Abstract
Podocyte damage and apoptosis are thought to be important if not essential in the development of glomerulosclerosis. Female estrogen receptor knockout mice develop glomerulosclerosis at 9 months of age due to excessive ovarian testosterone production and secretion. Here, we studied the pathogenesis of glomerulosclerosis in this mouse model to determine whether testosterone and/or 17β-estradiol directly affect the function and survival of podocytes. Glomerulosclerosis in these mice was associated with the expression of desmin and the loss of nephrin, markers of podocyte damage and apoptosis. Ovariectomy preserved the function and survival of podocytes by eliminating the source of endogenous testosterone production. In contrast, testosterone supplementation induced podocyte apoptosis in ovariectomized wild-type mice. Importantly, podocytes express functional androgen and estrogen receptors, which, upon stimulation by their respective ligands, have opposing effects. Testosterone induced podocyte apoptosis in vitro by androgen receptor activation, but independent of the TGF-β1 signaling pathway. Pretreatment with 17β-estradiol prevented testosterone-induced podocyte apoptosis, an estrogen receptor-dependent effect mediated by activation of the ERK signaling pathway, and protected podocytes from TGF-β1- or TNF-α-induced apoptosis. Thus, podocytes are target cells for testosterone and 17β-estradiol. These hormones modulate podocyte damage and apoptosis.
Keywords: apoptosis, disease and progression, glomerulosclerosis, podocyte, pathophysiology of renal
The main sex steroid hormones, 17β-estradiol (E2) and testosterone (T) modulate the development and progression of glomerulosclerosis (GS), a disease process largely determined by genetic traits, age, and sex. E2 via stimulation of the estrogen receptor (ER) α activates signaling pathways and regulates genes in glomerular/mesangial cells in a manner that is protective against GS.1,2 Accordingly, estrogen deficiency accelerates the progression3 and E2-replacement retards the development of GS in ovariectomized sclerosis-prone ROP Os/+ mice.4
In contrast, T from endogenous or exogenous sources exerts deleterious effects on the glomerulus. Female mice transgenic for an ERα gene deletion (αERKO) develop GS because of their elevated blood T levels that are eight times higher than those of their female littermates.5 Interestingly, the C57Bl6 (B6) background, which confers glomerular resistance to noxious stimuli such as diabetes or unilateral nephrectomy, does not protect female αERKO mice from the effects of high levels of circulating T.5 Ovariectomy (Ovx) prevents the onset of glomerular dysfunction in αERKO females by eliminating their endogenous T production.5 In contrast, T supplementation induces GS in ovariectomized B6 mice,5 whereas estrogen deficiency following Ovx had no deleterious effects on the glomerulus of B6 mice.
The cellular effects of estrogens and T in the pathogenesis of progressive kidney damage, in these and other studies, have focused on mesangial cells, for years considered the key player in the events leading to the progression of renal injury. In contrast, to our knowledge little data are available on the effects of sex hormones on podocytes,6 the cell type whose role may include initiation of progressive renal disease.7–11 The ability of the kidney to replace damaged or lost podocytes is limited as podocytes exhibit a reduced potential to regenerate via mitosis in the glomerulus.8,12 Thus, progressive podocyte damage characterized by foot process effacement, vacuolization, and detachment of podocytes from the glomerular basement membrane, which finally leads to the irreversible loss of podocytes are now considered important, if not essential, initial events in the development and progression of GS.7–12
In this study, we examined the effects of estrogens and T on podocyte damage and apoptosis. We found that female αERKO mice show podocyte damage and apoptosis, which was prevented by Ovx and reproduced in B6 mice by T supplementation. Moreover, we showed that cultured podocytes express androgen receptor (AR) and ER, and that estrogens and T have opposing effects on podocyte apoptosis. Finally, the involvement of the extracellular signal-regulated protein kinase (ERK) signaling pathway in these events was evaluated.
RESULTS
Podocyte injury in female αERKO mice
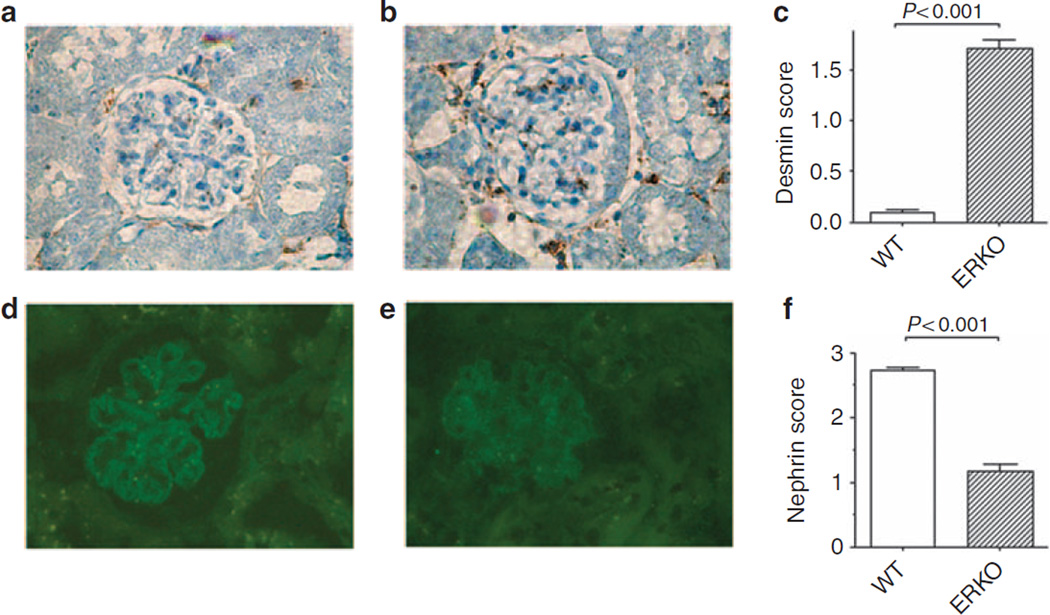
The expression of desmin, a marker of podocyte dedifferentiation and damage,13 was examined to evaluate podocyte injury in αERKO mice. Little or no expression of desmin was detected in wild-type (WT) littermates of αERKO mice (Figure 1a). In contrast, a strong positive signal was seen in glomeruli of αERKO mice (Figure 1b). Semi-quantitative evaluation of desmin staining confirmed that desmin neo-expression was higher in αERKO mice than in controls (Figure 1c).
Figure 1. Podocyte injury in female αERKO mice.
Immunohistochemical staining for desmin in glomeruli of female wild-type (WT) littermates (a) and female αERKO mice (b). Data are the mean ± s.e.m. (c). N = 5 mice/group. Immunofluorescence staining for nephrin in glomeruli of female wild-type littermates (d) and female αERKO mice (e). Data are the mean ± s.e.m. (f). N = 5 mice/group. Original magnifications: × 400. Student’s t-test was performed.
Nephrin, evaluated by indirect immunofluorescence, exhibited a glomerular epithelial staining pattern with a linear distribution along the peripheral capillary loops in WT littermates of αERKO mice (Figure 1d). In contrast, in glomeruli of αERKO mice (Figure 1e) there was a reduction of nephrin immunoreactivity (Figure 1f). Control sections incubated with the non-immune isotypic control antibody or with the appropriate labeled secondary antibody without the primary antibody were always negative (data not shown). In addition, we assessed the total number of podocytes per glomerulus by counting the cells expressing Wilms tumor (WT1), a specific marker of podocytes, and found that glomeruli of αERKO mice had a decreased number of WT1-positive cells compared with WT littermates (Table 1).
