regeneration after injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;58:1237–1246. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 47.Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol. 2004;287:C475–C483. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- 48.Mackey AL, Kjaer M, Dandanell S, et al. The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J Appl Physiol. 2007;103:425–431. doi: 10.1152/japplphysiol.00157.2007. [DOI] [PubMed] [Google Scholar]
- 49.Robertson TA, Grounds MD, Papadimitriou JM. Elucidation of aspects of murine skeletal muscle regeneration using local and whole body irradiation. J Anat. 1992;181(Pt 2):265–276. [PMC free article] [PubMed] [Google Scholar]
- 50.Stables MJ, Newson J, Ayoub SS, et al. Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic-susceptible and -resistant bacteria. Blood. 2010;116:2950–2959. doi: 10.1182/blood-2010-05-284844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadikot RT, Zeng H, Azim AC, et al. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur J Immunol. 2007;37:1001–1009. doi: 10.1002/eji.200636636. [DOI] [PubMed] [Google Scholar]