Table 1.
Wilms tumor (WT1)-positive cells per glomerulus in the different experimental groups
| αERKO | Wild type | αERKO/Ovx | Wild type/Ovx | B6/Ovx+T | B6/Ovx | |
|---|---|---|---|---|---|---|
| WT1-positive cells/glomerulus | 0.7 ± 0.06a,b | 3.0 ± 0.40 | 4.4 ± 0.0.58c | 3.9 ± 0.45d | 1.4 ± 0.01 | 2.3 ± 0.49e |
Abbreviation: Ovx, ovariectomy.
P < 0.005 compared with WT controls.
P < 0.0005 compared with αERKO/Ovx.
P < 0.005 compared with B6/Ovx+T.
P < 0.005 compared with B6/Ovx+T.
P < 0.05 compared with ERKO or ERKO/Ovx.
Increased podocyte apoptosis in female αERKO mice
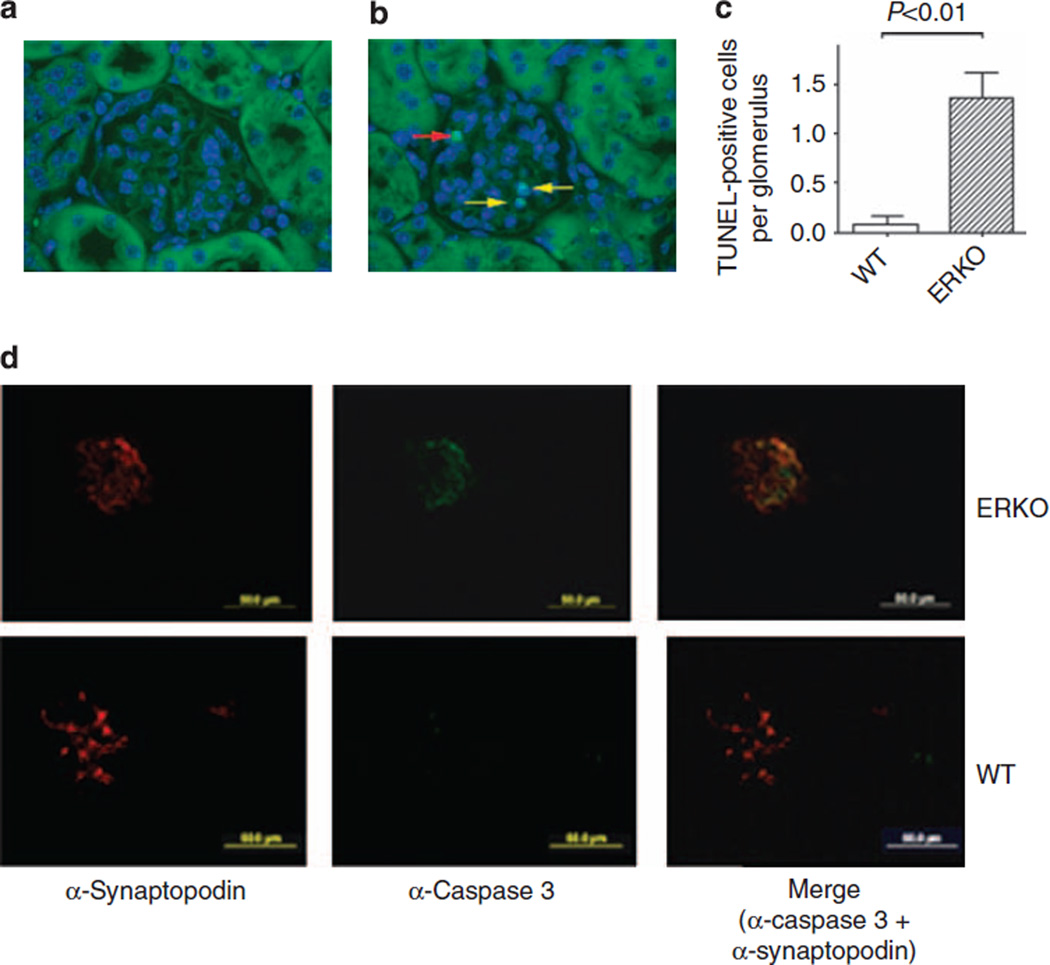
There was a 17-fold increase in the average of terminal dUTP nick-end labeling (TUNEL)-positive podocytes per glomerular section in αERKO mice compared with their WT littermates (Figure 2). On the contrary, no TUNEL-positive cells were detected among the other glomerular cell types or in the tubular compartment. In addition, we found increased podocyte-specific caspase 3 expression in αERKO glomeruli compared with WT littermates (Figure 2d). These results demonstrate that increased rates of apoptosis in podocytes are characteristic for this model of GS, and coincided with increased rates of podocyte damage as determined by desmin and nephrin immunostaining (see Figure 1).
Figure 2. Increased podocyte apoptosis in female αERKO mice.
Podocyte apoptosis in female wild-type (WT) littermates (a) and female αERKO mice (b), as detected by TUNEL assay. Yellow arrows indicate TUNEL-positive cells, whereas the red arrow indicates an artifactual staining of a red blood cell. Results are expressed as percentage of number of TUNEL-positive cells on the total number of glomerular cells and graphed as the mean ± s.e.m. (c). N = 5 mice/group. Original magnifications: × 400. Student’s t-test was performed.
(d) Representative images of caspase 3 immunofluorescence staining and colocalization with synaptopodin expression, used as podocyte marker, in female WT littermates and female αERKO mice. TUNEL, terminal dUTP nick-end labeling.
In vivo effects of T on podocytes
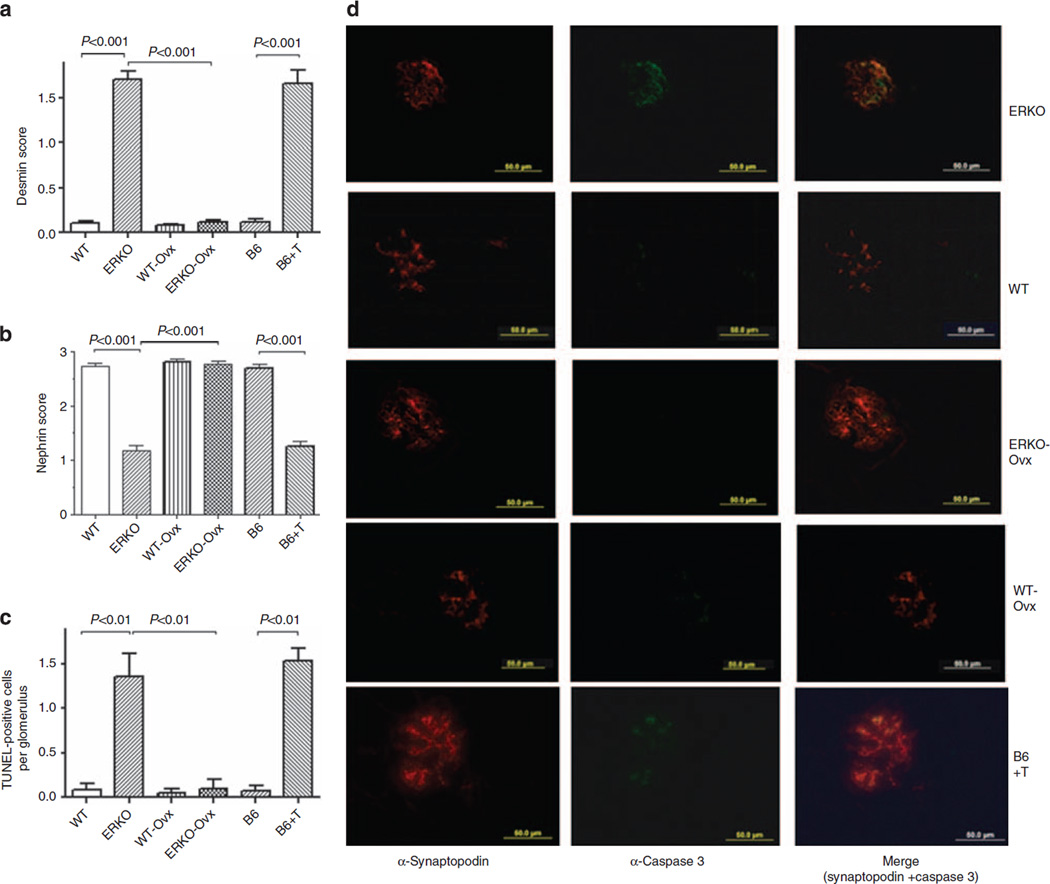
Ovx of αERKO mice reduced both podocyte damage, as shown by decreased desmin staining score and increased nephrin expression (Figure 3a and b), and podocyte loss (Table 1). In addition, the average of TUNEL-positive podocytes per glomerular section was significantly reduced in Ovx αERKO mice, returning to values similar to those measured in their WT littermates (Figure 3c). In contrast, Ovx of WT mice had neither effect on podocyte injury scores and apoptosis (Figure 3d), nor on podocyte number (Table 1). Finally, T supplementation of Ovx B6 mice induced a marked increase in both podocyte injury scores, as determined by desmin and nephrin immunostaining, and apoptosis rate (Figure 3d), as well as a significant reduction in the number of WT1 positive cells, compared with WT control glomeruli (Table 1). These data suggest that T action rather than estrogen deficiency has a predominant role in determining podocyte injury and apoptosis in vivo.
Figure 3. Testosterone induces in vivo podocyte injury and apoptosis in female αERKO mice.
Desmin (a) and nephrin (b) scores in glomeruli of female αERKO mice, their female wild-type (WT) littermates, ovariectomized female WT (WT-Ovx) and αERKO mice (ERKO-Ovx), and Ovx-B6 mice supplemented with T. Desmin and nephrin staining were measured as described in Materials and Methods and expressed as mean ± s.e.m. (c) Number of TUNEL-positive cells per glomerulus in the different experimental groups (mean ± s.e.m.). Sections were analyzed from 5 mice/group, 10 glomeruli/section. ANOVA with Newman-Keuls’ multicomparison test was performed. (d) Representative images of caspase 3 immunofluorescence staining and colocalization with synaptopodin expression, used as podocyte marker, in female αERKO mice, their female WT littermates, ovariectomized female WT (WT-Ovx) and αERKO mice (ERKO-Ovx), and Ovx-B6 mice supplemented with T. ANOVA, analysis of variance; Ovx, Ovariectomy; T, testosterone.
Estrogens protect podocytes from apoptosis induced by transforming growth factor (TGF)-β1 and tumor necrosis factor (TNF)-α
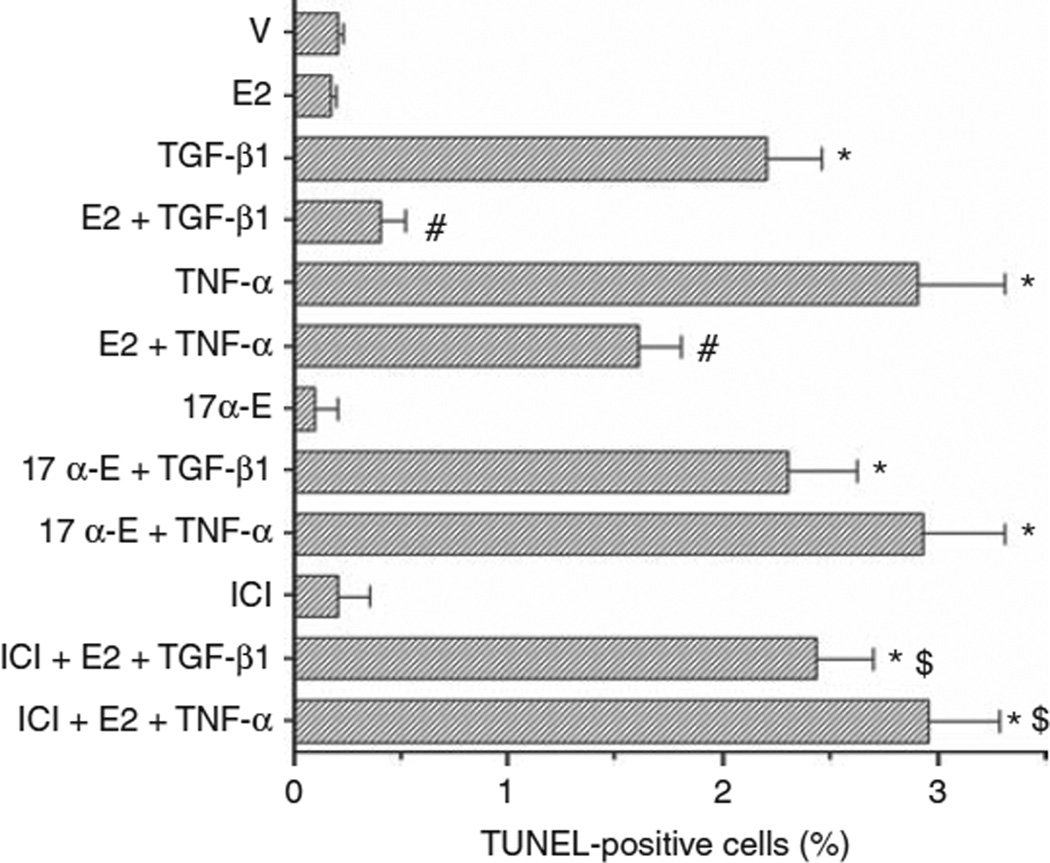
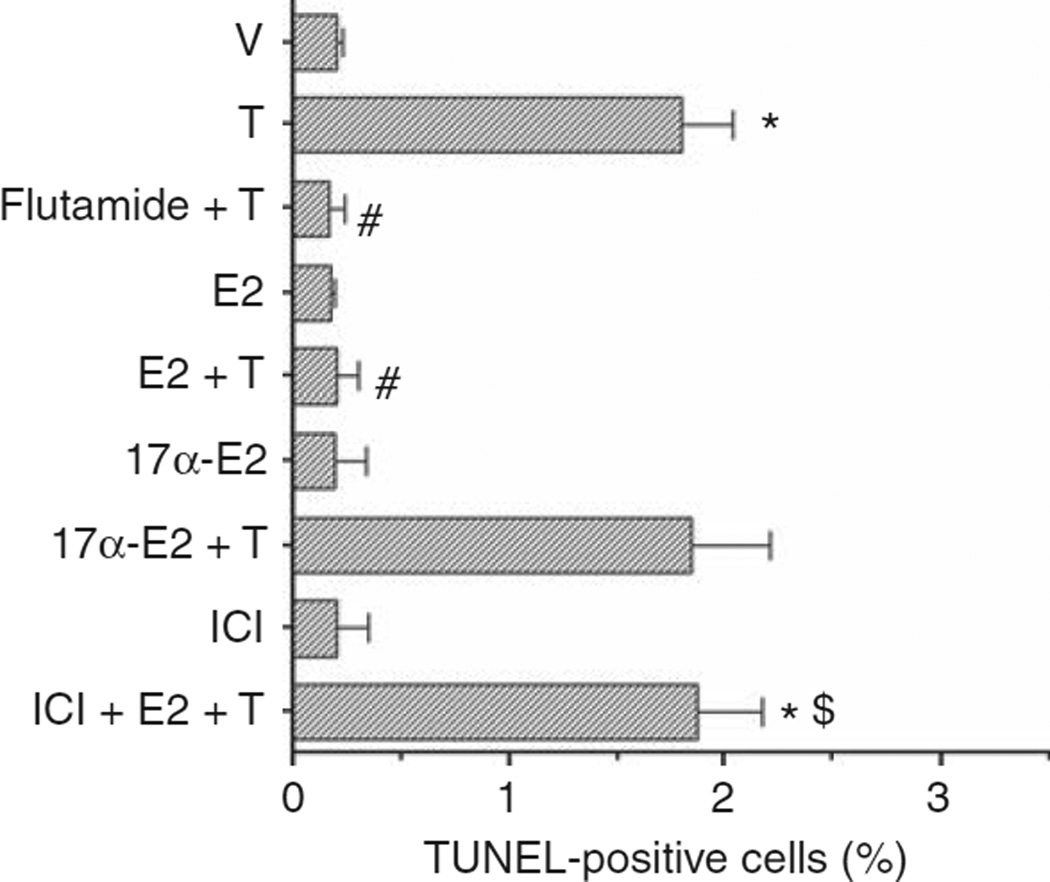
We tested whether E2 protects podocytes from apoptosis induced by TGF-β1 (10 ng/ml) and TNF-α (10 ng/ml). As expected, both TGF-β1 and TNF-α were able to induce apoptosis in cultured podocytes (Figure 4). Pretreatment of podocytes with physiological concentrations of E2 (1 nmol/l), significantly reduced the number of cells undergoing apoptosis after TGF-β1 and TNF-α stimulation (Figure 4). To determine whether E2-mediated protection from TGF-β1- and TNF-α-induced apoptosis was an ER-dependent effect, we incubated podocytes with the transcriptionally inactive stereoisomer 17α-estradiol, ICI 182780 (1 µmol/l), an antiestrogen compound devoid of agonist activity, or ICI, a complete ER antagonist, followed 1 h later by 1 nmol/l E2-containing medium. We found that equimolar concentrations of the transcriptionally inactive isomer 17α-estradiol did not protect cells from TGF-β1- and TNF-α-induced apoptosis. ICI abolished the protective effect of E2 on TGF-β1- and TNF-α-induced apoptosis (Figure 4), suggesting that this was an ER-dependent effect.
Figure 4. Estrogens protect podocytes from apoptosis induced by TGF-β1 and TNF-α.
Podocytes were treated in vitro with vehicle (V), TGF-β1 (10 ng/ml), TNF-α (10 ng/ml), E2 (1 nmol/l), 17α-estradiol (17α-E) (17α, 1 nmol/l), ICI 182780 (ICI, 1 µmol/l) or a combination. Apoptosis was measured in terms of TUNEL-positive cells (%) (see Materials and Methods for details). Data are the mean ± s.e.m. of 3–5 independent experiments. ANOVA with Newman-Keuls’ multicomparison test was performed. *P < 0.001 compared with vehicle alone, #P < 0.001 compared with TGF-β1 or TNF-α alone, respectively, $P < 0.001 compared with TGF-β1 + E2 or TNF-α + E2, respectively. Abbreviations: ANOVA, analysis of variance; E2, 17β-estradiol; TGF, transforming growth factor; TNF, tumor necrosis factor.
Expression of AR and ER in cultured podocytes
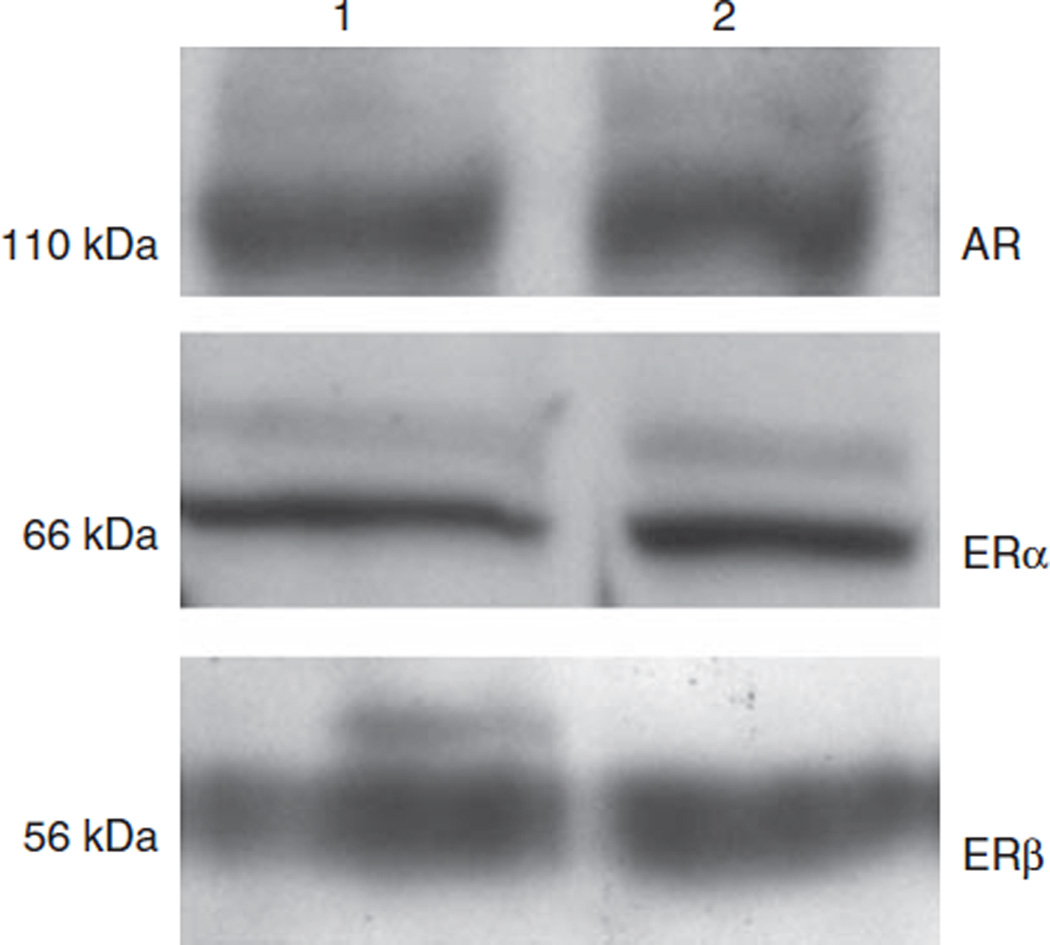
There were 250 ± 42 copies of ERα, 50 ± 10 copies of ERβ, and similar amounts of AR as that described for diabetic podocytes after estrogen treatment. ER and AR protein was expressed in cultured podocytes from female B6SJLF1/J (Figure 5). These data suggest that podocytes, as we had previously shown for mesangial cells,5 are target cells for sex steroid hormones.
Figure 5. Podocytes express androgen (AR) and estrogen receptors (ER).
Cell lysates were collected from cultured podocytes and western blot analysis performed as described in Materials and Methods. AR and ER expressions were detected at the expected molecular weights of 110 kDa (AR), 66 kDa (ERα), and 56 kDa (ERβ). Lane 1 and 2 podocyte cell lysates collected from two independent collections.
Proapoptotic effect of T on podocytes
The incubation of cultured podocytes with T (1 nmol/l) induced a ninefold increase in apoptosis rate as assessed by the TUNEL assay (Figure 6). The proapoptotic effect of T on podocytes was abolished by the administration of the androgen receptor antagonist flutamide (100 nmol/l, Figure 6), which restored the apoptosis rate to values similar to those observed in cells exposed to the vehicle alone (Figure 5). Pretreatment of podocytes with physiological concentrations of E2 (1 nmol/l), but not 17α-estradiol (1 nmol/l), significantly reduced the number of cells undergoing apoptosis after T stimulation (Figure 6). This was an ER-mediated effect since treatment with the complete ER antagonist ICI blocked the protective effects of E2 on T-induced apoptosis (Figure 6).
Figure 6. T induces in vitro podocyte apoptosis.
Podocytes were treated in vitro with vehicle (V), T (1 nmol/l), E2 (1 nmol/l), flutamide (100 nmol/l), ICI 182780 (ICI, 1 µmol/l), or a combination. Apoptosis was measured in terms of TUNEL-positive cells (%) (see Materials and Methods for details). Data are the mean ± s.e.m. of 3–5 independent experiments. ANOVA with Newman-Keuls’ multicomparison test was performed. *P < 0.001 compared with vehicle alone, #P < 0.001 compared with T alone, $P < 0.001 compared with E2 + T. ANOVA, analysis of variance; E2, 17β-estradiol; 17α-E2, 17α-estradiol; T, testosterone; TUNEL, terminal dUTP nick-end labeling.
Production of TGF-β1
Development of GS in αERKO mice was associated with increased TGF-β1 expression.5 As TGF-β1 has been shown to have a major role in inducing podocyte apoptosis,14 we evaluated whether T induced podocyte TGF-β1 production. No difference in TGF-β1 production was observed after T stimulation (data not shown).
Smad7 expression
Smad7 upregulation may induce apoptosis in podocytes independently from TGF-β1.14 We therefore examined by reverse transcription PCR whether (a) T directly induced Smad7 gene transcription, and (b) the proapoptotic effect of T in podocytes was mediated by increased Smad7 gene transcription. Reverse transcription PCR experiments showed that although TGF-β1 (10 ng/ml) induced a threefold increase in Smad7 gene expression, T stimulation did not modify Smad7 gene expression in podocytes after 1 and 3 h of incubation (data not shown).
ERK activation regulates estrogen effects on podocytes
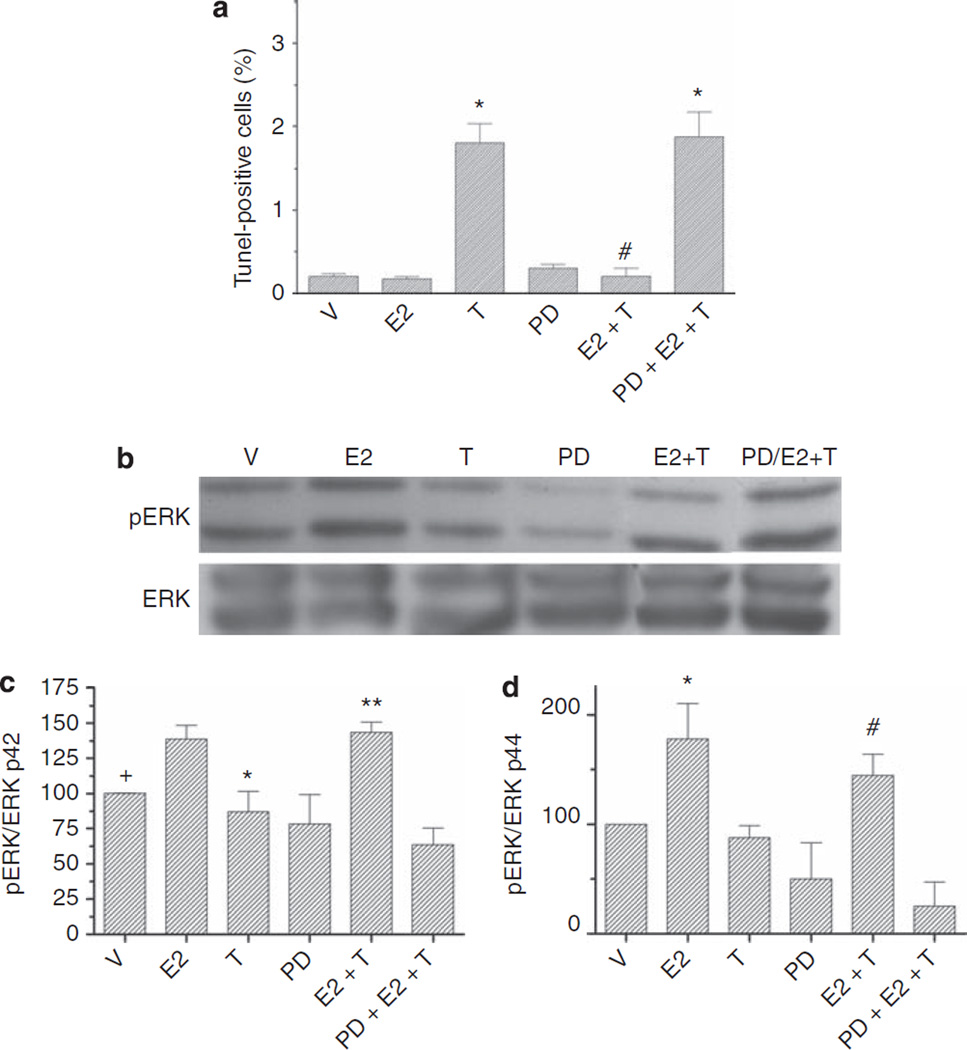
In many cell types, including mesangial cells, estrogens increase ERK phosphorylation via both nongenomic and genomic activation.15–17 Thus, we studied whether activation of ERK was involved in mediating the antiapoptotic effects of E2 on podocytes. Incubation of cultured podocytes with PD98059, the ERK kinase inhibitor, abolished the protective effect of E2 against T-induced apoptosis (Figure 7a). Western blot analysis confirmed that treatment with E2 increased ERK1/2 activation, both in the presence or absence of T (Figure 7), suggesting that the protective effect of estrogens on T-induced apoptosis was, at least partially, mediated by ERK activation.
Figure 7. E2-induced ERK activation protects podocytes from apoptosis.
Podocytes were treated with either vehicle (V), T (1 nmol/l), E2 (1 nmol/l), PD98059 (PD), or a combination. (a) Apoptosis was measured as % TUNEL-positive cells. Podocytes were counted as apoptotic only when nuclear labeling by DAPI (blue) and nuclear TUNEL labeling (green) colocalized resulting in turquoise nuclei. Data are expressed as the mean ± s.e.m. *P < 0.05 compared with control, #P < 0.05 compared with T alone. (b) Representative western blot analysis of pERK and total ERK in cultured podocytes. (c and d) Graphs represent the ratio of pERK/total ERK p42. Data are the mean ± s.e.m. of the percentage of the vehicle control value. N = 5 individual experiments. +P < 0.05 V compared with either E2 or E2 + T, *P < 0.05 T vs E2 or vs E2 + T, **P < 0.005 E2 + T vs PD + E2 + T (c) or pERK/totalERK p44 *P < 0.05 E2 vs V and T, and PD + E2 + T, #E2 + T vs T and PD + E2 + T (d). Abbreviations: DAPI, 4′-6-diamidino-2-phenylindole; E2, 17β-estradiol; ERK, extracellular signal-regulated protein kinase; T, testosterone; TUNEL, terminal dUTP nick-end labeling.
DISCUSSION
There is mounting evidence that sex steroid hormones affect and modulate the development and progression of GS; however, the molecular mechanism(s) underlying steroid hormone action on glomerular cells are only partially elucidated. In this study, we show, to our knowledge for the first time, that podocytes are target cells for T and E2, and that these sex steroid hormones are implicated in the pathogenesis of podocyte damage and apoptosis, thus influencing the pathophysiology of GS.
Podocytes have a pivotal role in the early functional and structural changes of kidney disease. In humans, podocyte loss closely correlates with the degree of progression in type 2 diabetic Pima Indians,18 type 1 diabetics,19 and IgA nephropathy patients.20 Induction of selective podocyte depletion by transgenic expression of CD25 or human diphteria toxin receptor and subsequent stimulation with anti-CD25 antibody or diphteria toxin,21,22 leads to pathological features of GS and progressive loss of renal function.
Experimental studies have focused on the molecular mechanisms underlying podocyte damage and loss, stressing the importance of podocyte apoptosis as a major factor leading to a decrease in podocyte number. Podocyte apoptosis has been detected during the early stages of progressive GS and peaks by the time of onset of proteinuria in TGF-β1-overexpressing,14 CD2AP-deficient,23 and diabetic Akita and db/db mice.24 In vitro, a variety of stimuli, including angiotensin II,25 oxygen radicals,26,27 mechanical stretch,28 cyclosporin A,29 TGF-β1, and related signaling molecules,14 are able to directly induce apoptosis in podocytes.
In this study we found that in female αERKO mice, podocyte damage and apoptosis participate in the development of GS. In these mice, the development of functional (increased urinary albumin excretion) and histological features (increased glomerular volume and ECM accumulation) of progressive GS5 were indeed associated with markers of podocyte damage, namely desmin neo-expression, nephrin loss, and reduced number of WT1-positive cells. Moreover, the rate of apoptosis in podocytes, as determined by TUNEL assay and podocyte specific caspase 3 expression, was significantly higher in female αERKO mice than in their WT littermates.
We previously reported that the development of GS in αERKO mice was related to their increased endogenous T production rather than to estrogen deficiency.5 Ovx prevented the onset of glomerular dysfunction in αERKO females, whereas T supplementation induced GS in ovariectomized B6 mice.5 In this study, we also found that Ovx prevented the decrease in podocyte number, evaluated as WT1-positive cells, in αERKO females, whereas T supplementation induced podocyte loss in ovariectomized B6 mice. Therefore, it would appear that podocyte damage and apoptosis in αERKO mice is mediated through androgen action rather than by estrogen deficiency. It cannot be excluded, however, that in other experimental or pathological settings, the lack of an estrogen protective effect per se could represent an important pathogenetic factor for the development of podocyte dysfunction and progressive GS.
The results of the present study extend our previously reported data on the glomerulus and mesangial cells as direct targets for T, by demonstrating, for the first time to our knowledge, the mRNA and protein expression of AR in podocytes. Effects of T on podocytes have been previously shown in a study by de Ruiter. Podocyte secretory activity (as evidenced by increased Golgi apparatus) was stimulated in vivo by T in male sticklebacks.30 Another important finding of our study is the demonstration that physiological concentrations of T directly induce apoptosis of podocytes in vivo and in vitro. This appears to be an AR-dependent effect as it was blocked by AR inhibition with flutamide. Verzola et al. have shown a direct proapoptotic effect of T in human tubular cells,31 but there are no previously published studies on glomerular cells.
We have previously shown that podocytes isolated from a model of type 2 diabetes and littermate controls express both ERα and ERβ subtypes.6 In addition, Gross et al. showed that estriol treatment prevented podocyte loss and injury in uninephrectomized ovariectomized rats.32 These data suggest that podocytes may be potential targets of estrogen action. Our results showed that the pretreatment of podocytes with physiological concentrations of E2, but not with its transcriptionally inactive stereoisomer 17α-estradiol, prevented podocyte apoptosis induced by T as well as by TGF-β1 and TNF-α. This was an ER-dependent effect, as ICI, a complete estrogen antagonist, abolished the protective effect of E2. Previous studies showed that estrogens are able to prevent apoptosis in a variety of cell types, including mesangial and tubular cells.31,33 As our results clearly showed that podocytes are direct targets of estrogen action, we speculate that the observed protective effects exerted in vivo by estrogen replacement therapy in several experimental models of renal disease3,4,34–36 may also be related to its action on podocytes. Moreover, the pretreatment of podocytes with E2 was able to prevent the apoptosis induced by T, suggesting that the balance between estrogens and Tmay have a relevant pathogenetic role in vivo. Interestingly, T production by the ovaries persists in women after menopause thereby changing the ratio of E2 to T in favor of T. This phenomenon may therefore potentially contribute to the faster progression of chronic kidney disease in women after menopause.37–41
We also evaluated whether T might induce TGF-β1 production, which has been shown to induce podocyte apoptosis.14 Although mesangial cells isolated from αERKO mice had increased TGF-β1 expression that was prevented by Ovx,35 we found no change in TGF-β1 production after podocyte stimulation with T. In addition, Smad7, the negative regulator of TGF-β1/Smad signaling42 was not upregulated by T, although it has been shown to induce apoptosis in podocytes both independently from and in cooperation with TGF-β1.14 Therefore, our data suggest that the T proapoptotic effect is not mediated by the regulation of podocyte TGF-β1 production; however, we can not exclude that, in vivo, TGF-β1 and T may have an additive or synergistic effect on podocyte apoptosis and GS progression.
Estrogens are known to induce activation of ERK via both nongenomic and genomic effects;15–17 thus, we studied whether ERK activation was involved in the antiapoptotic action of estrogens in podocytes. Western blot analysis revealed that treatment with E2 increased ERK1/2 activation; both in the presence or absence of T, suggesting this signaling pathway mediated the protective effect of estrogens on T-induced apoptosis.
Other groups have previously studied blood pressure levels in female αERKO mice and reported no difference in basal mean arterial pressure in both intact and ovariectomized αERKO mice compared with WT.43,44 Only Ovx αERKO mice treated with E2 had significantly smaller resting mean arterial pressure, suggesting an effect of estrogen on resting vascular tone possibly mediated by the ERβ subtype.43 On the other hand, it has been shown that ERα mediates the protective effects of estrogens against angiotensin II-induced hypertension.44 Therefore, we can not exclude that the lack of this protective effect may also contribute to the development of podocyte injury and GS in αERKO mice.
In conclusion, we showed that, in αERKO mice, the progression of GS is associated with the appearance of markers of podocyte damage and apoptosis, which, in this experimental model, are related to excessive androgen stimulation and not to estrogen deficiency. We characterized at the cellular level the opposing effects that estrogens and T exert on podocyte apoptosis. In particular, we showed that estrogens protect podocytes from apoptosis induced by TGF-β1, TNF-α, and T, and that T is able to directly induce podocyte apoptosis in vitro. These findings show that podocytes are direct targets of estrogen and androgen action and implicate sex hormones, for the first time, in the pathogenesis of podocyte damage and apoptosis.
MATERIALS AND METHODS
ICI 182780 (ICI) was obtained from Tocris (Ballwin, MO), and PD98059 (PD) from Calbiochem (La Jolla, CA). Antigen retrieval solution (Retrieve-ALL-1) was from Signet Laboratories (Dedlham, MA). The TUNEL assay (ApoTag) was from Oncor (Gaithersburg, MD). Antibodies anti-mouse WT1, anti-ERK and pERK, anti-AR, and protein A/G-agarose were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-caspase 3, anti-synaptopodin, and anti-desmin antibodies were from Cell Signaling Technology (Beverly, MA), Novus Biologicals (Littleton, CO), and DAKO (Gostrup, Denmark), respectively. An irrelevant IgG1 isotypic control antibody was purchased from Cedarlane (Hornby, Ontario, Canada). Phenol red-free DMEM/Ham’s F-12 medium (B medium) was purchased by Invitrogen Life Technologies (Grand Island, NY), and dextran–charcoal stripped fetal bovine serum (FBS) from HyClone (Pittsburgh, PA). TGF-β1 ELISA assay was from R&D Systems (Minneapolis, MN). All other reagents were purchased from Sigma–Aldrich (St Louis, MO).
Animals
Female ERKO mice and their WT littermates were obtained from Taconic farms (Germantown, NY).5 B6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Female αERKO, WT littermates, and B6 mice were ovariectomized at 3–4 months of age using the previously described procedure approved by the Committee for Animal Safety at the University of Miami School of Medicine.5 Placebo, E2 (0.05 mg/pellet), or T (12.5 mg/pellet) was administered to the mice via 90-day time-release pellets (Innovative Research of America, Sarasota, FL) as previously described.5 Mice were allowed free food and water and were killed at 9 months of age, as previously detailed.5 As we described previously, αERKO serum T levels were approximately eight times higher than that of female littermates. T supplementation of Ovx B6 mice increased T to levels approximately threefold higher than those of Sham B6 mice.
T and E2 levels were significantly decreased in Ovx αERKO mice. As expected, E2 supplementation resulted in increased uterine weight in WT littermate mice, but not in αERKO mice.5
Histopathology
Desmin immunostaining was performed by standard immunohistochemical procedure and analyzed by a renal pathologist blinded to the origin of the kidney slides using the following semiquantitative scoring system (0–3): score 0: no expression, score 1: weak expression, score 2: moderate expression, score 3: strong expression. The average value of the desmin score in 50 randomly selected nonsclerotic glomeruli in each animal was taken to represent the degree of podocyte injury.45
Nephrin staining was evaluated by indirect immunofluorescence in 2 µm-thick cryostat sections fixed in 3.5% paraformaldehyde for 15 min and then washed in phosphate-buffered saline, as previously described.46 Control experiments included incubation of sections with nonimmune isotypic control antibodies or the omission of primary antibodies followed by the appropriate labeled secondary antibodies. Nephrin staining in each glomerulus was scored from 0 to 3 by a renal pathologist blinded to the origin of the kidney slides and the average value of the nephrin score in 50 randomly selected glomeruli in each animal was taken.
For WT1 detection, slides were deparaffinized, exposed to antigen retrieval solution, blocked with 0.3% H2O2 for 30 min to block endogenous peroixidase, and incubated with a rabbit antimouse WT1 antibody overnight. Slides were then washed, exposed to a biotinylated rabbit antibody, followed by Vectastain elite ABC reagent.
Apoptosis detection
Apoptosis was evaluated by TUNEL assay following the manufacturer’s protocol. Podocytes were counted as apoptotic only when nuclear labeling by 4′-6-diamidino-2-phenylindole (blue) and nuclear TUNEL labeling (green) colocalized resulting in turquoise nuclei.14 Results are expressed as percentage of number of TUNEL-positive cells on the total number of glomerular cells. The expression of caspase 3, one of the caspases involved in the apoptosis signaling cascade, was also evaluated by standard immunofluorescence techniques. Synaptopodin expression, detected by indirect immunofluorescence, was used as a podocyte marker and the merge of caspase 3 and synaptopodin staining was considered a sign of podocyte specific expression of caspase 3.
Cell culture and experimental in vitro conditions
Glomerular epithelial cells isolated from 6-week-old female B6SJLF1/J mice were used at passages 23–25.47 At 3 days before apoptosis detection, cells were plated in eight-well Permanox slide at a density of 25 000 cells per well in phenol red-free B medium containing 20% dextran–charcoal stripped FBS.1 In the evening before the experiment, the medium was replaced with phenol red-free B medium containing 0.1% dextran–charcoal stripped FBS.
For induction of apoptosis, cells were incubated with vehicle control (0.001% ethanol), TGF-β1 (10 ng/ml), TNF-α (10 ng/ml), or T (1 nmol/l) for 24 h. In selected experiments, the cells were incubated with vehicle control (0.001% ethanol), E2 (1 nmol/l), ICI (1 µmol/l), ICI followed 1 h later by E2 (1 nmol/l), or 17α-estradiol (1 nmol/l) for 2 h before stimulation with TGF-β1 (10 ng/ml), TNF-α (10 ng/ml), or T (1 nmol/l) for 24 h. In some experiments, cells were exposed to 100 mmol/l flutamide for 4 h before incubation with T (1 nmol/l), or with 40 µmol/l PD for 30 min before incubation with E2 (1 nmol/l).
To evaluate Smad7 gene expression, cells were plated in 25 cm2 dishes at a concentration of 300 000 cells per dish in phenol red-free B medium containing 20% dextran–charcoal stripped FBS and grown for 3 days. In the evening before the experiment, the medium was replaced with phenol red-free B medium containing 0.1% dextran–charcoal stripped FBS. Cells were then incubated with vehicle control (0.001% ethanol), TGF-β1 (10 ng/ml), or T (1nmol/l) for 1 and 3 h. In selected experiments cells were incubated with vehicle control (0.001% ethanol) or E2 (1 nmol/l) for 2 h before stimulation with TGF-β1 (10 ng/ml) or T (1 nmol/l).
Isolation of RNA and quantitative analysis of RNA expression by real-time reverse transcription PCR
Total RNA was extracted from cultured podocytes isolated from female B6SJLF1/J mice using the guanidinium thiocyanate–phenol–-chloroform method as described. Amplification and quantification of target RNAs was performed on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA).1 Copy number for ERα, ERβ, and AR was determined as previously described.6 TaqMan ribosomal RNA control reagents to detect 18S ribosomal RNA served as an endogenous control to normalize for variations in the isolated RNA amount.
Western blot analysis
To determine the presence of AR and ER subtypes α and β, and to assess ERK activation in cultured podocytes, we performed western blot analysis as previously described.6
Statistical analysis
All data are expressed as mean ± s.e.m. Significance of differences between experimental groups was determined by analysis of variance in combination with Newman-Keuls’ multiple comparison test or by Student’s t-test. A P-value <0.05 was considered significant.
Acknowledgments
This work was supported by NIH Grant RO1AG17170-06 (to SJE and MK), and Progetto di Ricerca Sanitaria Finalizzata—Regione Piemonte (to EL).
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Potier M, Elliot SJ, Tack I, et al. Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol. 2001;12:241–251. doi: 10.1681/ASN.V122241. [DOI] [PubMed] [Google Scholar]
- 2.Potier M, Karl M, Zheng F, et al. Estrogen-related abnormalities in glomerulosclerosis-prone mice: reduced mesangial cell estrogen receptor expression and prosclerotic response to estrogens. Am J Path. 2002;160:1877–1885. doi: 10.1016/S0002-9440(10)61134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliot SJ, Karl M, Berho M, et al. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am J Pathol. 2003;162:1441–1448. doi: 10.1016/S0002-9440(10)64277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karl M, Berho M, Pignac-Kobinger J, et al. Differential effects of continuous and intermittent 17beta-estradiol replacement and tamoxifen therapy on the prevention of glomerulosclerosis: modulation of the mesangial cell phenotype in vivo. Am J Pathol. 2006;169:351–361. doi: 10.2353/ajpath.2006.051255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliot SJ, Berho M, Korach K, et al. Gender-specific effects of endogenous testosterone: Female alpha-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007;72:464–472. doi: 10.1038/sj.ki.5002328. [DOI] [PubMed] [Google Scholar]
- 6.Catanuto P, Doublier S, Fornoni A, et al. 17-estradiol and Tamoxifen upregulate estrogen receptor â and regulate podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009;75:1194–1201. doi: 10.1038/ki.2009.69. [DOI] [PubMed] [Google Scholar]
- 7.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 8.Barisoni L, Mundel P. Podocyte biology and the emerging understanding of podocyte diseases. Am J Nephrol. 2003;23:353–360. doi: 10.1159/000072917. [DOI] [PubMed] [Google Scholar]
- 9.Kretzler M. Role of podocytes in focal sclerosis: defining the point of no return. J Am Soc Nephrol. 2005;16:2830–2832. doi: 10.1681/ASN.2005080841. [DOI] [PubMed] [Google Scholar]
- 10.Ly J, Alexander M, Quaggin SE. A podocentric view of nephrology. Curr Opin Nephrol Hypertens. 2004;13:299–305. doi: 10.1097/00041552-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 12.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 13.Yaoita E, Kawasaki K, Yamamoto T, et al. Variable expression of desmin in rat glomerular epithelial cells. Am J Pathol. 1990;136:899–908. [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karl M, Potier M, Schulman IH, et al. Autocrine activation of the local insulin-like growth factor I system is up-regulated by estrogen receptor (ER)-independent estrogen actions and accounts for decreased ER expression in type 2 diabetic mesangial cells. Endocrinology. 2005;146:889–900. doi: 10.1210/en.2004-1121. [DOI] [PubMed] [Google Scholar]
- 16.Santen RJ, Song RX, McPherson R, et al. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80:239–256. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- 17.Singh M, Setalo G, Jr, Guan X, et al. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 19.Steffes MW, Schmidt D, McCrery R, et al. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 20.Lemley KV, Lafayette RA, Safai M, et al. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1485. doi: 10.1046/j.1523-1755.2002.00269.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsusaka T, Xin J, Niwa S, et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16:1013–1023. doi: 10.1681/ASN.2004080720. [DOI] [PubMed] [Google Scholar]
- 22.Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 23.Schiffer M, Mundel P, Shaw AS, et al. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J Biol Chem. 2004;279:37004–37012. doi: 10.1074/jbc.M403534200. [DOI] [PubMed] [Google Scholar]
- 24.Susztak K, Raff AC, Schiffer M, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 25.Ding G, Reddy K, Kapasi AA, et al. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol. 2002;283:F173–F180. doi: 10.1152/ajprenal.00240.2001. [DOI] [PubMed] [Google Scholar]
- 26.Sanwal V, Pandya M, Bhaskaran M, et al. Puromycin aminonucleoside induces glomerular epithelial cell apoptosis. Exp Mol Pathol. 2001;70:54–64. doi: 10.1006/exmp.2000.2345. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Takemura H, Noiri E, et al. Puromycin aminonucleoside induces apoptosis and increases HNE in cultured glomerular epithelial cells(1) Free Radic Biol Med. 2001;31:615–623. doi: 10.1016/s0891-5849(01)00641-4. [DOI] [PubMed] [Google Scholar]
- 28.Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 29.Fornoni A, Li H, Foschi A, et al. Hepatocyte growth factor, but not insulin-like growth factor I, protects podocytes against cyclosporin A-induced apoptosis. Am J Pathol. 2001;158:275–280. doi: 10.1016/S0002-9440(10)63966-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Ruiter AJ. Testosterone-dependent changes in vivo and in vitro in the structure of the renal glomeruli of the teleost Gasterosteus aculeatus L. Cell Tissue Res. 1981;219:253–266. doi: 10.1007/BF00210146. [DOI] [PubMed] [Google Scholar]
- 31.Verzola D, Gandolfo MT, Salvatore F, et al. Testosterone promotes apoptotic damage in human renal tubular cells. Kidney Int. 2004;65:1252–1261. doi: 10.1111/j.1523-1755.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- 32.Gross M-L, Adamczak M, Rabe T, et al. Beneficial effects of estrogens on indices of renal damage in uninephrectomized SHRsp rats. J Am Soc Nephrol. 2004;15:348–358. doi: 10.1097/01.asn.0000105993.63023.d8. [DOI] [PubMed] [Google Scholar]
- 33.Blush J, Lei J, Ju W, et al. Estradiol reverses renal injury in Alb/TGF-beta1 transgenic mice. Kidney Int. 2004;66:2148–2154. doi: 10.1111/j.1523-1755.2004.66005.x. [DOI] [PubMed] [Google Scholar]
- 34.Chin M, Isono M, Isshiki K, et al. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am J Pathol. 2005;166:1629–1636. doi: 10.1016/s0002-9440(10)62473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji H, Menini S, Mok K, et al. Gonadal steroid regulation of renal injury in renal wrap hypertension. Am J Physiol Renal Physiol. 2005;288:F513–F520. doi: 10.1152/ajprenal.00032.2004. [DOI] [PubMed] [Google Scholar]
- 36.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol. 2004;15:1546–1556. doi: 10.1097/01.asn.0000128219.65330.ea. [DOI] [PubMed] [Google Scholar]
- 37.Haroun MK, Jaar BG, Hoffman SC, et al. Risk factors for chronic kidney disease: a prospective study of 23 534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14:2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 38.Jafar TH, Schmid CH, Stark PC, et al. The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrol Dial Transplant. 2003;18:2047–2053. doi: 10.1093/ndt/gfg317. [DOI] [PubMed] [Google Scholar]
- 39.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 40.Seliger SL, Davis C, Stehman-Breen C. Gender and the progression of renal disease. Curr Opin Nephrol Hypertens. 2001;10:219–225. doi: 10.1097/00041552-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 41.US Renal Data System: USRDS 2000 Annual Data Report. 2. Bethesda, MD: The National Institutes of Health, NIDDK; 2000. [Google Scholar]
- 42.Nakao A, Afrakhte M, Moren A, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 43.Pamidimukkala J, Xue B, Newton LG, et al. Estrogen receptor-{alpha} mediates estrogen facilitation of baroreflex heart rate responses in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H1063–H1070. doi: 10.1152/ajpheart.01163.2003. [DOI] [PubMed] [Google Scholar]
- 44.Xue B, Pamidimukkala J, Lubahn DB, et al. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol. 2007;292:H1770–H1776. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 45.Hoshi S, Shu Y, Yoshida F, et al. Podocyte injury promotes progressive nephropathy in zucker diabetic fatty rats. Lab Invest. 2002;82:25–35. doi: 10.1038/labinvest.3780392. [DOI] [PubMed] [Google Scholar]
- 46.Doublier S, Ruotsalainen V, Salvidio G, et al. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723–1731. doi: 10.1016/S0002-9440(10)64128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacKay K, Striker LJ, Stauffer JW, et al. Transforming growth factor-beta. Murine glomerular receptors and responses of isolated glomerular cells. J Clin Invest. 1989;83:1160–1167. doi: 10.1172/JCI113996. [DOI] [PMC free article] [PubMed] [Google Scholar]